Polycyclic Aromatic Hydrocarbon Sorption by Functionalized Humic Acids Immobilized in Micro- and Nano-Zeolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Purification of Humic Acids
2.2. Functionalization of Humic Acids
2.3. Characterization of Humic Acids
2.4. Immobilization in Zeolite of Humic Acids
2.5. Sorption of PAHs into Pure and Chemically Modified HA
2.6. Sorption of PAHs in an HA-Zeolite Hybrid Sorbent
2.7. Statistical Analysis
3. Results
3.1. Elemental Composition of Humic Substances
3.2. Solubility, pH, and Surface Tension
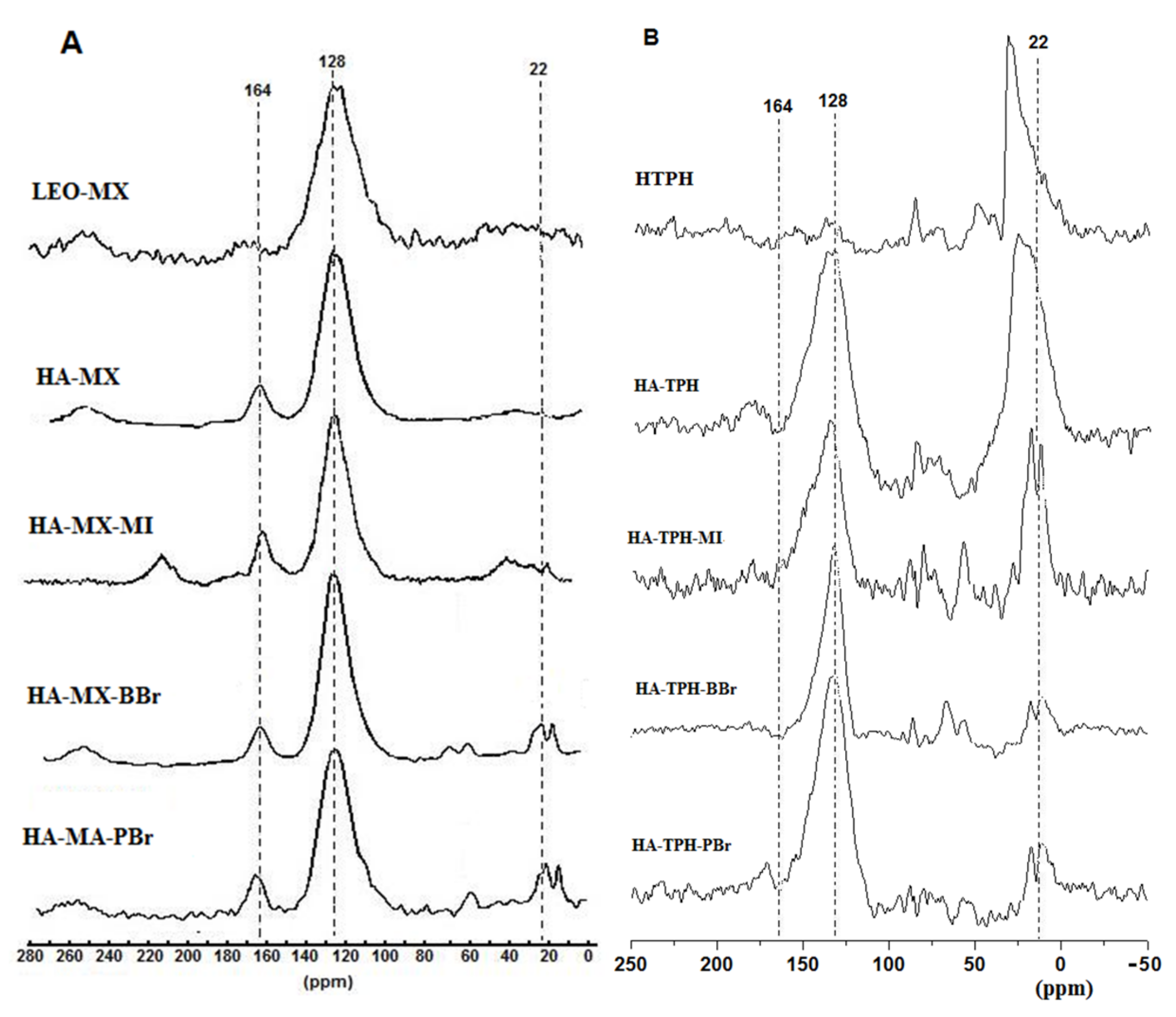
3.3. FTIR and 13C-NMR Spectra of the Tested Humic Acids
3.4. Sorption of PAHs in Selected Humic Acids
3.5. Immobilization in Zeolite of Humic Acids
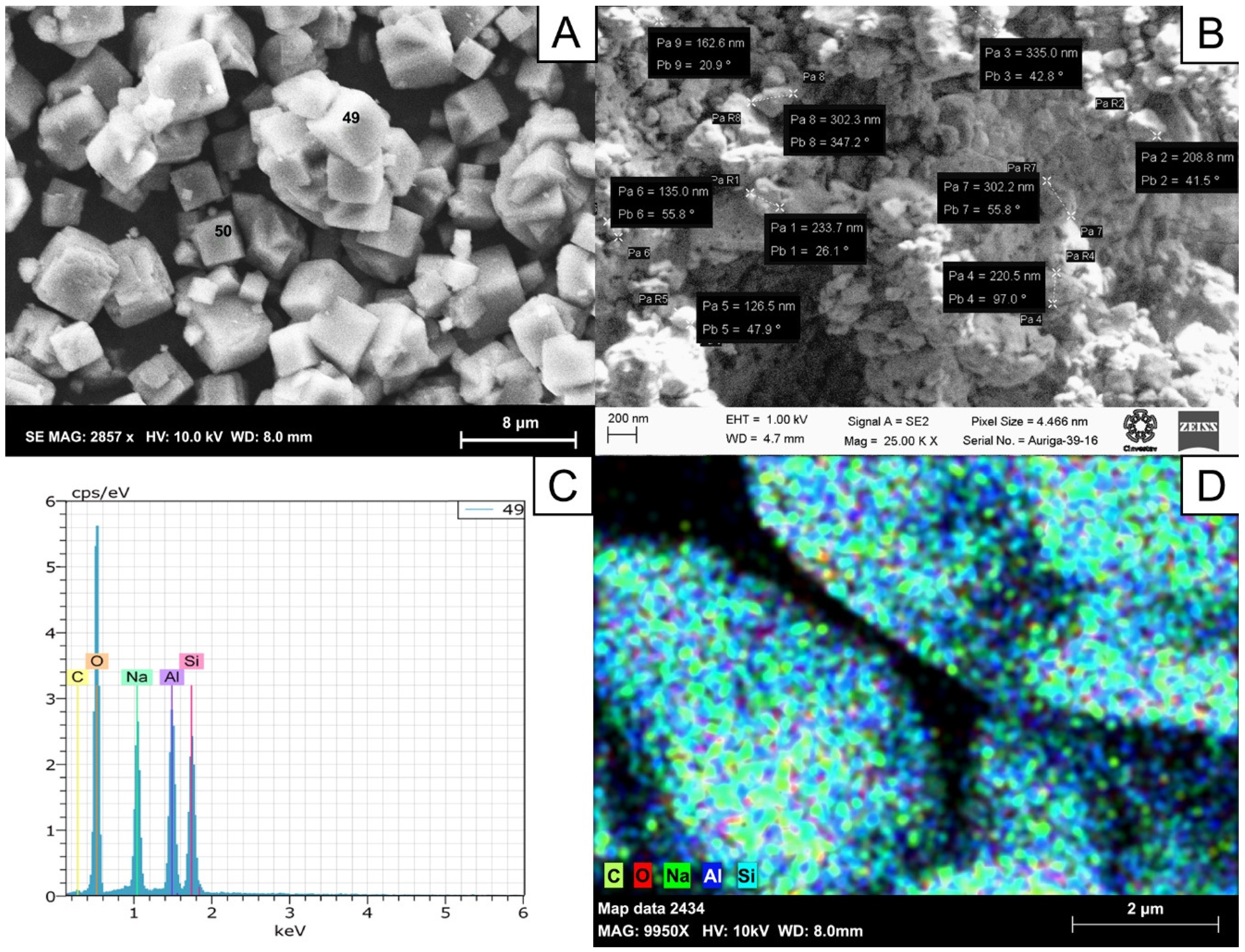
3.5.1. Characterization of Zeolite
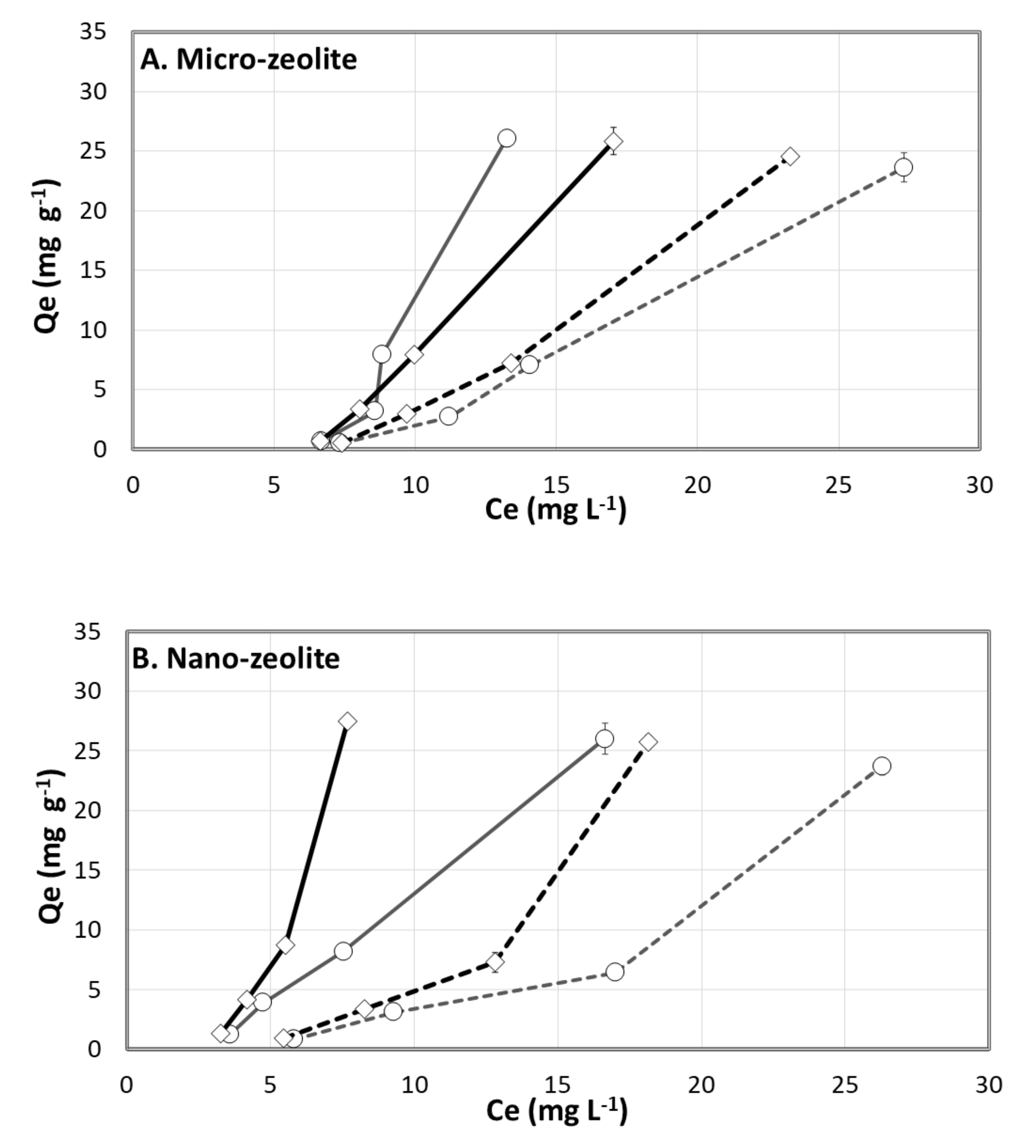
3.5.2. Sorption Isotherms of Humic Acids in Zeolite of Two Particle Sizes
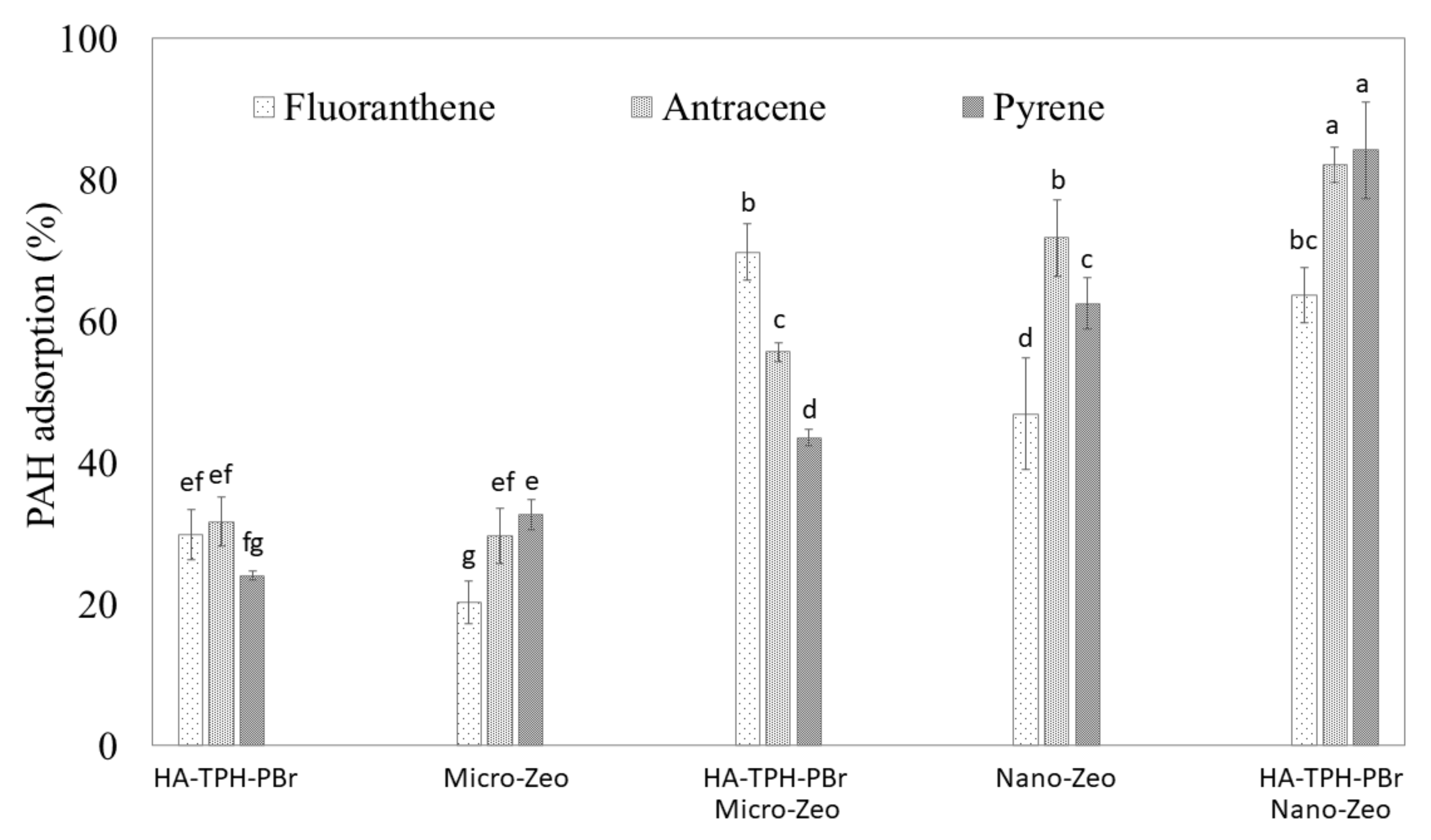
3.6. Sorption of PAHs in Functionalized HA Immobilized in Zeolite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D.; Salvestrini, S. Sorption of Organic Pollutants by Humic Acids: A Review. Molecules 2020, 25, 918. [Google Scholar] [CrossRef] [Green Version]
- Sakshi; Singh, S.; Haritash, A.K. Polycyclic aromatic hydrocarbons: Soil pollution and remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Sun, J.; Zhang, L.; Zhang, B.; Zhang, T.; Wang, J.; Xu, H.; Liu, P.; Zhang, N.; et al. Parent, alkylated, oxygenated and nitro polycyclic aromatic hydrocarbons from raw coal chunks and clean coal combustion: Emission factors, source profiles, and health risks. Sci. Total Environ. 2020, 721, 137696. