Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854)
Abstract
:1. Introduction
2. Materials and Methods
2.1. MPs Used in the Study
Form
2.2. Medaka Fish Maintenance and PS-MPs Exposure
2.3. Histological Analysis
2.4. The Determination of Intestinal Oxidative Stress and Permeability
2.5. The Determination of Brain Oxidative Stress and Neurotoxicity
2.6. Statistical Analysis
3. Results
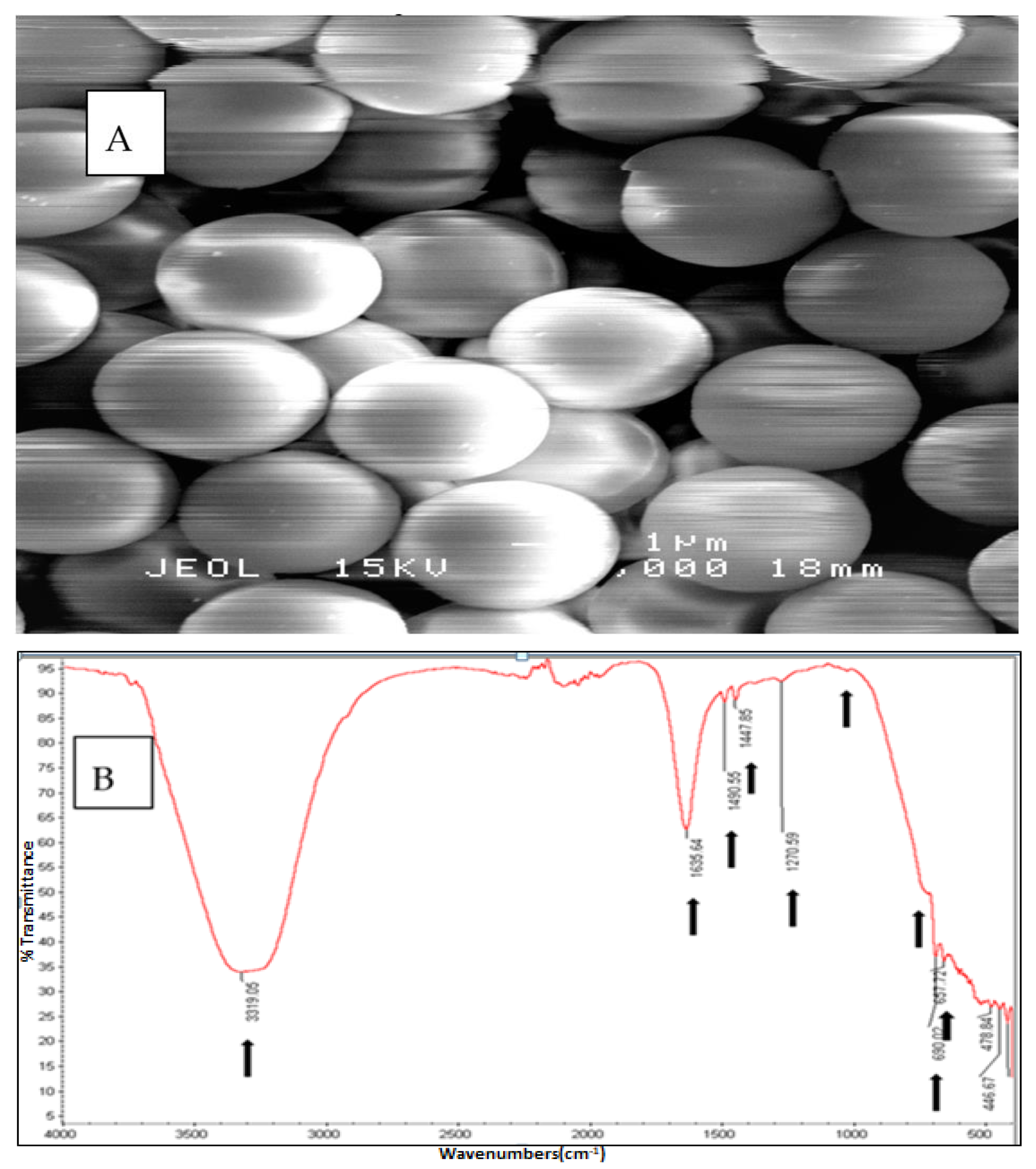
3.1. The Physical and Chemical Confirmation of PS-MPs Used in the Study
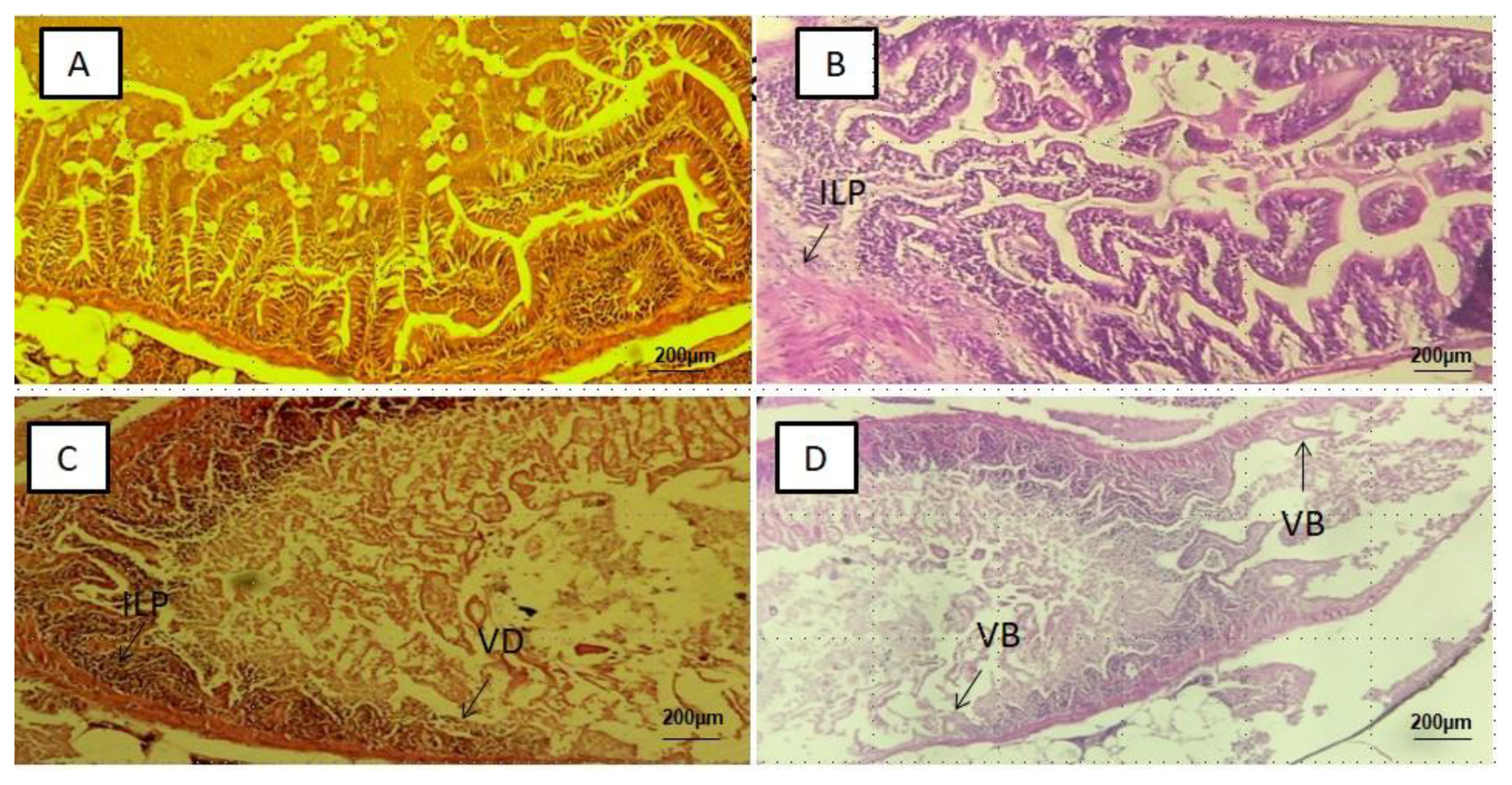
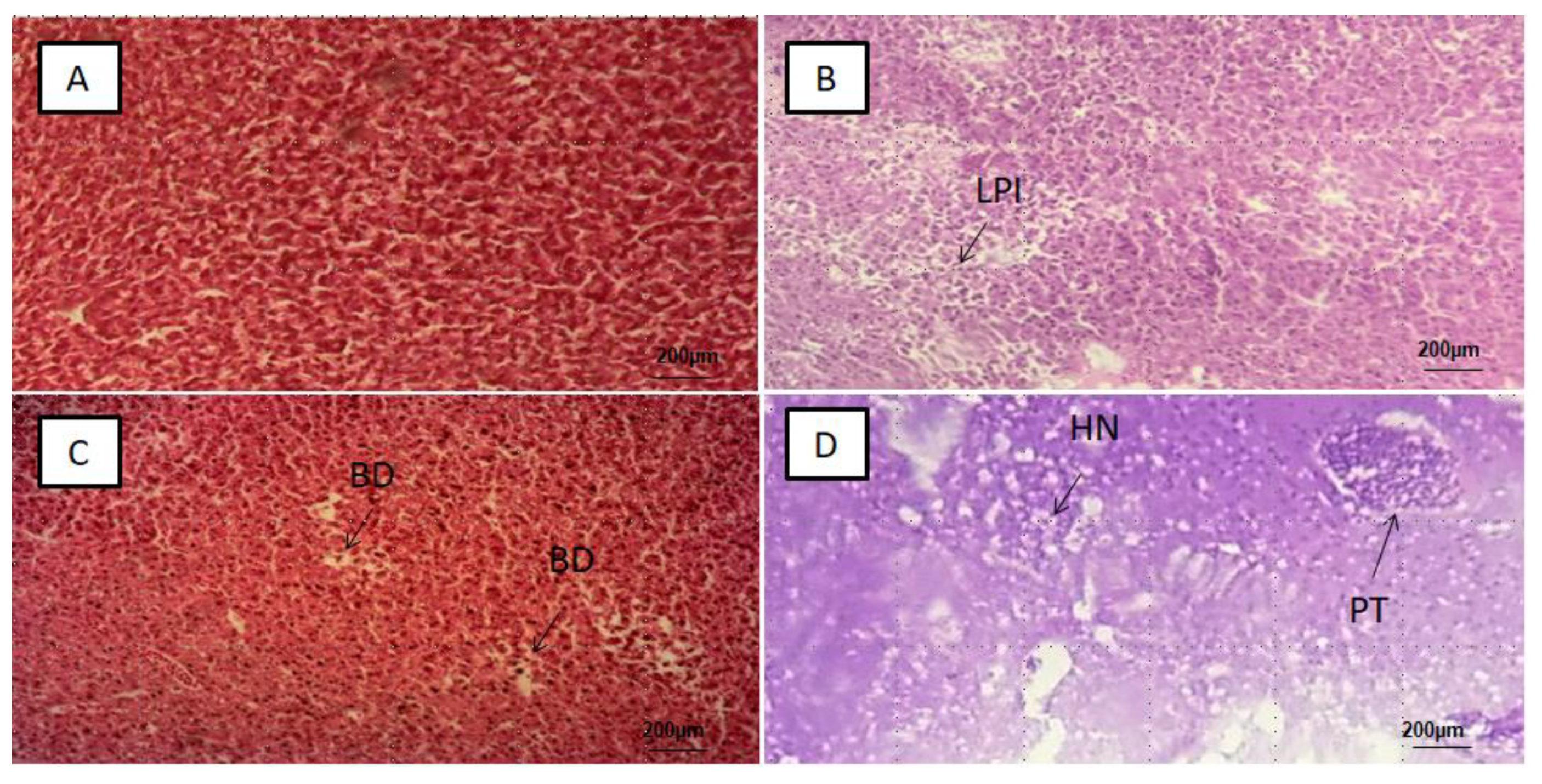
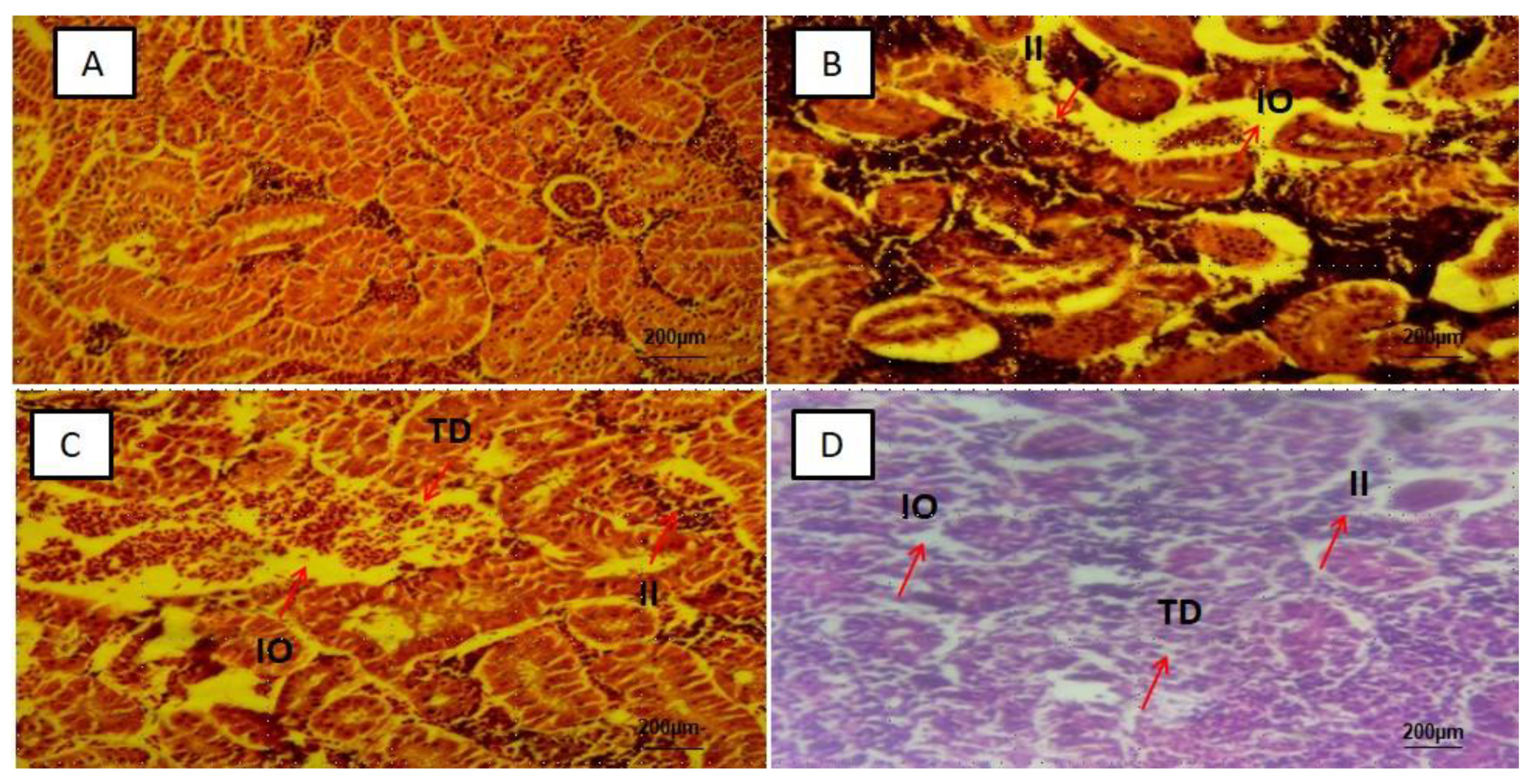
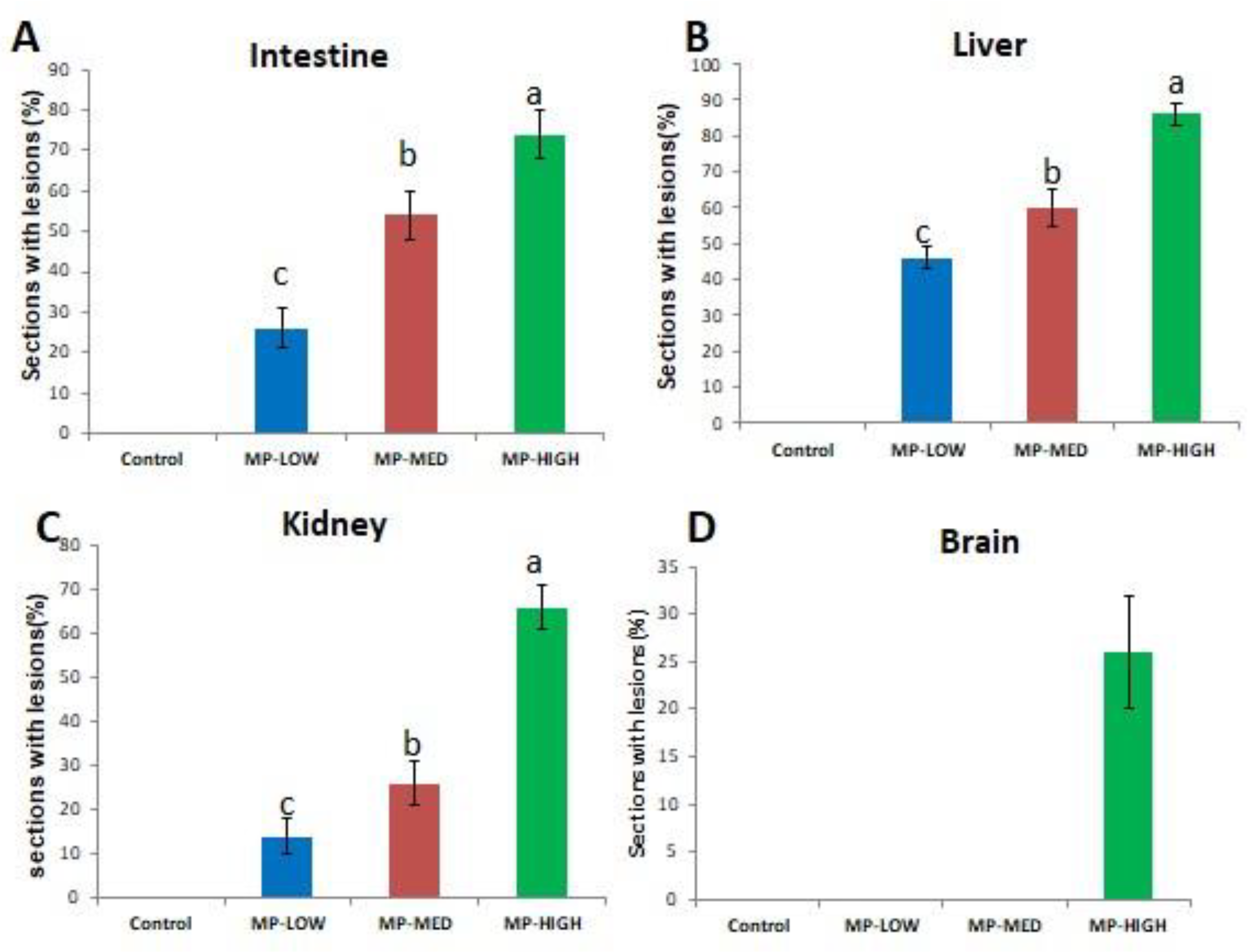
3.2. Histological Alterations in the Intestines, Liver, Kidney and Brain
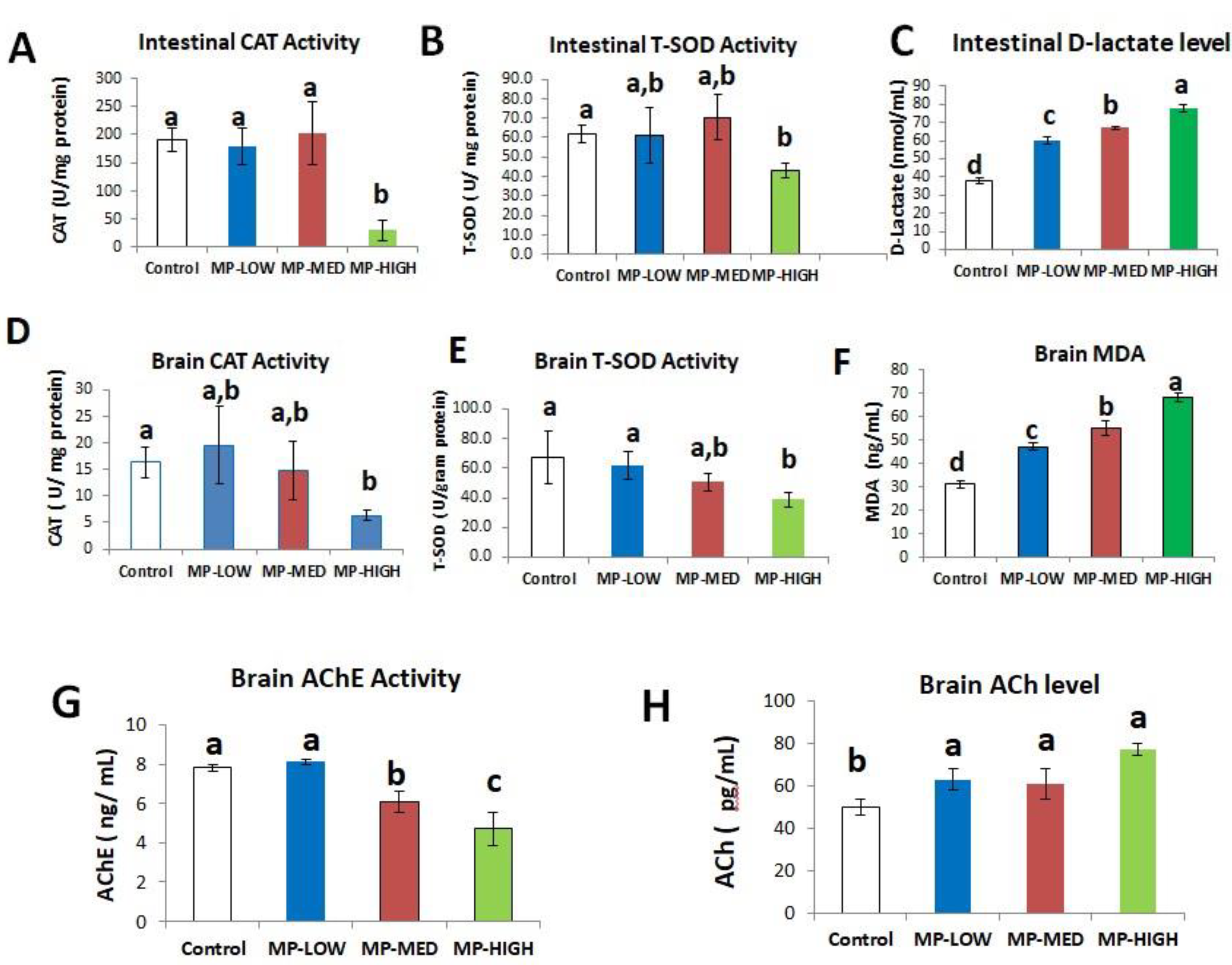
3.3. Intestinal Oxidative Stress and Increased Permeability Induced by PS-MPs
3.4. Brain Oxidative Stress and Neurotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Freshwater Microplastics; Emerging Environmental Contaminants? Springer Nature: Basingstoke, UK, 2018; Volume 58, ISBN 978-3-319-61614-8. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Kershaw, P.J. (Ed.) Sources, fate and effects of microplastics in the marine environment: Part 2 of a global assessment. (IMO, FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP). In Report Study GESAMP No. 90 (96 pp), Report Study GESAMP No. 93 96 P.; International Maritime Organization: London, UK, 2016; Volume 93. [Google Scholar]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Carbery, M.; Wayne, O.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 45–57. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharm. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of East China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [Green Version]
- Usman, S.; Razis, A.F.A.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Isa, N.M.; Nazarudin, M.F.; Zulkifli, S.Z.; Sutra, J.; Ibrahim, M.A. Microplastics pollution as an invisible potential threat to food safety and security, policy challenges and the way forward. Int. J. Environ. Res. Public Health 2020, 17, 9591. [Google Scholar] [CrossRef]
- Ajith, N.; Arumugam, S. Global distribution of microplastics and its impact on marine environment—A review. Environ. Sci. Pollut. Res. 2020, 27, 25970–25986. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, G.; Lu, L.; Zheng, Y.; Zhang, Q.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Low level of polystyrene microplastics decreases early developmental toxicity of phenanthrene on marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 385, 121586. [Google Scholar] [CrossRef]
- Cong, Y.; Jin, F.; Tian, M.; Wang, J.; Shi, H.; Wang, Y.; Mu, J. Ingestion, egestion and post-exposure effects of polystyrene microspheres on marine medaka (Oryzias melastigma). Chemosphere 2019, 228, 93–100. [Google Scholar] [CrossRef]
- Zhu, M.; Chernick, M.; Rittschof, D.; Hinton, D.E. Chronic dietary exposure to polystyrene microplastics in maturing Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2020, 220, 105396. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xu, J.; Zhu, L.; Peng, G.; Xu, P.; Li, D. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar. Pollut. Bull. 2019, 146, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, K.; Li, B.; Chen, Q.; Su, L.; Wu, C.; Hollert, H.; Shi, H. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Won, E.; Lee, S.; Souissi, S.; Jeong, C.; Lee, M.; Hwang, D.; Lee, J.; Kang, H.; Hwang, U.; Zhou, B. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Borza, C.; Muntean, D.; Dehelean, C.; Savoiu, G.; Serban, C.; Simu, G.; Andoni, M.; Butur, M.; Drag, S. Oxidative Stress and Lipid Peroxidation—A Lipid Metabolism Dysfunction. Lipid Metab. 2013. [Google Scholar] [CrossRef] [Green Version]
- Pacelli, C.; Giguère, N.; Bourque, M.J.; Lévesque, M.; Slack, R.S.; Trudeau, L.É. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr. Biol. 2015, 25, 2349–2360. [Google Scholar] [CrossRef] [Green Version]
- Reed, T.T. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011, 51, 1302–1319. [Google Scholar] [CrossRef]
- Sidorova, Y.; Domanskyi, A. Detecting Oxidative Stress Biomarkers in Neurodegenerative Disease Models and Patients. Methods Protoc. 2020, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Zahm, M.; Cabau, C.; Klopp, C.; Roques, C.; Bouchez, O.; Donnadieu, C.; Barrachina, C.; Journot, L.; Kawaguchi, M.; et al. Genome Sequence of the Euryhaline Javafish Medaka, Oryzias javanicus: A Small Aquarium Fish Model for Studies on Adaptation To Salinity. G3 Genes Genomes Genet. 2020, 10, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, Y.; Kanazawa, N.; Yamagishi, T.; Yonekura, K.; Tatarazako, N. Ecotoxicological Test Assay Using OECD TG 212 in Marine Java Medaka (Oryzias javanicus) and Freshwater Japanese Medaka (Oryzias latipes). Bull. Environ. Contam. Toxicol. 2018, 101, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Kawamata, M.; Imai, S.; Fukunaga, M.; Uno, S.; Kakuno, A. Java Medaka: A Proposed New Marine Test Fish for Ecotoxicology. Environ. Toxicol. 2008, 23, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Adamu, M.; Zahmir, S.; Noor, M.; Azmai, A.; Mohamat-yusuff, F.; Ismail, A. Embryonic toxicity of 3,4-dichloroaniline (3,4-DCA) on Javanese medaka. Toxicol. Rep. 2020, 7, 1039–1045. [Google Scholar] [CrossRef]
