Dye Adsorption Mechanism of Glass Fiber-Reinforced Plastic/Clay Ceramics and Influencing Factors
Abstract
1. Introduction
2. Materials and Methods
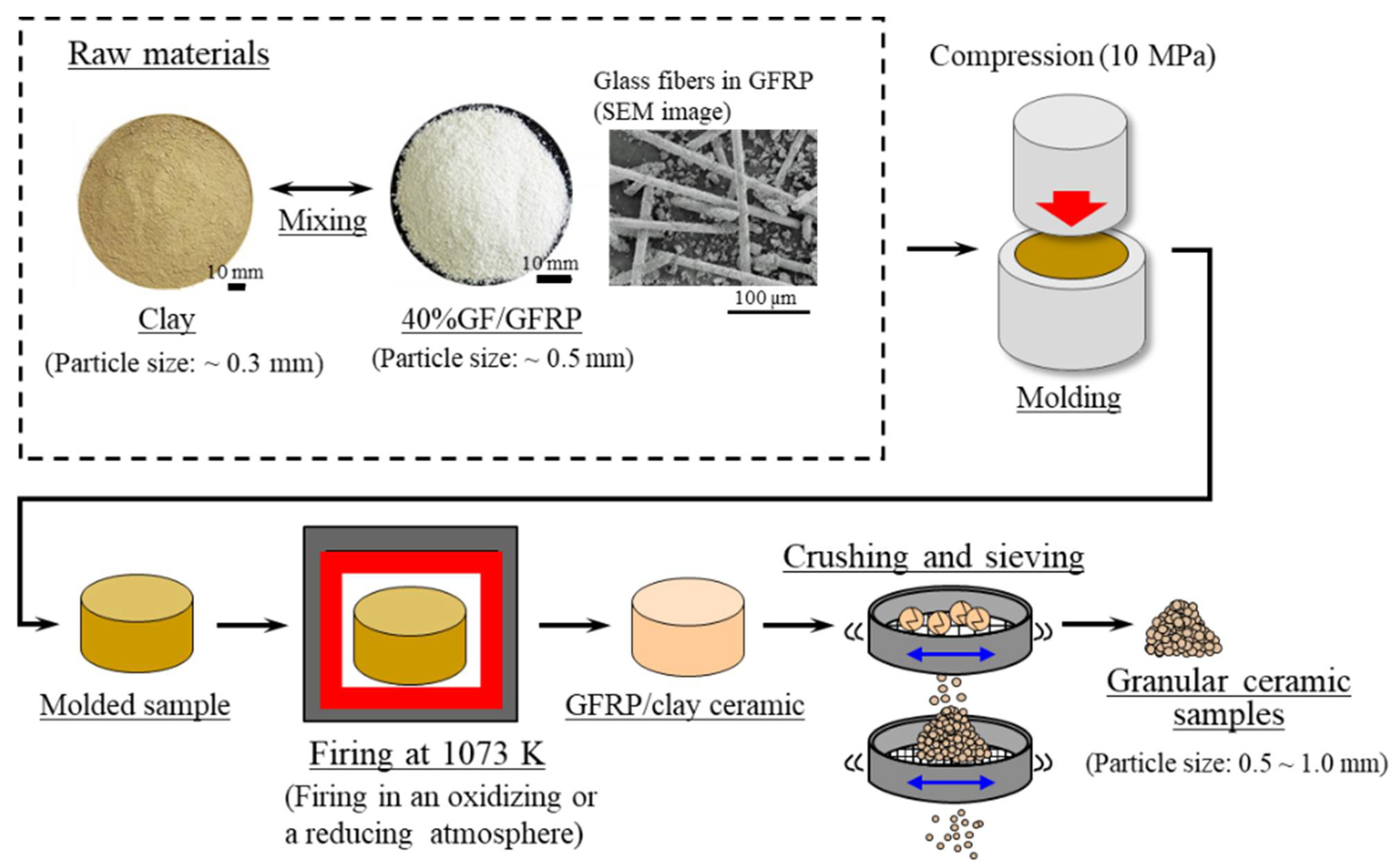
2.1. GFRP/Clay Ceramic Samples Used for Dye Adsorption Tests
- Clay was crushed with a rotary mill (New Power Mill ABS-W, Osaka Chemical Co., Ltd., Osaka, Japan) and then was sifted with a 0.3-mm mesh screen.
- GFRP was also crushed with the rotary mill and then was sifted with a 0.5-mm mesh screen.
- The crushed GFRP was mixed with the clay at the mass rates listed in Table 2.
- The GFRP/clay mixture was solidified by being pressed into a mold at 10 MPa. The molded samples had a diameter of 74 mm and a thickness of 50–60 mm.
- The molded samples were heated in an oxidizing or reducing atmosphere to 1073 K in an electric furnace (KY-4N, Kyoei Electric Kilns Co., Ltd., Tajimi, Japan). The samples were then held at the firing temperature for 1 h and allowed to cool to room temperature in the furnace. For oxidative firing, the samples were heated at 100 K h−1 to the firing temperature. For reductive firing, the samples were heated at 400 K h−1. The reducing atmosphere was obtained by closing the intake port attached to the bottom of the electric furnace.
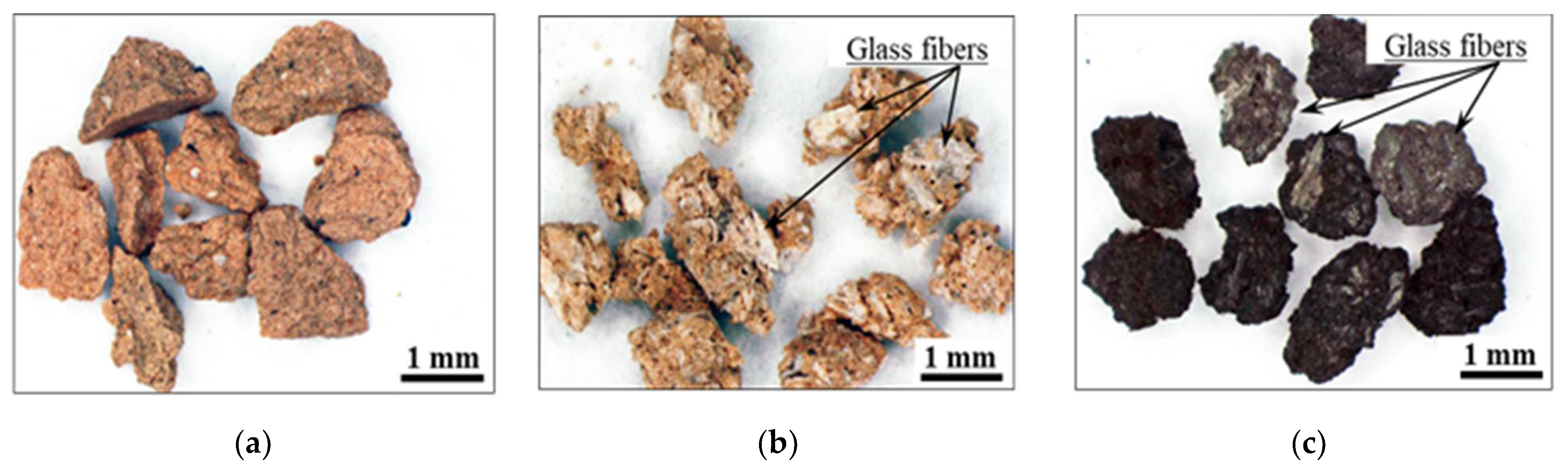
- The produced GFRP/clay samples were then crushed with a hammer, and particle sizes of 0.5–1.0 mm were selected.