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.S.; Li, L.Y. Removal of polycyclic aromatic hydrocarbons from aqueous media using modified clinoptilolite. J. Environ. Manag. 2020, 273, 111113. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Jácome-Francis, K. Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials. Sustainability 2021, 13, 6378. [Google Scholar] [CrossRef]
- Catrinescu, C.; Apreutesei, R.E.; Teodosiu, C. Surfactant-modified natural zeolites for environmental applications in water purification. Environ. Eng. Manag. J. 2008, 7, 149–161. [Google Scholar] [CrossRef]
- Lemić, J.; Tomašević-Čanović, M.; Adamović, M.; Kovačević, D.; Milićević, S. Competitive adsorption of polycyclic aromatic hydrocarbons on organo-zeolites. Microporous Mesoporous Mat. 2007, 105, 317–323. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamizhdurai, P.; Mangesh, V.L.; Ragupathi, C.; Krishnan, P.S.; Ramesh, A. Recent advances in the synthesis and applications of mordenite zeolite—Review. RSC Adv. 2021, 11, 250–267. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Pota, G.; Venezia, V.; Vitiello, G.; Di Donato, P.; Mollo, V.; Costantini, A.; Avossa, J.; Nuzzo, A.; Piccolo, A.; Silvestri, B.; et al. Tuning Functional Behavior of Humic Acids through Interactions with Stöber Silica Nanoparticles. Polymer 2020, 12, 982. [Google Scholar] [CrossRef] [Green Version]
- García-Díaz, C.; Nebbioso, A.; Piccolo, A.; Barrera-Cortés, J.; Martínez-Palou, R. Remediation of Hydrocarbon-Contaminated Soil by Washing with Novel Chemically Modified Humic Substances. J. Environ. Qual. 2015, 44, 1764–1771. [Google Scholar] [CrossRef]
