Pesticides: Behavior in Agricultural Soil and Plants
Abstract
:1. Introduction
- To protect plants or plant products from pests/diseases before or after the harvest (e.g., fungicides, insecticides, molluscicides, nematocides, rodenticides);
- To influence processes of plant life (e.g., substances affecting their growth, with the exception of nutrients);
- To preserve plant products (e.g., fumigants);
- To destroy undesirable plants and their parts or to prevent their growth (e.g., defoliants);
- To prevent undesirable growth of plants (e.g., herbicides).
2. Multi-Residual Methods to Determine Pesticides in Agricultural Products
3. Herbicide-Absorption and Translocation in the Soil-Plants Systems
- Poor characterization of soil profiles and compositions of the layers.
- Weather conditions not quantified (rainfall/irrigation pattern).
- No information on the crop growth during the experiment (soil cover/leaf area index, root development).
- Few soil-sampling field experiments, low number of samples and too shallow soil sampling (only the top layer).
- Too short duration of the experiments to study deeper movement.
- Too low sensitivity of the concentration measurements in soil (high determination limit).
- No site-specific measurements on adsorption and rate of transformation in soil as model inputs (e.g., data taken from handbooks and databases).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Pimentel, D. Pesticides and pest control. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 83–87. [Google Scholar]
- Zhang, W.-J.; van der Werf, W.; Pang, Y.A. simulation model for vegetable-insect pest-insect nucleopolyhedrovirus epidemic system. J. Environ. Entomol. 2011, 33, 283–301. [Google Scholar]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: New Delhi, India, 2014; ISBN 978-81-322-1688-9. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Top Pesticide Using Countries. Available online: https://www.worldatlas.com/articles/top-pesticide-consuming-countries-ofthe-world.html (accessed on 30 June 2021).
- Q&A on Pests and Pesticide Management. Available online: http://www.fao.org/news/story/en/item/1398779/icode/ (accessed on 9 July 2021).
- Regulation EC 1107/2009. Placing on the Market of PPPs. Available online: https://ec.europa.eu/food/plants/pesticides/legislation-plant-protection-products-ppps_en (accessed on 9 July 2021).
- SANTE/11813/2017. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. Available online: http://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 9 July 2021).
- Lehotary, S.J.; de Kok, A.; Hiemstra, M.; van Bodegraven, P. Validation of a fast and easy method for the determination of 229 pesticide residues in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J. AOAC Int. 2005, 88, 595–614. [Google Scholar] [CrossRef] [Green Version]
- Manual of Methods of Analysis of Foods. Pesticide Residues; Food Safety and Standards Authority of India Ministry of Health and Family Welfare Government of India: New Delhi, India, 2015; 270p.
- Dharam, P.; Shankar, A.U. Integrated Pest Management: Principles and Practice; CAB International: Oxfordshire, UK, 2012. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- AOAC Official Method 2007.01. Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate [Electronic Resource]. Available online: https://weber.hu/Downloads/SPE/QuEChERS/AOAC_2007_01.pdf (accessed on 9 July 2021).
- Ðurović, R.; Ðorđević, T. Modern Extraction Techniques for Pesticide Residues Determination in Plant and Soil Samples. Pesticides in the Modern World—Trends in Pesticides. Analysis. Available online: http://cdn.intechopen.com/pdfs/20992/InTech-Modern_extraction_tecniques_for_pesticide_residues_determination_in_plant_and_soil_samples.pdf (accessed on 5 July 2021).