2.2. Methodology of Dye Adsorption Tests
- Samples were washed with distilled water and were dried in an electric furnace at 373 K for over 24 h before the dye adsorption tests.
- MB, Orange II, and Congo-red powders were dissolved in distilled water to make aqueous solutions each with a concentration of 1 × 10−4 mol/L.
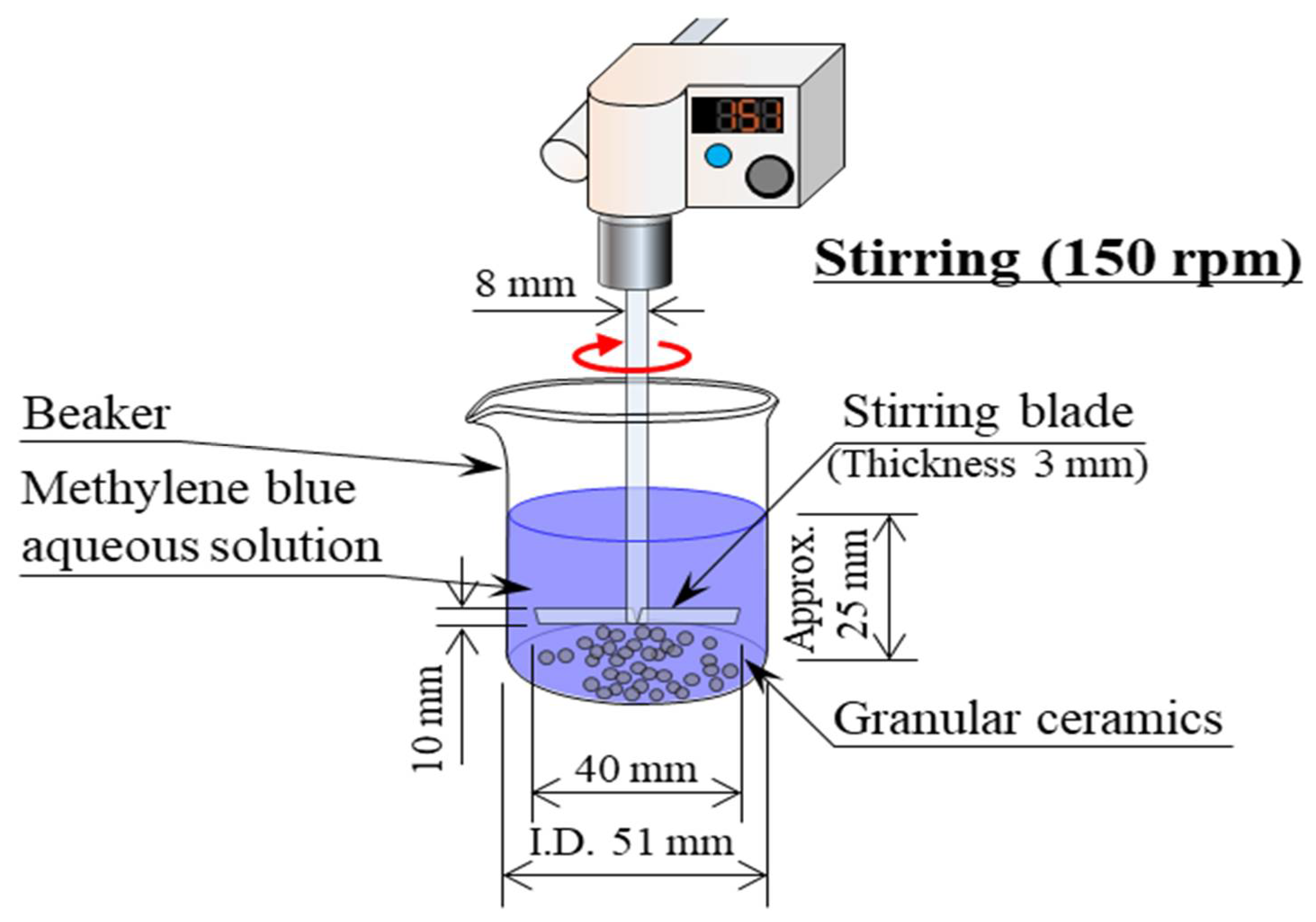
- Then, 1 g of the granular samples was placed in a beaker containing 50 mL of the aqueous solution, and the aqueous solution was stirred with a stirring device (EYLA ZZ-1010, Rikakikai Co., Ltd., Tokyo, Japan) at a speed of 150 rpm.
- The dye concentration and pH value of the aqueous solutions were measured after 1, 10, 30, 60, and 120 min.
- The dye concentration in the aqueous solution was measured using a drainage analyzer (NDR-2000, Nippon Denshoku Industries Co., Ltd., Tokyo, Japan). The absorbance of the aqueous solution was determined. Then, the dye concentration was calculated from a calibration curve that expressed the relationship between the absorbance and dye concentration of the aqueous solution. The pH of the aqueous solution was measured using a pH meter (HM-25R, DKK-TOA Corporation, Tokyo, Japan).