- Sannino, F.; Spaccini, R.; Savy, D.; Piccolo, A. Remediation of highly contaminated soils from an industrial site by employing a combined treatment with exogeneous humic substances and oxidative biomimetic catalysis. J. Hazard. Mater. 2013, 261, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loepert, P.N., Soltanpur, P.N., Tabatabai, M.A., Johnson, C.T., Sumner, M.E., Eds.; Soil Science Society of America Book Series; John Wiley & Sons: Madison, WI, USA, 1996; Volume 5, pp. 1018–1020. [Google Scholar]
- Fuentes, M.; González-Gaitano, G.; García-Mina, J.M. The usefulness of UV–visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org. Geochem. 2006, 37, 1949–1959. [Google Scholar] [CrossRef]
- Kang, S.; Xing, B. Phenanthrene Sorption to Sequentially Extracted Soil Humic Acids and Humins. Environ. Sci. Technol. 2005, 39, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zeledón-Toruño, Z.C.; Lao-Luque, C.; Heras, F.X.C.D.L.; Sardans, M.S. Removal of PAHs from water using an immature coal (leonardite). Chemosphere 2007, 67, 505–512. [Google Scholar] [CrossRef]
- Wang, S.; Terdkiatburana, T.; Tadé, M. Adsorption of Cu(II), Pb(II) and humic acid on natural zeolite tuff in single and binary systems. Sep. Purif. Technol. 2008, 62, 64–70. [Google Scholar] [CrossRef]
- Elsheikh, A.F.; Ahmad, U.K.; Ramli, Z. Investigations on humic acid removal from water using surfactant-modified zeolite as adsorbent in a fixed-bed reactor. Appl. Water Sci. 2016, 7, 2843–2856. [Google Scholar] [CrossRef] [Green Version]
- Salvestrini, S. New insights into the interaction mechanism of humic acids with phillipsite. React. Kinet. Mech. Catal. 2017, 120, 735–752. [Google Scholar] [CrossRef]