- Turner, J.A. (Ed.) The Pesticide Manual; BCPC: Alton, UK, 2015. [Google Scholar]
- Hrouzková, S.; Szarka, A. Development of a Modified QuEChERS Procedure for the Isolation of Pesticide Residues from Textile Samples, Followed by GC–MS Determination. Separations 2021, 8, 106. [Google Scholar] [CrossRef]
- Kolberg, D.I.; Prestes, O.D.; Adaime, M.B.; Zenella, R. Development of a fast multiresidues method for the determination of pesticides in dry samples (wheat grain, flour and bran) using QuEChERS based method and GC-MS. Food Chem. 2011, 125, 1436–1442. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Herrera-Herrera, A.V.; Ravelo-Pérez, L.M.; Hernández-Borges, J. Sample-preperation methods for pesticide residue analysis in cereals and derivatives. Trends Analyt. Chem. 2012, 38, 32–51. [Google Scholar] [CrossRef]
- Rakitskii, V.N.; Hai, D.N.; Fedorova, N.E.; Bereznyak, I.V.; Tung, L.V.; Egorchenkova, O.E.; Grechina, M.S. Safety of imported agricultural products: Pesticide residues. Health Care Russ. Fed. 2020, 64, 150–157. [Google Scholar] [CrossRef]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human health Effects. In Encyclopedia of Environmental Health; Nrjagu, J.O., Ed.; Elsevier: Burlington, MA, USA, 2011; pp. 438–453. [Google Scholar]
- Al-Khshemawee, H.; Agarwal, M.; Ren, Y. Detection of Mediterranean Fruit Fly Larvae Ceratitis capitata (Diptera: Tephritidae) in Different Types of Fruit by HS-SPME GC-MS Method. J. Biosci. Med. 2017, 5, 154–169. [Google Scholar] [CrossRef] [Green Version]
- The Use of Pesticides in Developing Countries and Their Impact on Health and the Right to Food. Requested by the DEVE Committee. Available online: https://www.europarl.europa.eu (accessed on 2 July 2021).
- WHO Pesticide Evaluation Scheme 50 Years of Global Leadership; World Health Organization: Geneva, Switzerland, 2010; ISBN 978 92 4 159927. Available online: https://apps.who.int/iris/handle/10665/44305 (accessed on 2 July 2021).
- Pesticide Manual Online. Available online: http://www.bcpc.org/product/bcpc-online-pesticide-manual-latest-version?gclid=Cj0KCQjwub-HBhCyARIsAPctr7zu_0RMssrYna6MGW9GzYVWYr6m1YlesKu3ZkhUZANjHG1uTZTcnkYaAq9aEALw_wcB (accessed on 15 June 2021).
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total. Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Verger, P.J.P.; Boobis, A.R. Reevaluate pesticides for food security and safety. Science 2013, 341, 717–718. [Google Scholar] [CrossRef]
- Mascarelli, A. Growing up with pesticides. Science 2013, 341, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Sharon, M.; Bhawana, M.; Anita, S.; Gothecha, V.K. A short review on how pesticides affect human health. Int. J. Ayurvedic Herb. Med. 2012, 2, 935–946. [Google Scholar]
- Wickerham, E.L.; Lozoff, B.; Shao, J.; Kaciroti, N.; Xia, Y.; Meeker, J.D. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ. Int. 2012, 47, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med Sci. Monit. 2004, 10, 141–147. [Google Scholar]
- Lushchaka, V.I.; Matviishyna, T.M.; Husaka, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar]
- Wilkinson, C.F.; Christoph, G.R.; Julien, E.; Kelley, J.M.; Kronenberg, J.; McCarthy, J.; Reiss, R. Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: How to cumulate? Regul. Toxicol. Pharmacol. 2000, 31, 30–43. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, G.E. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Pimentel, D.; Culliney, T.W.; Bashore, T. Public Health Risks Associated with Pesticides and Natural Toxins in Foods. In IPM World Textbook; Regents of the University of Minnesota: Comstock Hall, Ithaca, NY, USA, 2013. [Google Scholar]
- Hodgson, E. Metabolism of pesticides. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 893–921. [Google Scholar]
- Hodgson, E.; Goldstein, J.A. Metabolism of toxicants: Phase I reactions and pharmacogenetics. In Introduction to Biochemical Toxicology; Hodgson, E., Smart, R.C., Eds.; Wiley: New York, NY, USA, 2001; pp. 67–113. [Google Scholar]
- van Alphen, B.J.; Stoorvogel, J.J. Effects of soil variability and weather conditions on pesticide leaching—A farm-level evaluation. J. Environ. Qual. 2002, 31, 797–805. [Google Scholar] [PubMed]