3. Results
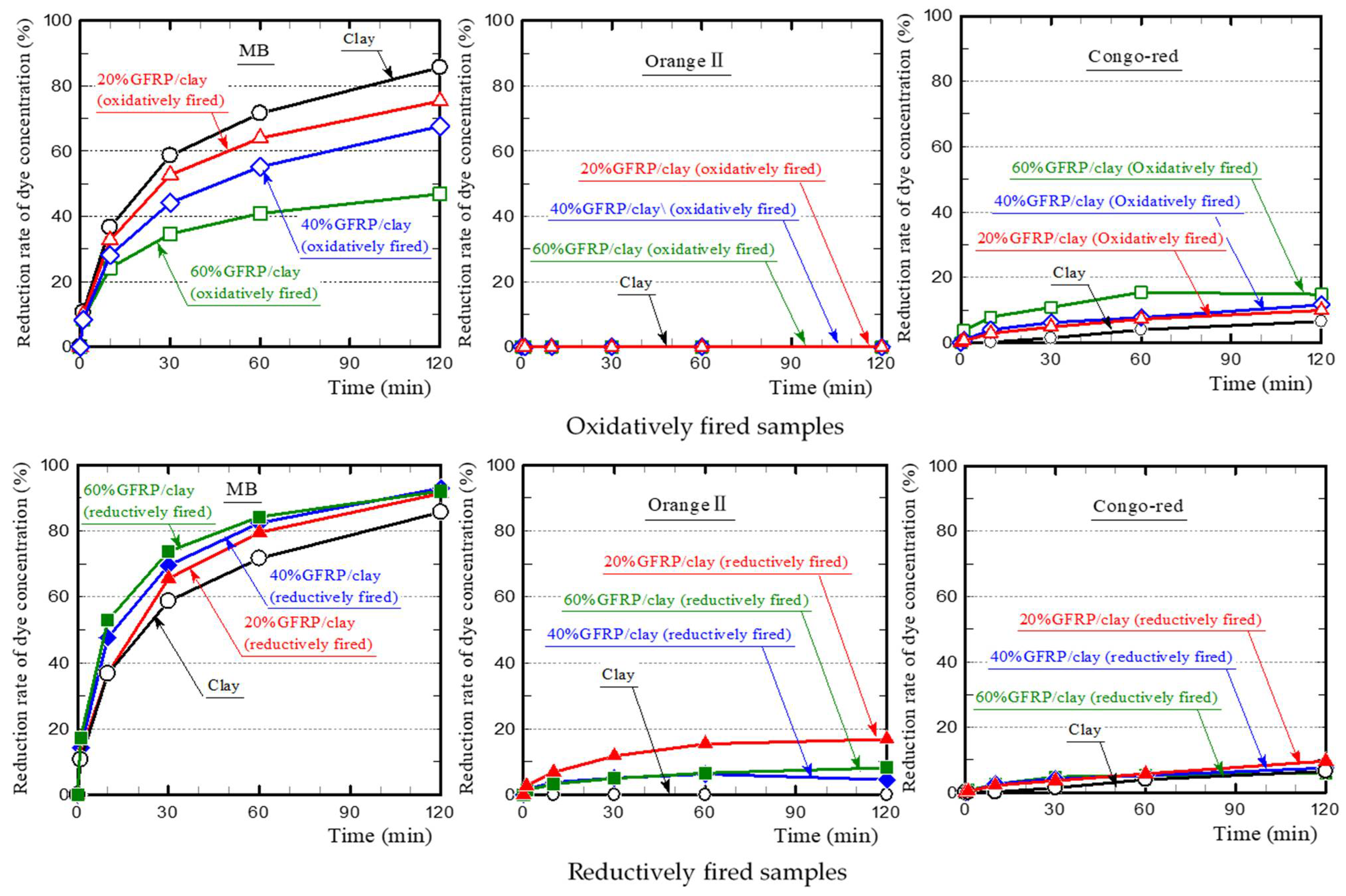
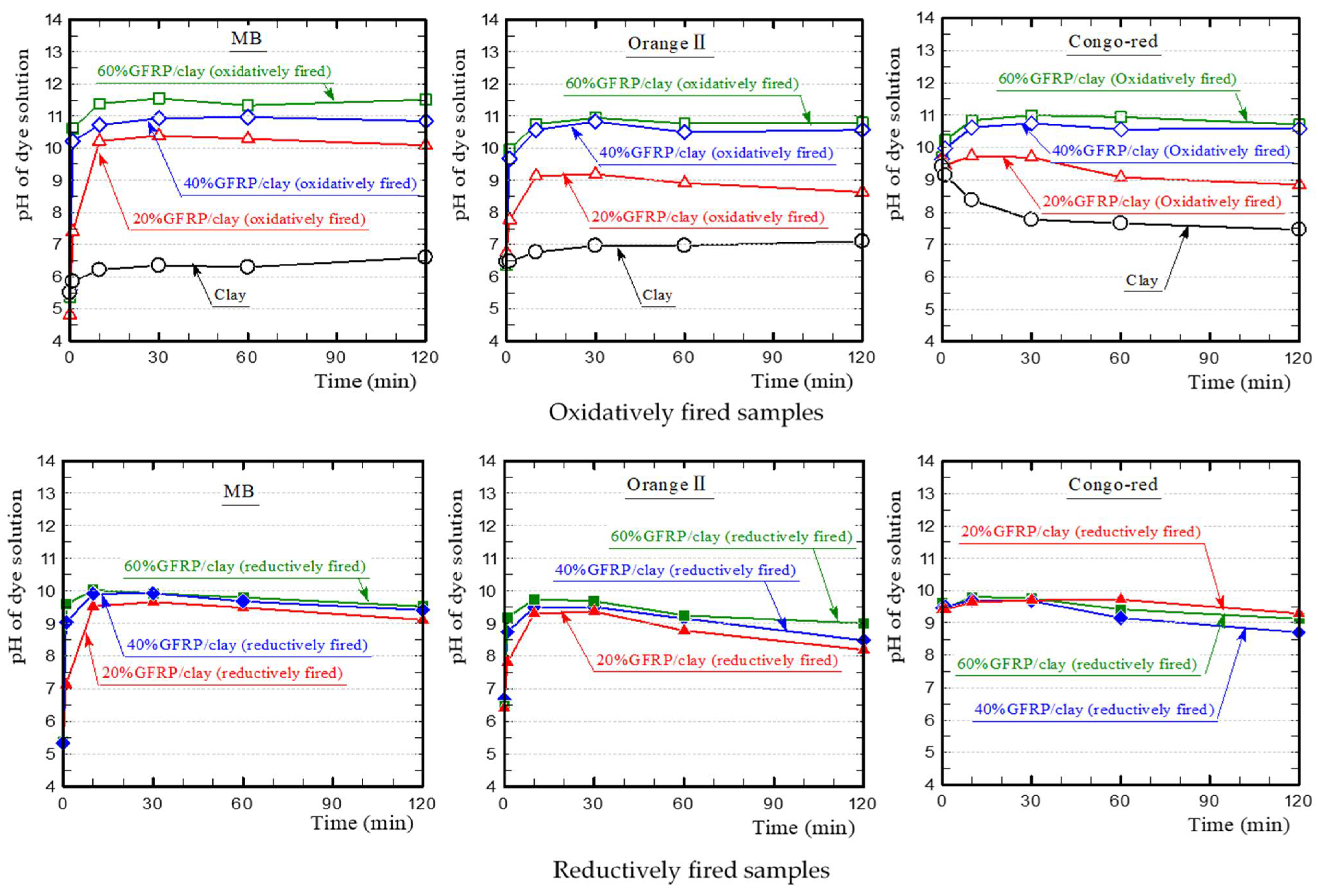
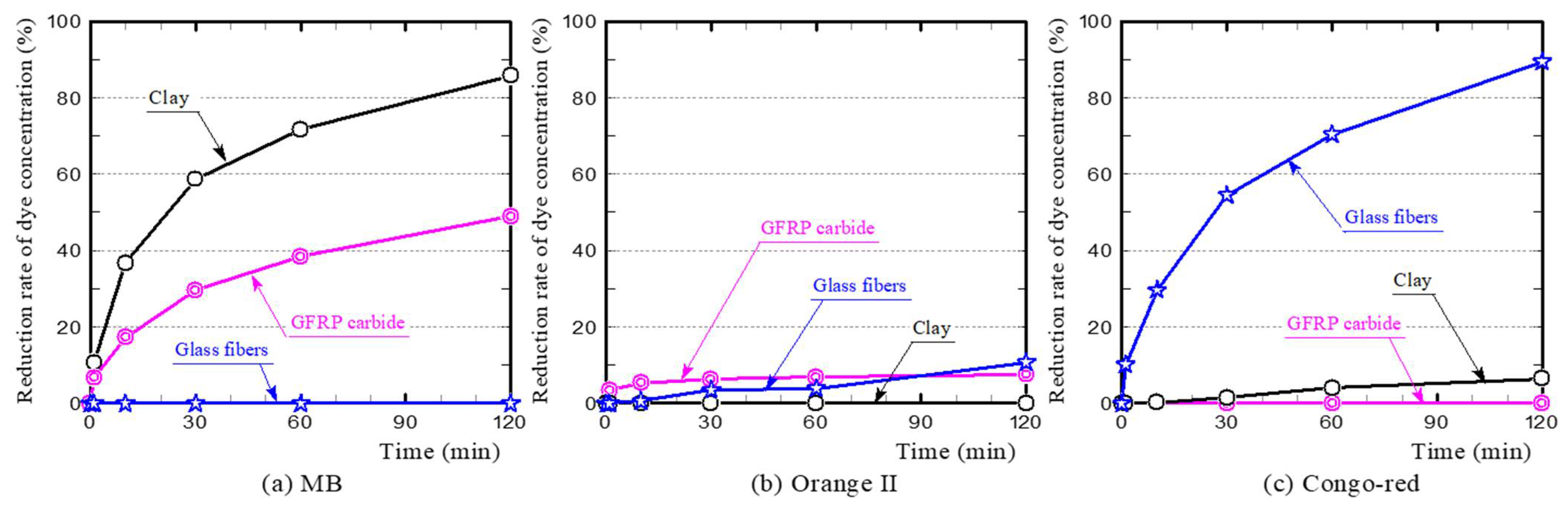
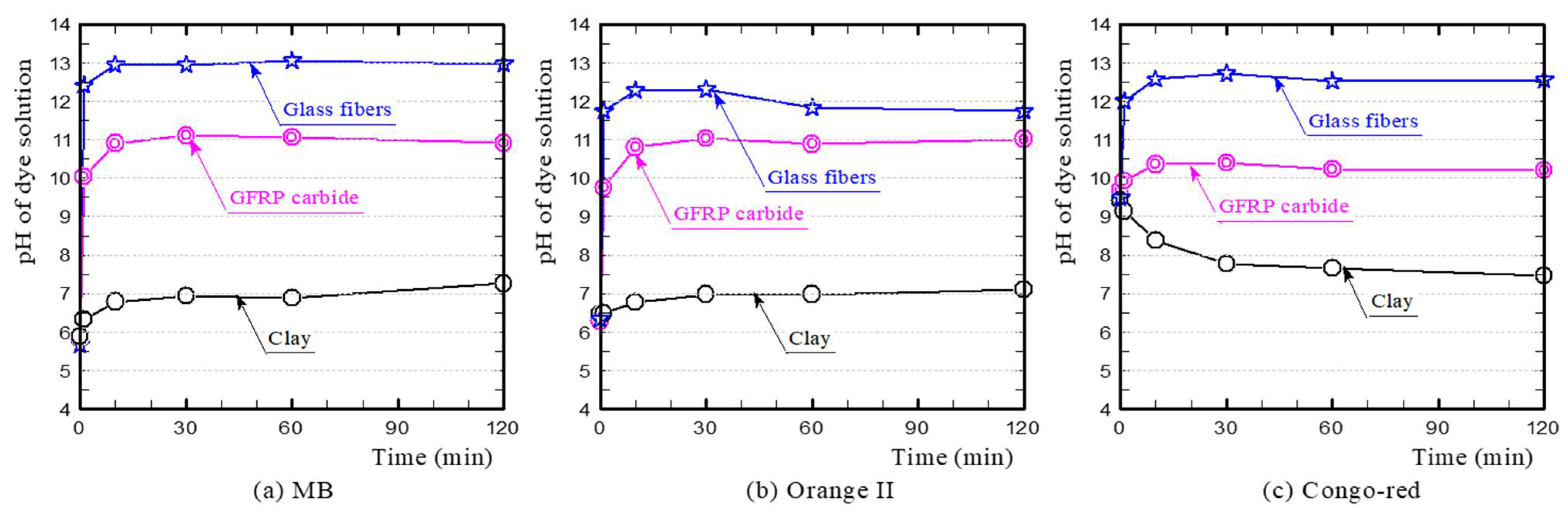
3.1. Dye Adsorption of GFRP/Clay Ceramics
3.2. Primary Influencing Factors for Dye Adsorption on the GFRP/Clay Ceramics
- (a)
- MB
- (b) Orange II
- (c) Congo-red
4. Discussion
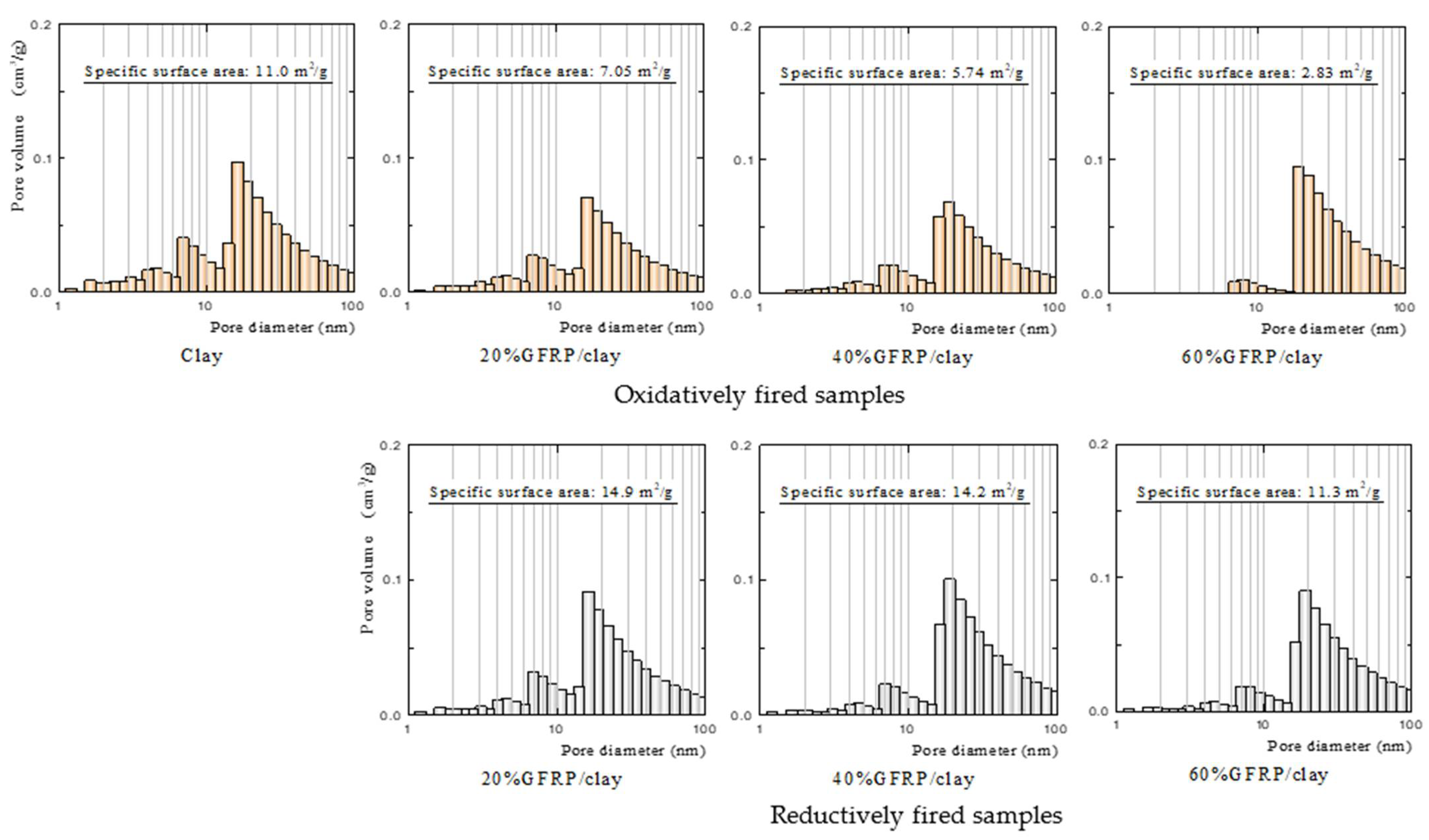
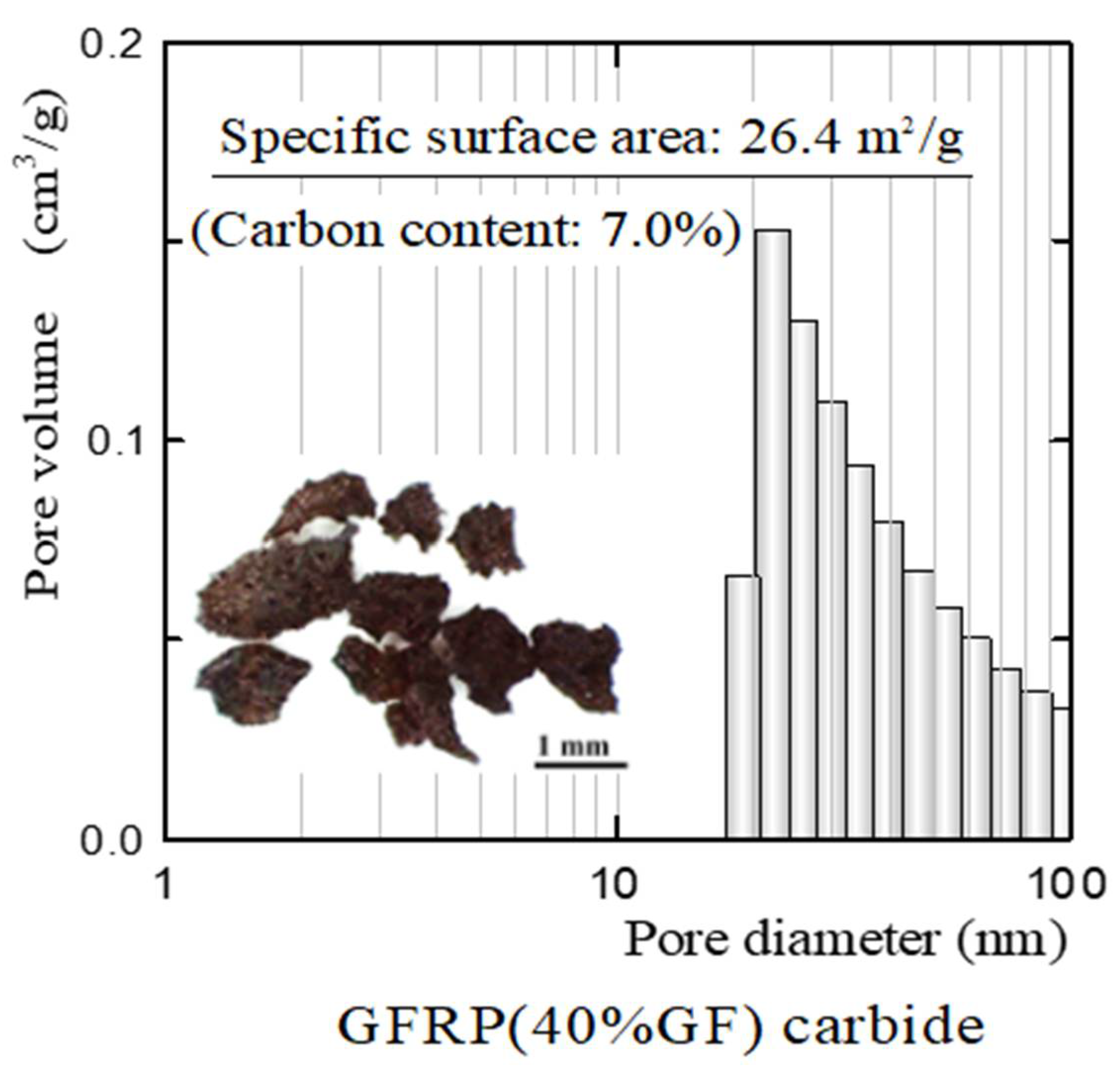
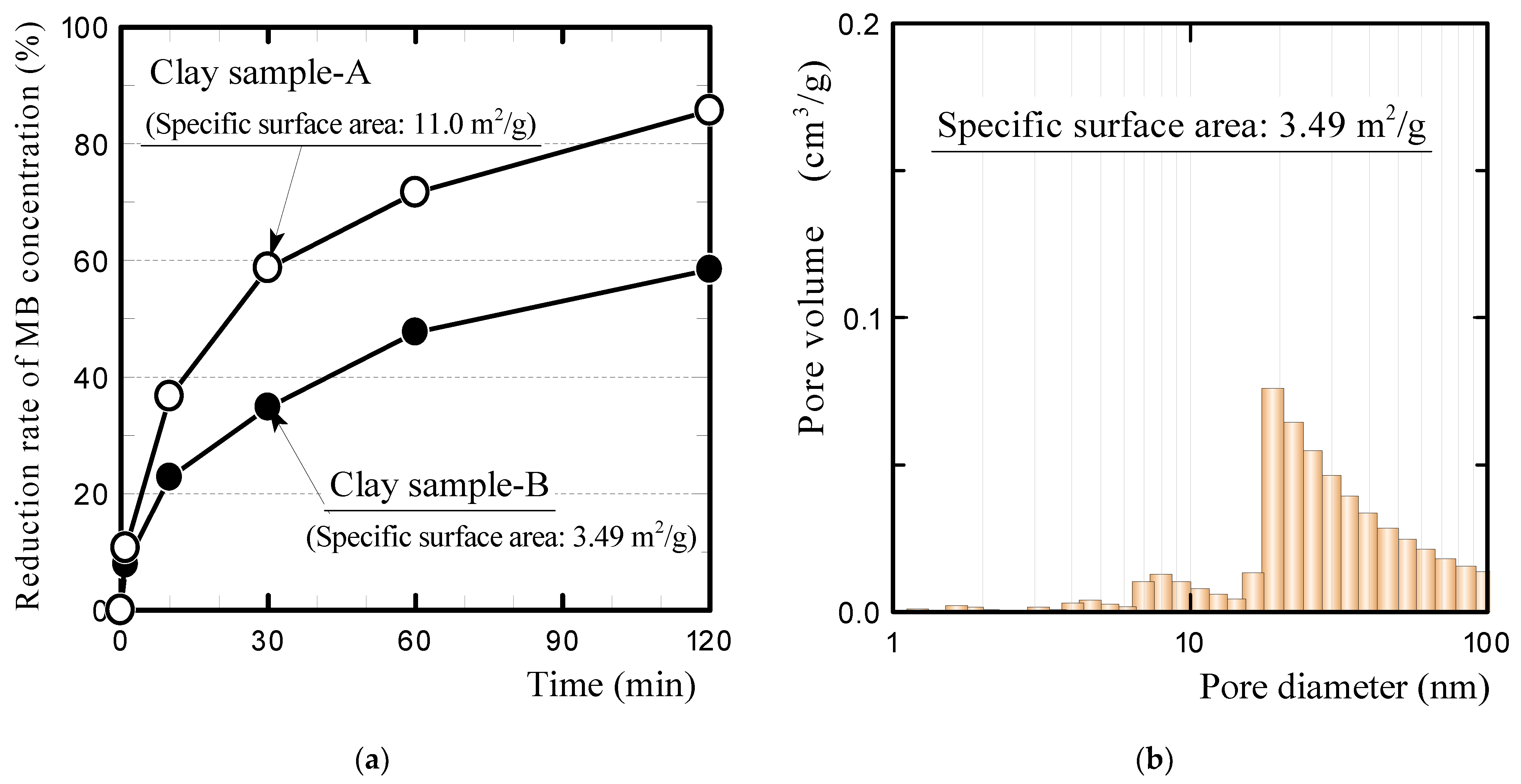
- The plastic carbide residue in the reductively fired GFRP/clay ceramic structure increases the specific surface area of the ceramic. The high specific surface area of the ceramic increases the physical and chemical adsorption of MB. In addition, the high porosity facilitates MB movement into the ceramic body.
- The reductively fired GFRP/clay ceramic samples exhibited a high adsorption capacity for only basic dyes. This indicates that the adsorption mechanism is dominated by cation exchange with clay components.
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazdanbakhsh, A.; Bank, L.C. A Critical Review of Research on Reuse of Mechanically Recycled FRP Production and End-of-Life Waste for Construction. Polymers 2014, 6, 1810–1826. [Google Scholar] [CrossRef]
- Castro, A.C.M.; Carvalho, J.P.; Ribeiro, M.C.S.; Meixedo, J.P.; Silva, F.J.; Fiúza, A.; Dinis, M.L. An integrated recycling approach for GFRP pultrusion wastes: Recycling and reuse assessment into new composite materials using Fuzzy Boolean Nets. J. Clean. Prod. 2014, 66, 420–430. [Google Scholar] [CrossRef]
- Denkena, B.; Dittrich, M.-A.; Rahner, B.-H. Smart and energy-efficient dust suction concept for milling of fibre-reinforced plastics. Prod. Eng. 2017, 11, 723–729. [Google Scholar] [CrossRef]
