An Experimental and Theoretical Study of Dye Properties of Thiophenyl Derivatives of 2-Hydroxy-1,4-naphthoquinone (Lawsone)
Abstract
1. Introduction
2. Results
2.1. Colorimetric Study
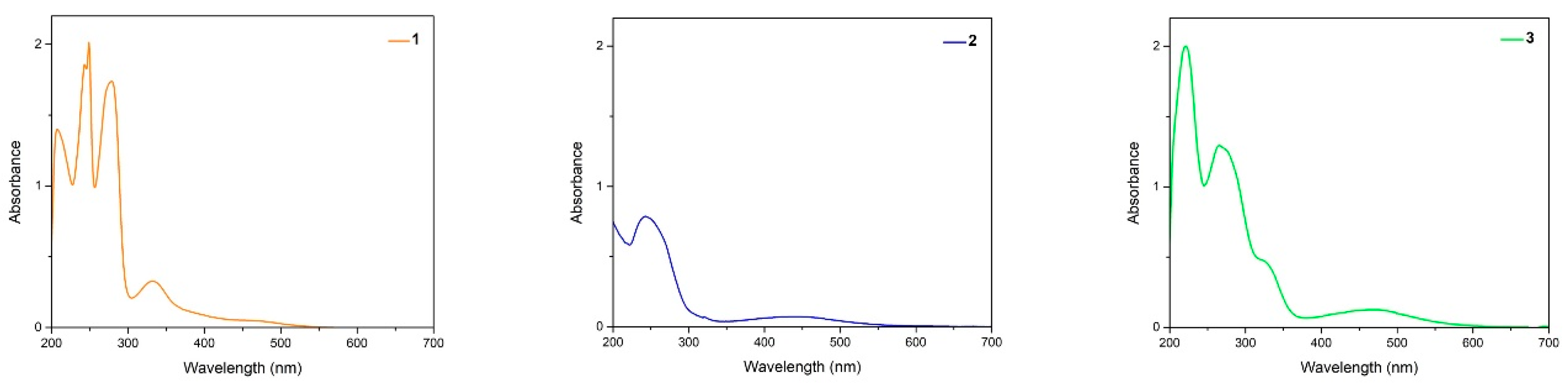
2.2. UV/Vis Spectroscopy
2.3. Theoretical Calculations
2.3.1. Electronic and Spectroscopic Study
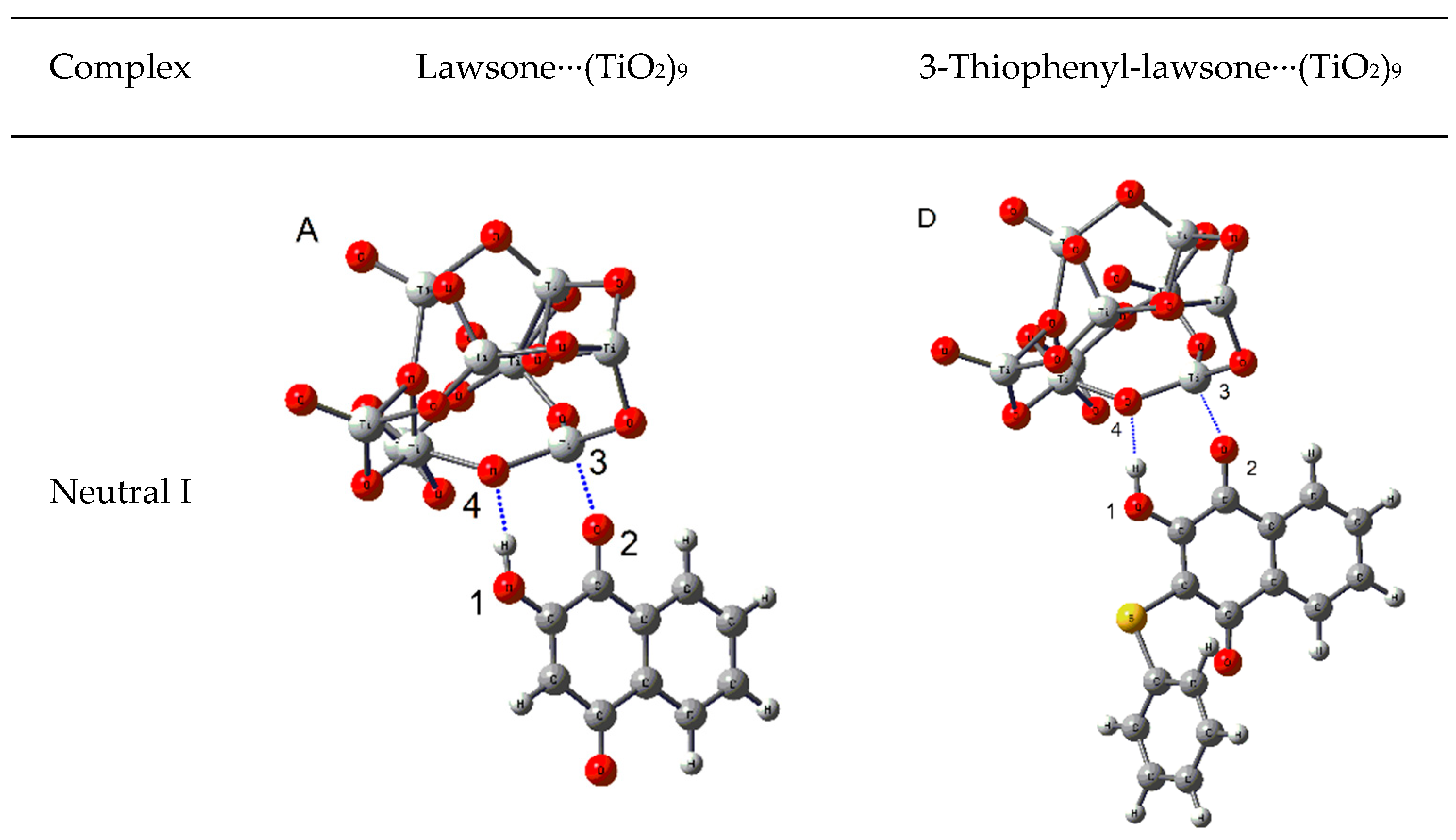
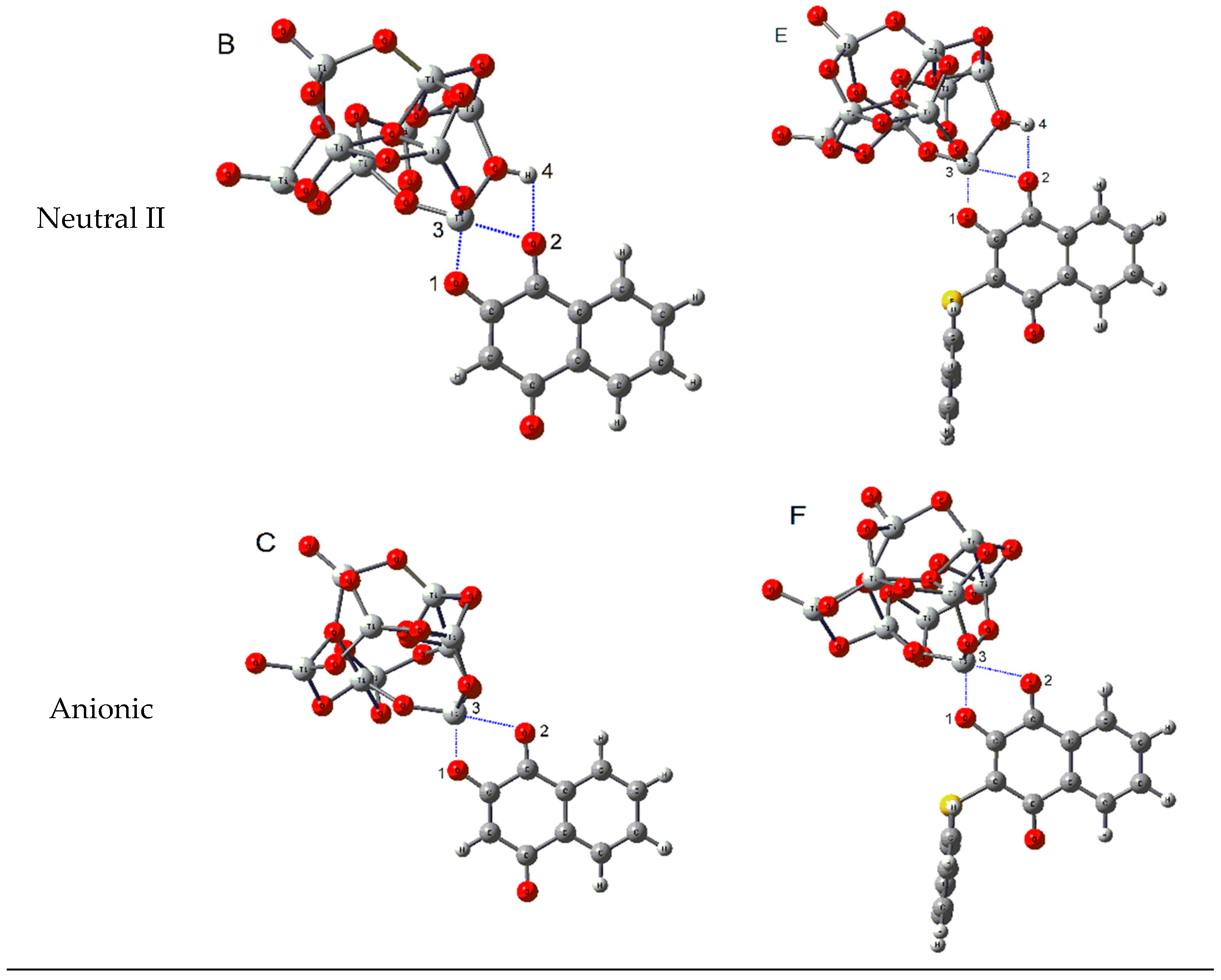
2.3.2. Adsorption of Lawsone and 3-Thiophenyl-lawsone on a TiO2 Model
3. Materials and Methods
3.1. Synthesis
3.2. Color Evaluation by Nix Pro Sensor
3.3. Measurement of the UV/Vis Spectrum
3.4. Computational Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, M.V.C.; Lardy, B.; Rousset, F.; Hazane-Puch, F.; Zhang, L.; Trocme, C.; Serrander, L.; Krause, K.-H.; Morel, F. Quinone compounds regulate the level of ROS production by the NADPH oxidase Nox4. Biochem. Pharmacol. 2013, 85, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Buettner, G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010, 49, 919–962. [Google Scholar] [CrossRef] [PubMed]
- Hillard, E.A.; de Abreu, F.C.; Ferreira, D.C.M.; Jaouen, G.; Goulart, M.O.F.; Amatore, C. Electrochemical parameters and techniques in drug development, with an emphasis on quinones and related compounds. Chem. Commun. 2008, 23, 2612–2628. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal Pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Neta, P. Radiation chemistry of quinonoid compounds. Quinonoid Compd. 1988, 2, 879–898. [Google Scholar]
- Martha, R.; Mubarok, M.; Darmawan, W.; Syafii, W.; Dumarcay, S.; Charbonnier, C.G.; Gérardin, P. Biomolecules of Interest Present in the Main Industrial Wood Species Used in Indonesia-A Review. J. Renew. Mater. 2021, 9, 399–449. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. Organic quinones towards advanced electrochemical energy storage: Recent advances and challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Islam, A.; Islam, S.; Hossain, A.; Nahar, K. Improving dyeability and antibacterial activity of Lawsonia inermis L on jute fabrics by chitosan pretreatment. Text. Cloth. Sustain. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Sauriasari, R.; Wang, D.-H.; Takemura, Y.; Tsutsui, K.; Masuoka, N.; Sano, K.; Horita, M.; Wang, B.-L.; Ogino, K. Cytotoxicity of lawsone and cytoprotective activity of antioxidants in catalase mutant Escherichia coli. Toxicology 2007, 235, 103–111. [Google Scholar] [CrossRef]
- Mirza, S.; Akbar, Z.; Ahmad, M.S. A Simple Nondestructive, Cost-Effective Method for Differentiation of Protein Crystals from Salt Crystals by Using a Natural Dye. Cryst. Growth Des. 2019, 19, 3612–3615. [Google Scholar] [CrossRef]