- Hernández, C.G.; Barrera-Cortés, J.; García-Rojas, C.M.C.; Tinoco, M.D.C.; Ponce-Noyola, T.; Domínguez, M.S.; Gómez, B.C. Weathered Railroad Diesel Removed from a Loamy Sand Soil by Means of Mono-rhamnolipids. Soil Sediment Contam. Int. J. 2021, 30, 350–372. [Google Scholar] [CrossRef]
- Droussi, Z.; Ouatmane, A.; D’Orazio, V.; Hafidi, M. Elemental and Spectroscopic Characterization of Humic-acid-like Compounds during Composting of Olive Mill By-products. J. Hazard. Mater. 2009, 163, 1289–1297. [Google Scholar] [CrossRef]
- Klavins, M.; Purmalis, O. Surface Activity of Humic Substances. In Recent Advances in Continuum Mechanics, Hydrology and Ecology; Mladenov, V., Kralov, I., Tashev, T., Popescu, F., Hernández López, J.I., Kattel, G., Tavares Martins, A.M., Eds.; Energy, Environmental and Structural Engineering Series; WSEAS: Athens, Greece, 2013; Volume 14, pp. 146–151. [Google Scholar]
- Maryganova, V.V.; Bambalov, N.N.; Strigutskii, V.P.; Parmon, S.V. Changes in the Composition of Humic Substances Depending on the Depth of Peat Occurrence. Solid Fuel Chem. 2013, 47, 153–164. [Google Scholar] [CrossRef]
- Ramírez-Coutiño, V.A.; Torres-Bustillos, L.G.; Godínez Mora Tovar, L.A.; Guerra-Sánchez, R.J.; Rodríguez-Valadez, F.J. pH effect on surfactant properties and supramolecular structure of humic substances obtained from sewage sludge composting. Rev. Int. Contam. Ambient. 2013, 29, 191–199. [Google Scholar]
- Davies, G.; Ghabbour, E.; Steelink, C. Humic Acids: Marvelous Products of Soil Chemistry. J. Chem. Educ. 2001, 78, 1609–1614. [Google Scholar] [CrossRef]
- Da Silva, R.R.; Lucena, G.N.; Machado, Â.F.; De Freitas, G.A.; Matos, A.; Abrahão, W.A.P. Spectroscopic and elementary characterization of humic substances in organic substrates. Comun. Sci. 2018, 9, 264–274. [Google Scholar] [CrossRef]