- Mohamat-Yusuff, F.; Sarah-Nabila, A.G.; Zulkifli, S.Z.; Azmai, M.N.A.; Ibrahim, W.N.W.; Yusof, S.; Ismail, A. Acute toxicity test of copper pyrithione on Javanese medaka and the behavioural stress symptoms. Mar. Pollut. Bull. 2018, 127, 150–153. [Google Scholar] [CrossRef]
- Masato, K.; Kenji, M.; Kiyoshi, N.; Minoru, T. (Eds.) Medaka, Biology, Management and Experimental Protocol, 1st ed.; Wiley Black-Well: Ames, IA, USA, 2009. [Google Scholar]
- Ahrendt, C.; Perez-venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M. Microplastic ingestion cause intestinal lesions in the intertidal fi sh Girella laevifrons. Mar. Pollut. Bull. 2019, 151, 110795. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubular, P.; Cells, E.; Mice, M.C.B.L.; Wang, Y.; Lee, Y.; Hsu, Y.; Chiu, I.; Huang, C.C.; Huang, C.; Chia, Z. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney. Environ. Health Perspect. 2021, 129, 1–18. [Google Scholar]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef] [Green Version]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Rahiman, S.A.; Matteoli, G. Intestinal permeability, inflammation and the role of nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbǎ, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative stress and the microbiota-gut-brain axis. Oxid. Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef] [Green Version]
- Iheanacho, S.C.; Igberi, C.; Amadi-Eke, A.; Chinonyerem, D.; Iheanacho, A.; Avwemoya, F. Biomarkers of neurotoxicity, oxidative stress, hepatotoxicity and lipid peroxidation in Clarias gariepinus exposed to melamine and polyvinyl chloride. Biomarkers 2020, 25, 603–610. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a Biomarker in Environmental and Occupational Medicine: New Insights and Future Perspectives. BioMed. Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, S.; Abdull Razis, A.F.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Mat Isa, N.; Nazarudin, M.F. Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). Int. J. Environ. Res. Public Health 2021, 18, 9449. https://doi.org/10.3390/ijerph18189449
Usman S, Abdull Razis AF, Shaari K, Amal MNA, Saad MZ, Mat Isa N, Nazarudin MF. Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). International Journal of Environmental Research and Public Health. 2021; 18(18):9449. https://doi.org/10.3390/ijerph18189449
Chicago/Turabian StyleUsman, Sunusi, Ahmad Faizal Abdull Razis, Khozirah Shaari, Mohammad Noor Azmai Amal, Mohd Zamri Saad, Nurulfiza Mat Isa, and Muhammad Farhan Nazarudin. 2021. "Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854)" International Journal of Environmental Research and Public Health 18, no. 18: 9449. https://doi.org/10.3390/ijerph18189449
APA StyleUsman, S., Abdull Razis, A. F., Shaari, K., Amal, M. N. A., Saad, M. Z., Mat Isa, N., & Nazarudin, M. F. (2021). Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). International Journal of Environmental Research and Public Health, 18(18), 9449. https://doi.org/10.3390/ijerph18189449