- Khadtare, S.S.; Ware, A.P.; Salunke-Gawali, S.; Jadkar, S.R.; Pingale, S.S.; Pathan, H.M. Dye sensitized solar cell with lawsone dye using a ZnO photoanode: Experimental and TD-DFT study. RSC Adv. 2015, 5, 17647–17652. [Google Scholar] [CrossRef]
- Fantacci, S.; De Angelis, F. Ab initio modeling of solar cell dye sensitizers: The hunt for red photons continues. Eur. J. Inorg. Chem. 2019, 2019, 743–750. [Google Scholar] [CrossRef]
- Kurpiers, J.; Ferron, T.; Roland, S.; Jakoby, M.; Thiede, T.; Jaiser, F.; Albrecht, S.; Janietz, S.; Collins, B.A.; Howard, I.A. Probing the pathways of free charge generation in organic bulk heterojunction solar cells. Nat. Commun. 2018, 9, 2038. [Google Scholar] [CrossRef] [PubMed]
- Makoye, A.; Pogrebnoi, A.; Pogrebnaya, T. Lawsone isomers, lawsone ether and bilawsone for dye-sensitized solar cells applications: DFT and UV–Vis studies. J. Mol. Graph. Model. 2020, 94, 107457. [Google Scholar] [CrossRef] [PubMed]
- Chaiamornnugool, P.; Tontapha, S.; Phatchana, R.; Ratchapolthavisin, N.; Kanokmedhakul, S.; Sang-aroon, W.; Amornkitbamrung, V. Performance and stability of low-cost dye-sensitized solar cell based crude and pre-concentrated anthocyanins: Combined experimental and DFT/TDDFT study. J. Mol. Struct. 2017, 1127, 145–155. [Google Scholar] [CrossRef]
- Sandquist, C.; McHale, J.L. Improved efficiency of betanin-based dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2011, 221, 90–97. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Radha, N.; Maheswari, G.; Anandan, S.; Manoharan, S.; Williams, R.V. Betalain and anthocyanin dye-sensitized solar cells. J. Appl. Electrochem. 2016, 46, 929–941. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; Cerdán-Pasarán, A.; López-Luke, T.; Rodríguez-Delgado, M.; Cárdenas-Chávez, D.L.; Cepeda-Pérez, E.; Ornelas-Soto, N. Astaxanthin from Haematococcus pluvialis as a natural photosensitizer for dye-sensitized solar cell. Algal Res. 2017, 26, 15–24. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K. Extraction, preparation and application of pigments from Cordyline fruticosa and Hylocereus polyrhizus as sensitizers for dye-sensitized solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 23–31. [Google Scholar] [CrossRef]
- Nan, H.; Shen, H.-P.; Wang, G.; Xie, S.-D.; Yang, G.-J.; Lin, H. Studies on the optical and photoelectric properties of anthocyanin and chlorophyll as natural co-sensitizers in dye sensitized solar cell. Opt. Mater. 2017, 73, 172–178. [Google Scholar] [CrossRef]