- van den Bosch, H.; Boesten, J.J.T.I. Validation of the PESTLA Model: Field Test for Leaching of Two Pesticides in a Humic Sandy soil in Vredepeel (The Netherlands); Report 82; The Winand Staring Centre for Integrated Land, Soil and Water Research: Wageningen, The Netherlands, 1994. [Google Scholar]
- Allen, G.; Halsall, C.J.; Ukpebor, J.; Paul, N.D.; Ridall, G.; Wargent, J.J. Increased occurrence of pesticide residues on crops grown in protected environments compared to crops grown in open field conditions. Chemosphere 2015, 119, 1428–1435. [Google Scholar] [CrossRef]
- Bojac’a, C.R.; Arias, L.A.; Ahumada, D.A.; Casilimas, H.A.; Schrevens, E. Evaluation of pesticide residues in open field and greenhouse tomatoes from Colombia. Food Control 2013, 30, 400–403. [Google Scholar] [CrossRef]
- Bondareva, L.; Fedorova, N. Herbicide absorption and translocation in plants and soil using radioisotopes. In Natural Resource Management and Environmental Security; Dinesh, S., Ed.; Integrated Publications: New Delhi, India, 2021; pp. 75–91. [Google Scholar]
- Available online: https://www.iaea.org/publications/5298/use-of-isotopic-tracers-in-studies-of-herbicide-performance-on-grasses-and-sedges (accessed on 2 July 2021).
- Available online: https://www.icrp.org/publication.asp?id=ICRP%20Publication%20130 (accessed on 2 July 2021).
- Kniss, A.R.; Vassios, J.D.; Nissen, S.J.; Ritz, C. Nonlinear regression analysis of herbicide absorption studies. Weed Sci. 2011, 59, 601–610. [Google Scholar] [CrossRef]
- Ahmad, K.S. Adsorption of rimsulfuron in selected soils and its removal via activated carbon. Rev. Roum. Chim. 2019, 64, 299–310. [Google Scholar] [CrossRef]
- Mendes, K.F.; Martins, B.A.B.; Reis, F.C.; Dias, A.C.R.; Tornisielo, V.L. Methodologies to study the behavior of herbicides on plants and the soil using radioisotopes. Planta Daninh 2017, 35, 1–21. [Google Scholar] [CrossRef]
- Nandula, V.K.; Vencill, W.K. Herbicide absorption and translocation in plants using radioisotopes. Weed Sci. 2015, 63, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Wanga, Z.; Yang, L.; Cheng, P.; Yuc, Y.; Zhang, Z.; Li, H. Adsorption, degradation and leaching migration characteristics of chlorothalonil in different soils. Eur. J. Remote. Sens. 2021, 5, 238–247. [Google Scholar] [CrossRef]
- Garba, J.; Samsuri, A.W.; Othman, R.; Hamdani, M.S.A. Adsorption-desorption and leaching potential of glyphosate and aminomethylphosphonic acid in acidic Malaysian soil amended with cow dung and rice husk ash. Environ. Monit. Assess. 2018, 190, 676. [Google Scholar] [CrossRef]
- Hearon, S.E.; Wang, M.C.; Phillips, T.D. Strong adsorption of dieldrin by parent and processed montmorillonite clays. Environ. Toxicol. Chem. 2019, 30, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yan, D.D.; Wang, X.N.; Wang, X.L.; Fang, W.S.; Zhang, D.Q.; Ouyang, C.B.; Wang, Q.X.; Cao, A.C. Soil fumigation alters adsorption and degradation behavior of pesticides in soil. Environ. Pollut. 2019, 246, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Ishag, A.E.S.A.; Abdelbagi, A.O.; Hammad, A.M.A.; Elsheikh, E.A.E. Biodegradation of chlorpyrifos, malathion, and dimethoate by three strains of bacteria isolated from pesticide-polluted soils in Sudan. J. Agric. Food Chem. 2016, 64, 8491–8498. [Google Scholar] [CrossRef]
- Jaime, V.; Kah, M.; Colin, D.B. Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manag. Sci. 2008, 64, 703–710. [Google Scholar]
- White, J.C. Plant-facilitated mobilization and translocation of weathered 2,2-bis (p-chlorophenyl)-1,1-dichloroethylene (p,p’-DDE) from an agricultural soil. Environ. Toxicol. Chem. 2001, 20, 2047. [Google Scholar]
- Wang, W.; Xu, W.; Zhou, K.; Xiong, Z. Research progressing of present contamination of Cd in soil and restoration method. Wuhan Univ. J. Nat. Sci. 2015, 20, 430–444. [Google Scholar] [CrossRef]
- EWG’s. Shopper’s Guide to Pesticides in Produce. Dirty Dozen. 2019. Available online: https://www.ewg.org/foodnews/dirty-dozen.php (accessed on 2 July 2021).