- López, F.A.; Martín, M.I.; García-Díaz, I.; Rodríguez, O.; Alguacil, F.J.; Romero, M. Recycling of Glass Fibers from Fiberglass Polyester Waste Composite for the Manufacture of Glass-Ceramic Materials. J. Environ. Prot. 2012, 3, 740–747. [Google Scholar] [CrossRef]
- Yang, Y.; Boom, R.; Irion, B.; van Heerden, D.-J.; Kuiper, P.; de Wit, H. Recycling of composite materials. Chem. Eng. Process. Process. Intensif. 2012, 51, 53–68. [Google Scholar] [CrossRef]
- Nagaoka, T. Value-Added Recycling of Disposal Plastics. J. Jpn. Soc. Technol. Plast. 2016, 49, 175–179. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kaizu, K.; Takeda, T.; Miyagi, H.; Kawamura, R.; Ikeda, K. Development of High Strength Porous Tile by Recycling of Waste Glass Fiber Reinforced Plastics. Trans. Jpn. Soc. Mech. Eng. Ser. A 2010, 76, 1507–1513. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nakazono, T.; Oyamada, M.; Yuji, T.; Ando, T.; Ikeda, K.; Kaizu, K.; Kobayashi, T.; Fukuyama, H.; Kawasaki, H. Development of High-Strength Porous Tiles Produced by Recycling Glass Fibers in Waste GFRP—Influence of Particle Size of GFRP on Tile Properties. J. JSEM 2011, 11, 241–248. (In Japanese) [Google Scholar] [CrossRef]
- Kinoshita, H.; Kaizu, K.; Hasegawa, S.; Ando, T.; Kawamura, R.; Ikeda, K.; Kobayashi, T.; Fukuyama, H. Production and Material Properties of Ceramic from Waste Glass Fiber Reinforced Plastic. J. Environ. Eng. 2013, 8, 27–40. [Google Scholar] [CrossRef][Green Version]
- Kinoshita, H.; Kaizu, K.; Yasuda, Y.; Nakazono, T.; Kobayashi, T.; Fukuyama, H.; Kawamura, R.; Ikeda, K.; Kawasaki, H. Development of Greening Plant Consists of Moss and Porous Ceramic Base Material of High Strength Made by Recycling Waste GFRP. J. JSEM 2013, 13, 100–106. (In Japanese) [Google Scholar] [CrossRef]