- Brogdon, P.; Cheema, H.; Delcamp, J.H. Near-infrared-absorbing metal-free organic, porphyrin, and phthalocyanine sensitizers for panchromatic dye-sensitized solar cells. ChemSusChem 2018, 11, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.; Pesala, B. Plasmonic enhancement of betanin-lawsone co-sensitized solar cells via tailored bimodal size distribution of silver nanoparticles. Sci. Rep. 2020, 10, 8240. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.; Pesala, B. Performance enhancement of betanin solar cells co-sensitized with indigo and lawsone: A Comparative Study. ACS Omega 2019, 4, 18023. [Google Scholar]
- Bhuiyan, M.A.R.; Islam, A.; Ali, A.; Islam, M.N. Color and chemical constitution of natural dye henna (Lawsonia inermis L.) and its application in the coloration of textiles. J. Clean. Prod. 2017, 167, 14–22. [Google Scholar] [CrossRef]
- Pallipurath, A.; Skelton, J.M.; Delori, A.; Duffy, C.; Erxleben, A.; Jones, W. Crystalline adducts of the Lawsone molecule (2-hydroxy-1,4-naphthaquinone): Optical properties and computational modelling. CrystEngComm 2015, 17, 7684–7692. [Google Scholar] [CrossRef][Green Version]
- Monroy-Cárdenas, M.; Méndez, D.; Trostchansky, A.; Martínez-Cifuentes, M.; Araya-Maturana, R.; Fuentes, E. Synthesis and Biological Evaluation of Thio-Derivatives of 2-Hydroxy-1, 4-Naphthoquinone (Lawsone) as Novel Antiplatelet Agents. Front. Chem. 2020, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Alebeid, O.K.; Pei, L.; Elhassan, A.; Zhou, W.; Wang, J. Cleaner dyeing and antibacterial activity of wool fabric using Henna dye modified with Acacia nilotica pods. Clean Technol. Environ. Policy 2020, 22, 2223–2230. [Google Scholar] [CrossRef]
- Räisänen, R.; Nousiainen, P.; Hynninen, P.H. Emodin and dermocybin natural anthraquinones as high-temperature disperse dyes for polyester and polyamide. Text. Res. J. 2001, 71, 922–927. [Google Scholar] [CrossRef]
- Patil, S.R.; Choudhary, A.S.; Sekar, N. Disperse styryl and azo dyes for polyester and nylon fibre: Synthesis, optical properties having the 1, 2, 4-triketo naphthoquinone skeleton. Fibers Polym. 2015, 16, 1068–1074. [Google Scholar] [CrossRef]
- Kathawate, L.; Joshi, P.V.; Dash, T.K.; Pal, S.; Nikalje, M.; Weyhermüller, T.; Puranik, V.G.; Konkimalla, V.B.; Salunke-Gawali, S. Reaction between lawsone and aminophenol derivatives: Synthesis, characterization, molecular structures and antiproliferative activity. J. Mol. Struct. 2014, 1075, 397–405. [Google Scholar] [CrossRef]
- Mahkam, M.; Kafshboran, H.R.; Nabati, M. Synthesis and characterization of novel colored polymers based on lawsone natural compound. Des. Monomers Polym. 2014, 17, 784–794. [Google Scholar] [CrossRef]
- Zollinger, H. Color. Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 3906390233. [Google Scholar]
- Chandrakalavathi, T.; Sudha, V.; Sindhuja, M.; Harinipriya, S.; Jeyalakshmi, R. Photosonoelectrochemical analysis of Lawsonia inermis (henna) and artificial dye used in tattoo and dye industry. J. Photochem. Photobiol. A Chem. 2018, 360, 44–57. [Google Scholar] [CrossRef]