- Fooken, U.; Liebezeit, G. An IR study of humic acids isolated from sediments and soils. Senckenberg. Marit. 2003, 32, 183–189. [Google Scholar] [CrossRef]
- Djomgoue, P.; Njopwouo, D. FT-IR Spectroscopy Applied for Surface Clays Characterization. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1994; 512p. [Google Scholar]
- Motta, F.L.; Melo, B.A.G.; Santana, M.H.A. Deprotonation and protonation of humic acids as a strategy for the technological development of pH-responsive nanoparticles with fungicidal potential. New Biotechnol. 2016, 33, 773–780. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Hamdi, H.; Revel, J.-C. Elemental analysis, FTIR and 13C-NMR of humic acids from sewage sludge composting. Agronomy 2004, 24, 13–18. [Google Scholar] [CrossRef]
- Reddy, S.B.; Nagaraja, M.; Kadalli, G.; Champa, B. Fourier Transform Infrared (FTIR) Spectroscopy of Soil Humic and Fulvic Acids Extracted from Paddy Land Use System. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 834–837. [Google Scholar] [CrossRef]
- Plaza, C.; Xing, B.; Fernández, J.M.; Senesi, N.; Polo, A. Binding of polycyclic aromatic hydrocarbons by humic acids formed during composting. Environ. Pollut. 2009, 157, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Saparpakorn, P.; Kim, J.H.; Hannongbua, S. Investigation on the Binding of Polycyclic AromaticHydrocarbons with Soil Organic Matter: A Theoretical Approach. Molecules 2007, 12, 703–715. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Gao, Q.; Chen, Y.; Li, S.; Yang, Y.; Werner, D.; Tao, S.; Wang, X. Association of 16 priority polycyclic aromatic hydrocarbons with humic acid and humin fractions in a peat soil and implications for their long-term retention. Environ. Pollut. 2017, 230, 882–890. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Leone, V.; Canzano, S.; Iovino, P.; Salvestrini, S.; Capasso, S. A novel organo-zeolite adduct for environmental applications: Sorption of phenol. Chemosphere 2013, 91, 415–420. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, Z.; Lin, J.; Qiu, Y.; Zhao, J. Removal of humic acid from aqueous solution by cetylpyridinium bromide modified zeolite. J. Environ. Sci. 2010, 22, 1327–1334. [Google Scholar] [CrossRef]
- Tashauoei, H.R.; Attar, M.H.; Amin, M.M.; Kamali, M.; Nikaeen, M.; Dastjerdi, M.V. Removal of cadmium and humic acid from aqueous solutions using surface modified nanozeolite A. Int. J. Environ. Sci. Technol. 2010, 7, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Lin, J.; Qiu, Y.; Gao, N.; Zhu, Z. Adsorption of humic acid from aqueous solution on bilayer hexadecyltrimethyl ammonium bromide-modified zeolite. Front. Environ. Sci. Eng. China 2011, 5, 65–75. [Google Scholar] [CrossRef]
- Capasso, S.; Salvestrini, S.; Coppola, E.; Buondonno, A.; Colella, C. Sorption of humic acid on zeolitic tuff: A preliminary investigation. Appl. Clay Sci. 2005, 28, 159–165. [Google Scholar] [CrossRef]
- Wołowiec, M.; Muir, B.; Zięba, K.; Bajda, T.; Kowalik, M.; Franus, W. Experimental Study on the Removal of VOCs and PAHs by Zeolites and Surfactant-Modified Zeolites. Energy Fuels 2017, 31, 8803–8812. [Google Scholar] [CrossRef]