- Kinoshita, H.; Yuji, T.; Yasuda, Y.; Yasui, K.; Sezaki, M. Environmental Harmony-Type Pavement Blocks Made from Clay and Waste GFRP. Int. J. Civ. Struct. Environ. Infrastruct. Eng. Res. Dev. 2016, 6, 51–60. [Google Scholar]
- Yasuda, Y.; Kinoshita, H.; Yasui, K.; Yuji, T.; Okamura, Y.; Sezaki, M.; Kawamura, R. Ceramics utilizing glass fiber-reinforced plastic as civil engineering materials to counteract the heat island phenomenon. Mech. Eng. J. 2016, 3, 16–00078. [Google Scholar] [CrossRef]
- Materials Science Society of Japan. Global Environment and Materials; Shokabo Co., Ltd.: Tokyo, Japan, 1999; pp. 61–74. (In Japanese) [Google Scholar]
- Yasui, K.; Goto, S.; Kinoshita, H.; Kamiunten, S.; Yuji, T.; Okamura, Y.; Mungkung, N.; Sezaki, M. Ceramic waste glass fiber-reinforced plastic-containing filtering materials for turbid water treatment. Environ. Earth Sci. 2016, 75, 1135. [Google Scholar] [CrossRef]
- Yasuda, Y.; Iwasaki, H.; Yasui, K.; Tanaka, A.; Kinoshita, H. Development of walkway blocks with high water permeability using waste glass fiber-reinforced plastic. AIMS Energy 2018, 6, 1032–1049. [Google Scholar] [CrossRef]
- Yasui, K.; Sasaki, K.; Ikeda, N.; Kinoshita, H. Dye Adsorbent Materials Based on Porous Ceramics from Glass Fiber-Reinforced Plastic and Clay. Appl. Sci. 2019, 9, 1574. [Google Scholar] [CrossRef]
- Sato, T. Characteristics and Applications of Clays. Clay Sci. 2001, 41, 26–33. (In Japanese) [Google Scholar] [CrossRef]
- Wada, K. An Invitation to Soil Clay Mineralogy. Clay Sci. 1986, 26, 1–11. (In Japanese) [Google Scholar] [CrossRef]
- Sarma, G.K.; SenGupta, S.; Bhattacharyya, K.G. Methylene Blue Adsorption on Natural and Modified Clays. Sep. Sci. Technol. 2011, 46, 1602–1614. [Google Scholar] [CrossRef]