- Park, D.W.; Kim, K.G.; Choi, E.A.; Kang, G.R.; Kim, T.S.; Yang, Y.S.; Moon, S.J.; Ha, D.R.; Kim, E.S.; Cho, B.S. Pesticide residues in leafy vegetables, stalk and stem vegetables from South Korea: A long-term study on safety and health risk assessment. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2015, 33, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.S. Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegetables using GC-MS. Sci. Rep. 2019, 9, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaka, S.M.; Iqbal, N.; Saeed, Q.; Akrem, A.; Batool, M.; Khan, A.A.; Anwar, A.; Bibi, M.; Azeem, S.; Rizvi, D.E.N.; et al. Toxic effects of some insecticides, herbicides, and plant essential oils against Tribolium confusum Jacquelin du val (Insecta: Coleoptera: Tenebrionidae). Saudi J. Biol. Sci. 2019, 26, 1767–1771. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Responses of plants to pesticide toxicity: An overview. Planta Daninha 2019, 37, e019184291. [Google Scholar] [CrossRef] [Green Version]
- Hooven, L.A.; Chakrabarti, P.; Harper, B.J.; Sagili, R.R.; Harper, S.L. Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules 2019, 24, 4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crenna, E.; Jolliet, O.; Collina, E.; Sala, S.; Fantke, P. Characterizing honey bee exposure and effects from pesticides for chemical prioritization and life cycle assessment. Environ. Int. 2020, 138, 105642. [Google Scholar] [CrossRef] [PubMed]
- Benuszak, J.; Laurent, M.; Chauzat, M.-P. The exposure of honey bees (Apis mellifera; Hymenoptera: Apidae) to pesticides: Room for improvement in research. Sci. Total Environ. 2017, 587–588, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Assessing Pesticide Risks to Bees. Office of Pesticide Programs United States Environmental Protection Agency Washington, D.C. 20460. Available online: https://www.epa.gov/sites/production/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf (accessed on 2 July 2021).