- Manni, A.; Saviano, G.; De Casa, G.; Rotatori, M.; Guarnieri, A.; Guerriero, E. Natural zeolites for PAH removal from liquid effluents. Organohalogen Compd. 2007, 69, 2938–2941. [Google Scholar]
- Akbari, Z.; Ghiassi, R. Remediation of Soil and Groundwater Polluted by Polycyclic Aromatic Hydrocarbons: A Review. Am. J. Civ. Environ. Eng. 2017, 2, 8–20. [Google Scholar]
- Vidal, C.B.; Barros, A.L.; Moura, C.P.; de Lima, A.C.; Dias, F.S.; Vasconcellos, L.C.; Fechine, P.B.; Nascimento, R. Adsorption of polycyclic aromatic hydrocarbons from aqueous solutions by modified periodic mesoporous organosilica. J. Colloid Interface Sci. 2011, 357, 466–473. [Google Scholar] [CrossRef] [Green Version]


| Humic Acids | %C (±SD) | %H (±SD) | % N (±SD) | |||
|---|---|---|---|---|---|---|
| -MX- | -TPH- | -MX- | -TPH- | -MX- | -TPH- | |
| HA sources | 13.9 (±0.2) g | 40.9 (±0.2) f | 1.31 (±0.001) g | 3.76 (±0.11) d | 0.22 (±0.03) g | 0.75 (±0.01) f |
| Pure HAs | 57.5 (±0.5) bcd | 46.5 (±0.3) e | 2.63 (±0.11) f | 3.14 (±0.11) e | 1.10 (±0.01) b | 1.01 (±0.01) e |
| Functionalized humic acids: | ||||||
| MI | 62.3 (±0.11) d | 57.3 (±0.2) cd | 3.79 (±0.01) f | 4.58 (±0.11) c | 1.41 (±0.02) d | 1.57 (±0.01) b |
| BBr | 58.9 (±0.3) b | 66.1 (±0.5) bcd | 3.12 (±0.01) ef | 5.53 (±0.06) a | 1.14 (±0.01) c | 1.26 (±0.02) a |
| PBr | 59.2 (±0.2) bc | 58.2 (±0.4) a | 2.97 (±0.08) ef | 6.05 (±0.10) b | 1.25 (±0.01) d | 1.69 (±0.01) c |
| Humic Acids | Surface Tension (mN m−1) | pH of HA in Solution | NaOH (M) Required to Solubilize the Tested HA | |||
|---|---|---|---|---|---|---|
| -MX- | -TPH- | -MX- | -TPH- | -MX- | -TPH- | |
| HA sources | 64.7 (±0.2) bc | 64.7 (±0.2) bc | 9.3 (±0) e | 9.2 (±0) e | 0.0 (±0) | 0.0 (±0) |
| Pure HAs | 64.8 (±0.7) bc | 60.3 (±1.1) de | 9 (±0) f | 11.5 (±0) d | 0.0 (±0) | 0.016 (±0) |