- Iimura, K. Adosorption of Mechyren Blue by Various Clays and Zeorite. Clay Sci. 1966, 6, 14–21. [Google Scholar] [CrossRef]
- Sekar, N. Handbook of Textile and Industrial Dyeing, Principles, Processes and Types of Dyes; Woodhead Publishing Series in Textiles; Elsevier: Cambridge, UK, 2011; Volume 1, pp. 486–514. [Google Scholar]
- Imada, K. Dye Chemistry for Dyeing Technicians (9). J. Text. Mach. Soc. Jpn. 2003, 54, 352–356. [Google Scholar] [CrossRef]
- Imada, K. Dye Chemistry for Dyeing Technicians (6). J. Text. Mach. Soc. Jpn. 2002, 55, 358–362. [Google Scholar] [CrossRef]
- Imada, K. Dye Chemistry for Dyeing Technicians (2). J. Text. Mach. Soc. Jpn. 2001, 54, 463–467. [Google Scholar] [CrossRef]
- Tanaka, M. Clay Hand Book; Gihoudou Shuppan Co., Ltd.: Tokyo, Japan, 1980; pp. 408–445. (In Japanese) [Google Scholar]
- Kondo, F.; Saito, Y.; Kanayama, M.; Nakazono, T. Settling and Dispersion-Flocculation Characteristics and Zeta Potential of Chlorite. Trans. JSIDRE 2003, 2003, 83–89. [Google Scholar] [CrossRef]
- Ogura, Y.; Takesako, H.; Nakabayashi, K. The hydroponic tomatoes using charge characteristics of clay minerals. Eco-Engineering 2014, 26, 11–17. [Google Scholar] [CrossRef]
- Acemioğlu, B. Adsorption of Congo red from aqueous solution onto calcium-rich fly ash. J. Colloid Interface Sci. 2004, 274, 371–379. [Google Scholar] [CrossRef]
- Wagner, M.; Eicheler, C.; Helmreich, B.; Hilbig, H.; Heinz, D. Removal of Congo Red from Aqueous Solutions at Hardened Cement Paste Surfaces. Front. Mater. 2020, 7, 567130. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic Degradation of Azo Dye Reactive Violet 5 on Fe-Doped Titania Catalysts under Visible Light Irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Novel Activated Carbon Monoliths for Methane Adsorption Obtained from Coffee Husks. Mater. Sci. Appl. 2011, 2, 331–339. [Google Scholar] [CrossRef]