- Dhaouadi, K.; Meliti, W.; Dallali, S.; Belkhir, M.; Ouerghemmi, S.; Sebei, H.; Fattouch, S. Commercial Lawsonia inermis L. dried leaves and processed powder: Phytochemical composition, antioxidant, antibacterial, and allelopathic activities. Ind. Crops Prod. 2015, 77, 544–552. [Google Scholar] [CrossRef]
- Kavitha, S.; Praveena, K.; Lakshmi, M. A new method to evaluate the feasibility of a dye in DSSC application. Int. J. Energy Res. 2017, 41, 2173–2183. [Google Scholar]
- Ouared, I.; Rekis, M.; Trari, M. Phenothiazine based organic dyes for dye sensitized solar cells: A theoretical study on the role of π-spacer. Dye. Pigment. 2021, 190, 109330. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Dye aggregation in dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 19541–19559. [Google Scholar] [CrossRef]
- Choi, H.; Baik, C.; Kang, S.O.; Ko, J.; Kang, M.; Nazeeruddin, M.K.; Grätzel, M. Highly efficient and thermally stable organic sensitizers for solvent-free dye-sensitized solar cells. Angew. Chem. 2008, 120, 333–336. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Photophysical and electrochemical properties, and molecular structures of organic dyes for dye-sensitized solar cells. ChemPhysChem 2012, 13, 4032–4080. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Dye-Sensitized Solar Cells; CRC press: Boca Raton, FL, USA, 2010; ISBN 143980866X. [Google Scholar]
- Ghamdi, S.N.A.L.; Ghamdi, H.A.A.L.; El-Shishtawy, R.M.; Asiri, A.M. Advances in phenothiazine and phenoxazine-based electron donors for organic dye-sensitized solar cells. Dye. Pigment. 2021, 194, 109638. [Google Scholar] [CrossRef]
- Lu, Y.; Song, H.; Li, X.; Ågren, H.; Liu, Q.; Zhang, J.; Zhang, X.; Xie, Y. Multiply wrapped porphyrin dyes with a phenothiazine donor: A high efficiency of 11.7% achieved through a synergetic coadsorption and cosensitization approach. ACS Appl. Mater. Interfaces 2019, 11, 5046–5054. [Google Scholar] [CrossRef]
- Zhao, D.; Lu, Q.; Su, R.; Li, Y.; Zhao, M. Light harvesting and optical-electronic properties of two quercitin and rutin natural dyes. Appl. Sci. 2019, 9, 2567. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Song, P.; Ma, F. An experimental and theoretical investigation of the electronic structures and photoelectrical properties of ethyl red and carminic acid for DSSC application. Materials 2016, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Madili, N.; Pogrebnoi, A.; Pogrebnaya, T. Theoretical design of complex molecule via combination of natural lawsone and synthetic indoline D131 dyes for dye sensitized solar cells application. Comput. Chem. 2018, 6, 87. [Google Scholar] [CrossRef]