| Functionalized humid acids: | ||||||
| MI | 62.4 (±1.1) cd | 58 (±2) e | 12.1 (±0) bc | 12.0 (±0) c | 0.02 (±0) | 0.013 (±0) |
| BBr | 67.3 (±0.1) ab | 63.2 (±0.1) cd | 12.1 (±0) bc | 12.2 (±0) ab | 0.02 (±0) | 0.013 (±0) |
| PBr | 68.7 (±0.1) a | 62 (±0.2) cd | 12.3 (±0.1) a | 12.1 (±0.1) bc | 0.02 (±0) | 0.016 (±0) |
| Sorbent | Fluoranthene (mg g−1) | Anthracene (mg g−1) | Pyrene (mg g−1) |
|---|---|---|---|
| HA-TPH-PBr | 1.9 ± 0.3 ef | 2.0 ± 0.2 ef | 1.6 ± 0.1 fg |
| Micro-Zeo | 1.3 ± 0.3 g | 1.9 ± 0.3 ef | 2.0 ± 0.2 e |
| (HA-TPH-PBr)-(Micro-Zeo) | 4.4 ± 0.04 b | 3.5 ± 0.1 c | 2.7 ± 0.1 d |
| Nano-Zeo | 2.93 ± 0.5 d | 4.5 ± 0.1 b | 3.8 ± 0.2 c |
| (HA-TPH-PBr)-(Nano-Zeo) | 4.0 ± 0.2 bc | 5.13 ± 0.1 a | 5.3 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-Mora, G.; Barrera-Cortés, J.; Valdez-Castro, L.; Solorza-Feria, O.; García-Díaz, C. Polycyclic Aromatic Hydrocarbon Sorption by Functionalized Humic Acids Immobilized in Micro- and Nano-Zeolites. Sustainability 2021, 13, 10391. https://doi.org/10.3390/su131810391
Robles-Mora G, Barrera-Cortés J, Valdez-Castro L, Solorza-Feria O, García-Díaz C. Polycyclic Aromatic Hydrocarbon Sorption by Functionalized Humic Acids Immobilized in Micro- and Nano-Zeolites. Sustainability. 2021; 13(18):10391. https://doi.org/10.3390/su131810391
Chicago/Turabian StyleRobles-Mora, Gabriela, Josefina Barrera-Cortés, Lucila Valdez-Castro, Omar Solorza-Feria, and César García-Díaz. 2021. "Polycyclic Aromatic Hydrocarbon Sorption by Functionalized Humic Acids Immobilized in Micro- and Nano-Zeolites" Sustainability 13, no. 18: 10391. https://doi.org/10.3390/su131810391