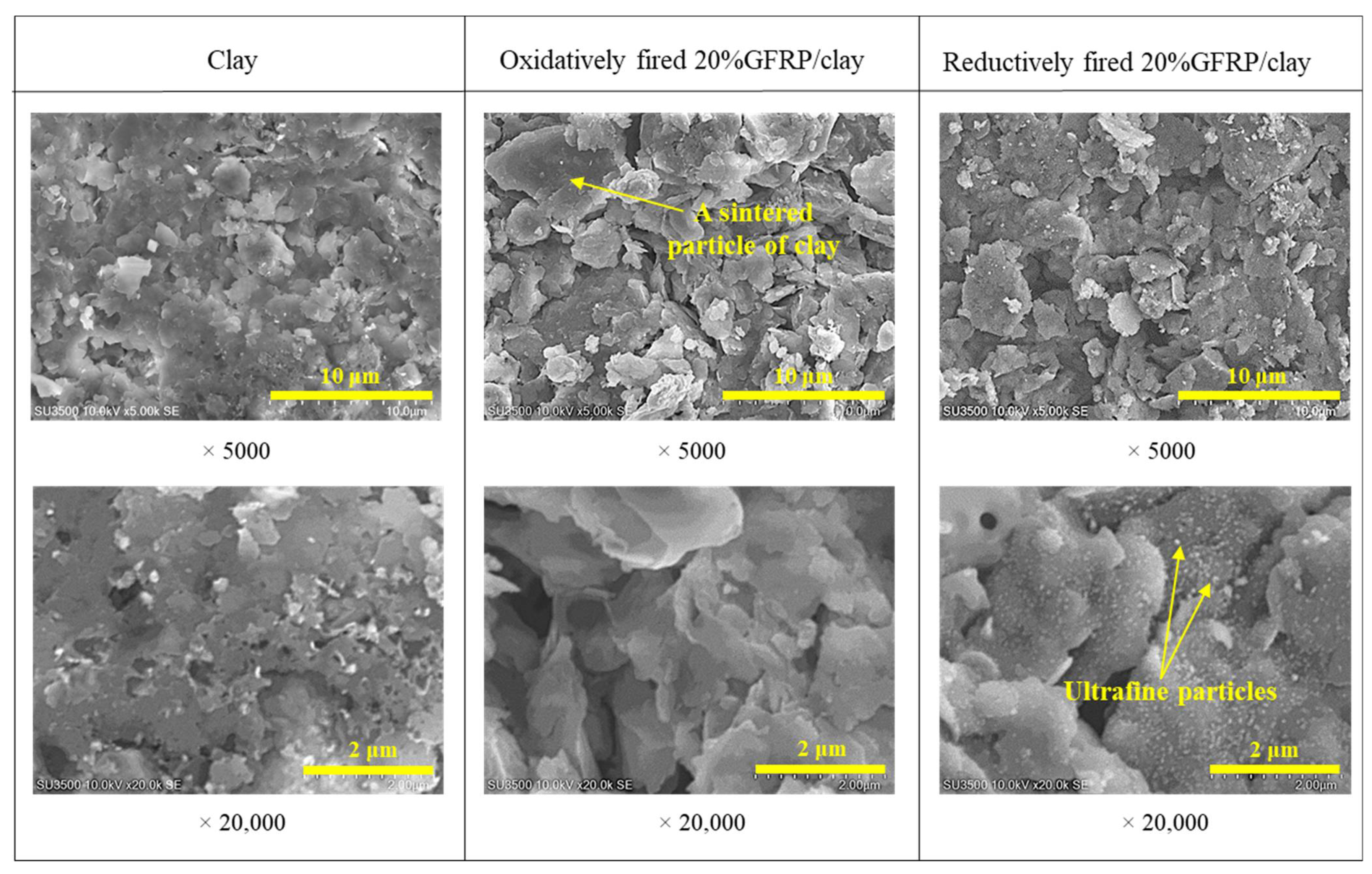


| Component | Raw Materials | |
|---|---|---|
| Clay (Mass%) | 40% GF/GFRP (Mass%) | |
| SiO2 | 65.8 | 54.9 |
| Al2O3 | 21.9 | 16.3 |
| Fe2O3 | 4.79 | 0.77 |
| K2O | 3.37 | 0.15 |
| MgO | 1.67 | - |
| CaO | 1.31 | 26.7 |
| TiO2 | 0.87 | 0.56 |
| others | 0.29 | 0.62 |
| Samples | Mixing Ratios of GFRP (Mass %) | Firing Conditions | |
|---|---|---|---|
| Oxidatively fired ceramics | Clay | 0 | Samples were heated at 100 K h−1 to 1073 K in an oxidizing atmosphere and then held at the firing temperature for 1 h |
| 20% GFRP/clay | 20 | ||
| 40% GFRP/clay | 40 | ||
| 60% GFRP/clay | 60 | ||
| Reductively fired ceramics | 20% GFRP/clay | 20 | Samples were heated at 400 K h−1 to 1073 K in a reducing atmosphere and then held at the firing temperature for 1 h |
| 40% GFRP/clay | 40 | ||
| 60% GFRP/clay | 60 | ||
| Component | Oxidatively Fired | Reductively Fired | ||||
|---|---|---|---|---|---|---|
| 20% GFRP/Clay | 40% GFRP/Clay | 60% GFRP/Clay | 20% GFRP/Clay | 40% GFRP/Clay | 60% GFRP/Clay | |
| SiO2 | 62.6 | 59.1 | 50.0 | 62.2 | 61.2 | 56.2 |
| Al2O3 | 22.1 | 20.7 | 17.7 | 18.5 | 9.13 | 4.79 |
| Fe2O3 | 4.87 | 4.16 | 4.09 | 6.13 | 7.56 | 7.34 |
| K2O | 3.26 | 2.91 | 2.00 | 3.73 | 3.77 | 3.11 |
| MgO | 1.66 | 1.75 | 1.51 | 2.24 | 2.43 | 2.14 |
| CaO | 4.02 | 9.93 | 23.2 | 5.34 | 12.9 | 22.7 |
| TiO2 | 0.86 | 0.80 | 1.03 | 1.21 | 1.56 | 1.49 |
| Others | 0.58 | 0.71 | 0.45 | 0.65 | 1.46 | 2.22 |
| Samples | Carbon Content (%) | |
|---|---|---|
| Oxidatively fired ceramics | Clay | 0.06 |
| 20% GFRP/clay | 0.24 | |
| 40% GFRP/clay | 0.25 | |
| 60% GFRP/clay | 0.26 | |
| Reductively fired ceramics | 20% GFRP/clay | 0.85 |