- Oprea, C.I.; Frecuş, B.; Minaev, B.F.; Gîrţu, M.A. DFT study of electronic structure and optical properties of some Ru-and Rh-based complexes for dye-sensitized solar cells. Mol. Phys. 2011, 109, 2511–2523. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, D.; Li, X.; Xu, Y. DA-π-A based organic dyes for efficient DSSCs: A theoretical study on the role of π-spacer. Comput. Mater. Sci. 2019, 161, 163–176. [Google Scholar] [CrossRef]
- Fujisawa, J.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Jose, R.; Thavasi, V.; Ramakrishna, S. Metal oxides for dye-sensitized solar cells. J. Am. Ceram. Soc. 2009, 92, 289–301. [Google Scholar] [CrossRef]
- Sang-aroon, W.; Saekow, S.; Amornkitbamrung, V. Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2012, 236, 35–40. [Google Scholar] [CrossRef]
- Prinzisky, C.; Meyenburg, I.; Jacob, A.; Heidelmeier, B.; Schröder, F.; Heimbrodt, W.; Sundermeyer, J. Optical and Electrochemical Properties of Anthraquinone Imine Based Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2016, 2016, 756–767. [Google Scholar] [CrossRef]
- Sánchez-de-Armas, R.; Oviedo Lopez, J.A.; San-Miguel, M.; Sanz, J.F.; Ordejón, P.; Pruneda, M. Real-time TD-DFT simulations in dye sensitized solar cells: The electronic absorption spectrum of alizarin supported on TiO2 nanoclusters. J. Chem. Theory Comput. 2010, 6, 2856–2865. [Google Scholar] [CrossRef]
- Sánchez-de-Armas, R.; Oviedo, J.; San Miguel, M.Á.; Sanz, J.F. Direct vs indirect mechanisms for electron injection in dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 11293–11301. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, H.-C.; Zhong, R.-L.; Wang, L.; Su, Z.-M. Promising heterocyclic anchoring groups with superior adsorption stability and improved IPCE for high-efficiency noncarboxyl dye sensitized solar cells: A theoretical study. Org. Electron. 2018, 54, 104–113. [Google Scholar] [CrossRef]
- Li, Y.; Song, P.; Yang, Y.; Ma, F.; Li, Y. Double-anchoring organic dyes for dye-sensitized solar cells: The opto-electronic property and performance. New J. Chem. 2017, 41, 12808–12829. [Google Scholar] [CrossRef]
- Urzúa-Leiva, R.; Pino-Rios, R.; Cárdenas-Jirón, G. The influence of antenna and anchoring moieties on the improvement of photoelectronic properties in Zn (ii)–porphyrin–TiO2 as potential dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2019, 21, 4339–4348. [Google Scholar] [CrossRef]
- Slimi, A.; Hachi, M.; Fitri, A.; Benjelloun, A.T.; Elkhattabi, S.; Benzakour, M.; Mcharfi, M.; Khenfouch, M.; Zorkani, I.; Bouachrine, M. Effects of electron acceptor groups on triphenylamine-based dyes for dye-sensitized solar cells: Theoretical investigation. J. Photochem. Photobiol. A Chem. 2020, 398, 112572. [Google Scholar] [CrossRef]