| 40% GFRP/clay | 0.99 | |
| 60% GFRP/clay | 1.12 | |
| Samples | Apparent Porosity (%) | Specific Surface Area (m2/g) | |
|---|---|---|---|
| Oxidatively fired ceramics | Clay | 31.9 | 11.0 |
| 20% GFRP/clay | 38.2 | 7.05 | |
| 40% GFRP/clay | 52.7 | 5.74 | |
| 60% GFRP/clay | 62.9 | 2.83 | |
| Reductively fired ceramics | 20% GFRP/clay | 43.1 | 14.9 |
| 40% GFRP/clay | 53.8 | 14.2 | |
| 60% GFRP/clay | 66.2 | 11.3 | |
| Samples | Apparent Porosity (%) | Specific Surface Area (m2/g) | Carbon Content (%) |
|---|---|---|---|
| Clay sample-B | 31.6 | 3.49 | 0.27 |
| 6% POM/clay | 29.5 | 7.32 | 0.46 |
| 12% POM/clay | 33.2 | 7.48 | 0.58 |
| 18% POM/clay | 46.7 | 7.19 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, H.; Sasaki, K.; Yasui, K.; Miyakawa, Y.; Yuji, T.; Misawa, N.; Mungkung, N. Dye Adsorption Mechanism of Glass Fiber-Reinforced Plastic/Clay Ceramics and Influencing Factors. Polymers 2021, 13, 3172. https://doi.org/10.3390/polym13183172
Kinoshita H, Sasaki K, Yasui K, Miyakawa Y, Yuji T, Misawa N, Mungkung N. Dye Adsorption Mechanism of Glass Fiber-Reinforced Plastic/Clay Ceramics and Influencing Factors. Polymers. 2021; 13(18):3172. https://doi.org/10.3390/polym13183172
Chicago/Turabian StyleKinoshita, Hiroyuki, Koya Sasaki, Kentaro Yasui, Yuko Miyakawa, Toshifumi Yuji, Naoaki Misawa, and Narong Mungkung. 2021. "Dye Adsorption Mechanism of Glass Fiber-Reinforced Plastic/Clay Ceramics and Influencing Factors" Polymers 13, no. 18: 3172. https://doi.org/10.3390/polym13183172
APA StyleKinoshita, H., Sasaki, K., Yasui, K., Miyakawa, Y., Yuji, T., Misawa, N., & Mungkung, N. (2021). Dye Adsorption Mechanism of Glass Fiber-Reinforced Plastic/Clay Ceramics and Influencing Factors. Polymers, 13(18), 3172. https://doi.org/10.3390/polym13183172