- Fadili, D.; Bouzzine, S.M.; Hamidi, M. Study of the structural and optoelectronic properties of dye solar cells based on phosphonic acid anchoring by DFT functionals. New J. Chem. 2021, 45, 2723–2733. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Zhang, R.; Hagfeldt, A.; Sun, L. Effect of different dye baths and dye-structures on the performance of dye-sensitized solar cells based on triphenylamine dyes. J. Phys. Chem. C 2008, 112, 11023–11033. [Google Scholar] [CrossRef]
- Frenking, G.; Shaik, S. The Chemical Bond: Fundamental Aspects of Chemical Bonding; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 3527664718. [Google Scholar]
- Oprea, C.I.; Gîrțu, M.A. Structure and electronic properties of TiO2 nanoclusters and dye–nanocluster systems appropriate to model hybrid photovoltaic or photocatalytic applications. Nanomaterials 2019, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO v. 6.0. Program; University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]

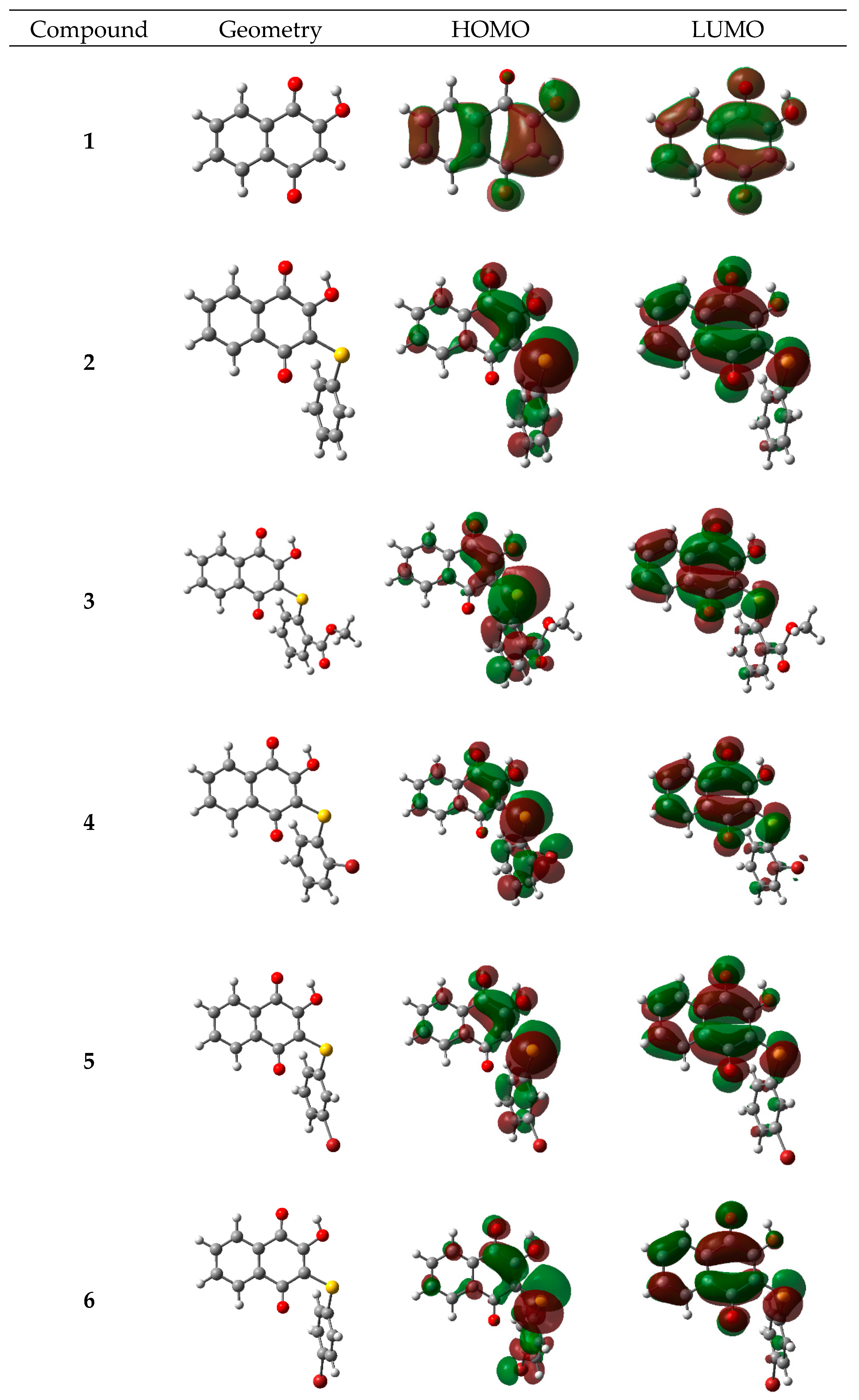
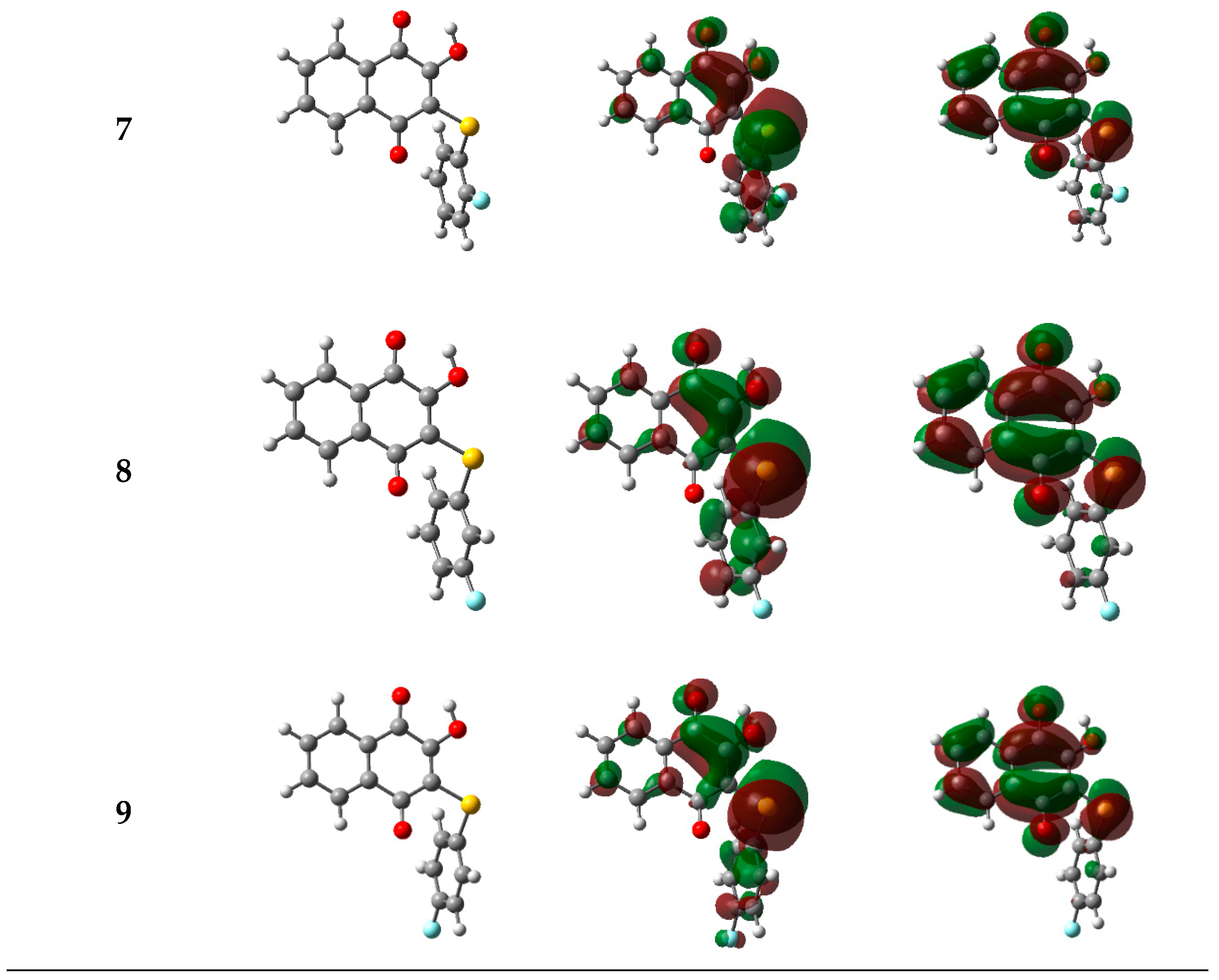

| Cpd | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| X | Lawsone | H | o-CO2Me | o-Br | m-Br | p-Br | o-F | m-Br | p-Br |
 | |||||||||
| Compound | L* | a* | b* | c* | h° | ΔE |
|---|---|---|---|---|---|---|
| 1 | 65.01 | 1.76 | 51.64 | 51.67 | 88.04 | - |
| 2 | 18.70 | 17.15 | 3.50 | 17.50 | 11.55 | 69.1 |
| 3 | 30.30 | 33.78 | 25.39 | 42.26 | 36.93 | 54.1 |
| 4 | 36.62 | 19.29 | 12.96 | 23.24 | 33.90 | 51.8 |
| 5 | 15.19 | 9.52 | 1.95 | 9.71 | 11.56 | 71.2 |
| 6 | 21.34 | 11.22 | −3.34 | 11.70 | 343.41 | 70.4 |
| 7 | 31.12 | 4.69 | −5.40 | 7.15 | 310.97 | 65,6 |
| 8 | 27.90 | 31.58 | 19.31 | 37.24 | 31.23 | 58.0 |
| 9 | 17.07 | 6.38 | 4.71 | 7.93 | 36.42 | 67.4 |
| Thio-Lawsone Derivatives | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| λ exp (nm) | 208 | 270 | 222 | 213 | 212 | 207 | 208 | 208 | 205 |
| 243 | 459 | 268 | 267 | 266 | 264 | 251 | 210 | 265 | |
| 249 | 273 | 465 | 467 | 478 | 280 | 252 | 277 | ||
| 279 | 325 | 463 | 279 | 473 | |||||
| 334 | 474 | 465 | |||||||
| Vacuum | Methanol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Excited State N°. | Eex, eV | λ, nm | f | LHE | Electronic Transition | Exited State No. | Eex, eV | λ, nm | f | LHE | Electronic Transition |
| Cpd 1 | Cpd 1 | ||||||||||
| ES 4 | 3.69 | 336 | 0.058 | 0.125 | H-2→ L (91%) | 4 | 3.57 | 348 | 0.075 | 0.159 | H-2→L (47%) |
| ES 5 | 4.31 | 288 | 0.177 | 0.334 | H-3→ L (88%) | 5 | 3.20 | 295 | 0.249 | 0.436 | H-3→L (92%) |
| Cpd 2 | Cpd 2 | ||||||||||
| ES 1 | 2.11 | 587 | 0.052 | 0.112 | H→L (98%) | 1 | 2.20 | 562 | 0.059 | 0.127 | H→L (98%) |
| ES 8 | 3.93 | 315 | 0.172 | 0.327 | H→L + 1 (88%) | 8 | 4.13 | 300 | 0.205 | 0.376 | H→L + 1 (96%) |
| Cpd 3 | Cpd 3 | ||||||||||
| ES 1 | 1.99 | 624 | 0.053 | 0.114 | H→L (99%) | 1 | 2.15 | 577 | 0.059 | 0.127 | H→L (99%) |
| ES 7 | 3.79 | 327 | 0.196 | 0.364 | H→L + 1 (77%) | 7 | 3.90 | 318 | 0.201 | 0.370 | H→L + 1 (96%) |
| Vacuum | Methanol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MO | EMO eV | Transition | Eex | Eg | ESOP | MO | EMO eV | Transition | Eex | Eg | ESOP |
| Cpd 1 | Cpd 1 | ||||||||||
| H-3 | −8.13 | H-3→L | 4.31 | 4.54 | −3.83 | H-3 | −8.04 | H-3→L | 3.20 | 4.48 | −4.84 |
| H-2 | −7.87 | H-2→L | 3.69 | 4.28 | −4.18 | H-2 | −7.76 | H-2→L | 3.57 | 4.20 | −4.19 |
| H | −7.4 | H→L | 3.13 | 3.81 | −4.27 | H | −7.36 | H→L | 3.06 | 3.80 | −4.30 |
| L | −3.59 | L | −3.56 | ||||||||
| L + 1 | −1.76 | L + 1 | −1.64 | ||||||||
| Cpd 2 | Cpd 2 | ||||||||||
| H-4 | −7.84 | H-4→L | 3.67 | 4.31 | −4.17 | H-3 | −7.74 | H-3→L | 3.55 | 4.15 | −4.19 |
| H-1 | −7.15 | H-1→L | 2.77 | 3.62 | −4.38 | H-1 | −7.29 | H-1→L | 2.88 | 3.70 | −4.41 |
| H | −6.21 | H→L | 2.11 | 2.68 | −4.10 | H | −6.40 | H→L | 2.20 | 2.81 | −4.20 |
| L | −3.53 | L | −3.59 | ||||||||
| L + 1 | −1.75 | L + 1 | −1.69 | ||||||||
| Cpd 3 | Cpd 3 | ||||||||||
| H-6 | −8.11 | H-6→L | 3.94 | 4.47 | −4.17 | H-3 | −7.76 | H-3→L | 3.53 | 4.12 | −4.23 |
| H-5 | −7.93 | H-5→L | 3.65 | 4.29 | −4.28 | H-1 | −7.51 | H-1→L | 2.95 | 3.87 | −4.56 |
| H | −6.2 | H→L | 1.99 | 2.56 | −4.21 | H | −6.40 | H→L | 2.15 | 2.76 | −4.25 |
| L | −3.64 | L | −3.64 | ||||||||
| L + 1 | −1.85 | L + 1 | −1.83 | ||||||||
| Cpd 4 | Cpd 4 | ||||||||||
| H-4 | −7.92 | H-4→L | 3.65 | 4.27 | −4.27 | H-3 | −7.77 | H-3→L | 3.53 | 4.13 | −4.24 |
| H-1 | −6.95 | H-1→L | 2.76 | 3.30 | −4.19 | H-1 | −7.14 | H-1→L | 2.91 | 3.50 | −4.23 |
| H | −6.22 | H→L | 2.01 | 2.57 | −4.21 | H | −6.40 | H→L | 2.15 | 2.76 | −4.25 |
| L | −3.65 | L | −3.64 | ||||||||
| L + 1 | −1.86 | L + 1 | −1.74 | ||||||||
| Cpd 5 | Cpd 5 | ||||||||||
| H-4 | −7.94 | H-4→L | 3.66 | 4.28 | −4.28 | H-3 | −7.76 | H-3→L | 3.54 | 4.14 | −4.22 |
| H-2 | −7.41 | H-2→L | 2.81 | 3.75 | −4.60 | H-2 | −7.46 | H-2→L | 2.91 | 3.84 | −4.55 |
| H | −6.39 | H→L | 2.15 | 2.73 | −4.24 | H | −6.49 | H→L | 2.25 | 2.87 | −4.24 |
| L | −3.66 | L | −3.62 | ||||||||
| L + 1 | −1.86 | L + 1 | −1.72 | ||||||||
| Cpd 6 | Cpd 6 | ||||||||||
| H-1 | −7.12 | H-1→L | 2.75 | 3.48 | −4.37 | H-3 | −7.75 | H-3→L | 3.53 | 4.14 | −4.22 |
| H | −6.33 | H→L | 2.13 | 2.69 | −4.20 | H-1 | −7.20 | H-1→L | 2.85 | 3.59 | −4.35 |
| L | −3.64 | H | −6.44 | H→L | 2.23 | 2.83 | −4.21 | ||||
| L + 1 | −1.84 | L | −3.61 | ||||||||
| L + 1 | −1.71 | ||||||||||
| Cpd 7 | Cpd 7 | ||||||||||
| H-4 | −7.89 | H-4→L | 3.65 | 4.29 | −4.24 | H-3 | −7.76 | H-3→L | 3.54 | 4.14 | −4.22 |
| H-1 | −7.11 | H-1→L | 2.75 | 3.51 | −4.36 | H-1 | −7.24 | H-1→L | 2.88 | 3.62 | −4.36 |
| H | −6.31 | H→L | 2.17 | 2.71 | −4.14 | H | −6.48 | H→L | 2.25 | 2.86 | −4.23 |
| L | −3.6 | L | −3.62 | ||||||||
| L + 1 | −1.8 | L + 1 | −1.71 | ||||||||
| Cpd 8 | Cpd 8 | ||||||||||
| H-4 | −7.93 | H-4→L | 3.66 | 4.29 | −4.27 | H-3 | −7.76 | H-3→L | 3.55 | 4.15 | −4.21 |
| H-2 | −7.4 | H-2→L | 2.81 | 3.76 | −4.59 | H-2 | −7.46 | H-2→L | 2.92 | 3.85 | −4.54 |
| H | −6.36 | H→L | 2.15 | 2.72 | −4.21 | H | −6.47 | H→L | 2.25 | 2.86 | −4.22 |
| L | −3.64 | L | −3.61 | ||||||||
| L + 1 | −1.84 | L + 1 | −1.71 | ||||||||
| Cpd 9 | Cpd 9 | ||||||||||
| H-1 | −7.17 | H-1→L | 2.75 | 3.56 | −4.42 | H-3 | −7.74 | H-3→L | 3.54 | 4.15 | −4.20 |
| H | −6.37 | H→L | 2.17 | 2.76 | −4.20 | H-1 | −7.26 | H-1→L | 2.84 | 3.67 | −4.42 |
| L | −3.61 | H | −6.48 | H→L | 2.27 | 2.89 | −4.21 | ||||
| L + 1 | −1.82 | L | −3.59 | ||||||||
| L + 1 | −1.69 | ||||||||||
| Complex | EHOMO (eV) | ELUMO (eV) | Egap (eV) | Eads (kcal/mol) |
|---|---|---|---|---|
| (A) Neutral lawsone∙∙∙(TiO2)9 | −7.97 | −4.84 | 3.13 | −19.94 |
| (B) Neutral deprotonated lawsone∙∙∙(TiO2)9 | −7.74 | −4.42 | 3.32 | −44.60 |
| (C) Anionic deprotanated lawsone∙∙∙(TiO2)9 | −5.08 | −1.61 | 3.47 | −97.39 |
| (D) Neutral 3-thiophenyl-lawsone∙∙∙(TiO2)9 | −6.92 | −4.68 | 2.24 | −17.79 |
| (E) Neutral deprotonated 3-thiophenyl-lawsone∙∙∙(TiO2)9 | −6.62 | −4.30 | 2.32 | −46.30 |
| (F) Anionic deprotanated 3-thiophenyl-lawsone∙∙∙(TiO2)9 | −4.28 | −1.69 | 2.59 | −80.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-Cárdenas, M.; Forero-Doria, O.; Araya-Maturana, R.; Martínez-Cifuentes, M. An Experimental and Theoretical Study of Dye Properties of Thiophenyl Derivatives of 2-Hydroxy-1,4-naphthoquinone (Lawsone). Materials 2021, 14, 5587. https://doi.org/10.3390/ma14195587
Monroy-Cárdenas M, Forero-Doria O, Araya-Maturana R, Martínez-Cifuentes M. An Experimental and Theoretical Study of Dye Properties of Thiophenyl Derivatives of 2-Hydroxy-1,4-naphthoquinone (Lawsone). Materials. 2021; 14(19):5587. https://doi.org/10.3390/ma14195587
Chicago/Turabian StyleMonroy-Cárdenas, Matías, Oscar Forero-Doria, Ramiro Araya-Maturana, and Maximiliano Martínez-Cifuentes. 2021. "An Experimental and Theoretical Study of Dye Properties of Thiophenyl Derivatives of 2-Hydroxy-1,4-naphthoquinone (Lawsone)" Materials 14, no. 19: 5587. https://doi.org/10.3390/ma14195587
APA StyleMonroy-Cárdenas, M., Forero-Doria, O., Araya-Maturana, R., & Martínez-Cifuentes, M. (2021). An Experimental and Theoretical Study of Dye Properties of Thiophenyl Derivatives of 2-Hydroxy-1,4-naphthoquinone (Lawsone). Materials, 14(19), 5587. https://doi.org/10.3390/ma14195587