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Lebedev, S.I. Delayed Fluorescence and its Use to Assess the State of the Photosynthetic Apparatus of Plants Source: Plant Physiology, 3rd ed.; Science: Moscow, Russia, 1988. [Google Scholar]
- Strehler, B.L.; Arnold, W.A. Light production by green plants. J. Gen. Physiol. 1951, 34, 809–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radenović, C.N.; Marković, D.Z.; Jeremić, M. Delayed chlorophyll fluorescence in plant models. Photosynthetica 1994, 30, 1–24. [Google Scholar]
- Akita, S.; Yano, A.; Ishii, H.; Satoh, C.; Akai, N.; Nakata, M. Delayed fluorescence spectra of intact leaves photoexcited by sunlight measured with a multichannel Fourier-transform chemiluminescence spectrometer. Chem. Phys. Lett. 2013, 574, 120–123. [Google Scholar] [CrossRef]
- Malkin, S. Delayed Luminescence. In Photosynthesis I. Photosynthetic Electron Transport and Photophosphorilation; Trebst, A., Avron, M., Eds.; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Jursinic, P. Delayed Fluorescence: Current Concepts and Status. In Light Emission by Plants and Bacteria; Govindjee, A.J., Fork, D.J., Eds.; Academic Press: Orlando, FL, USA, 1986. [Google Scholar]
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.J.; Godderis, L.; Adam, B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020, 181, 108926. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health—A review. Toxicol Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumara, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
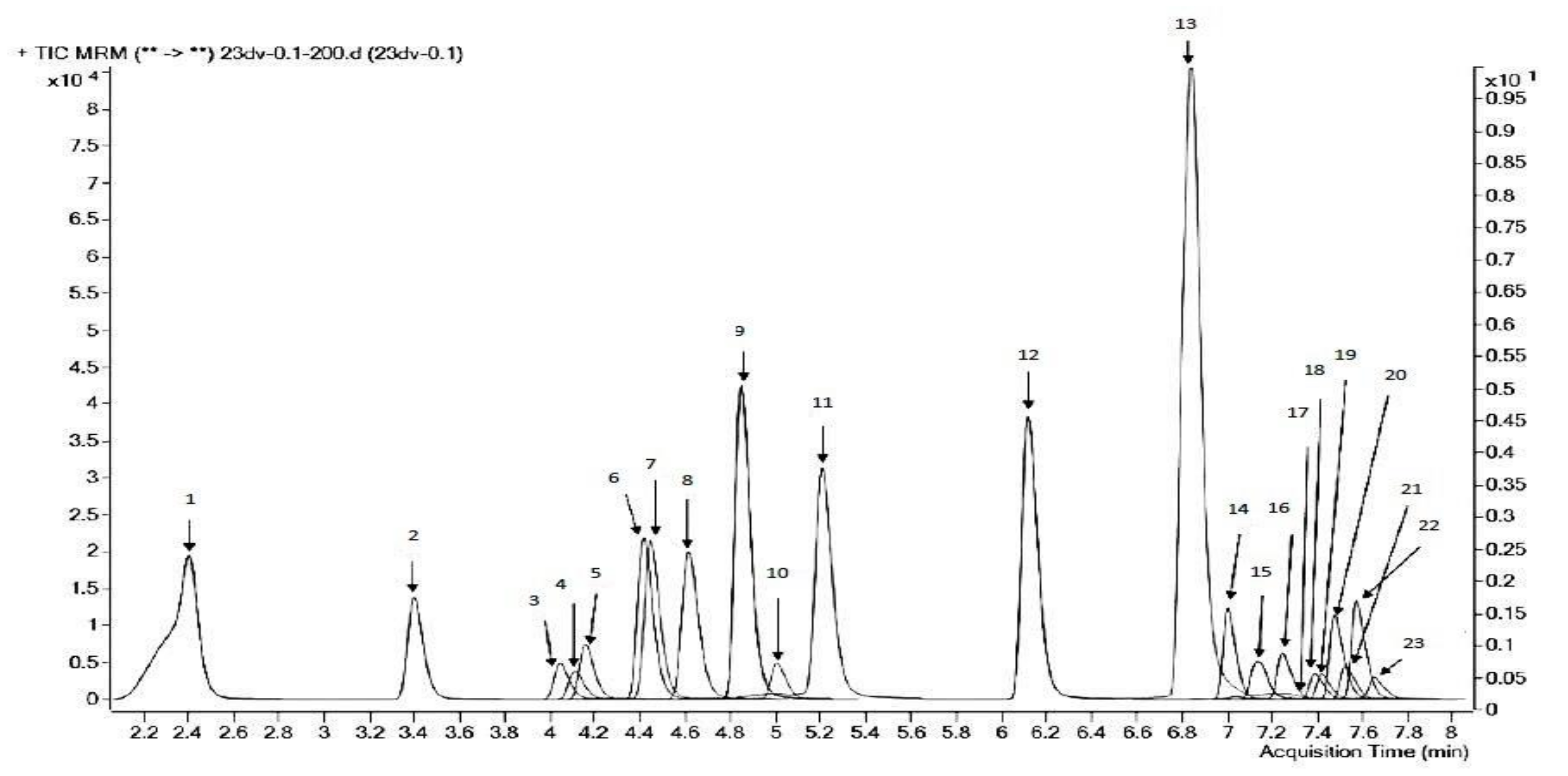
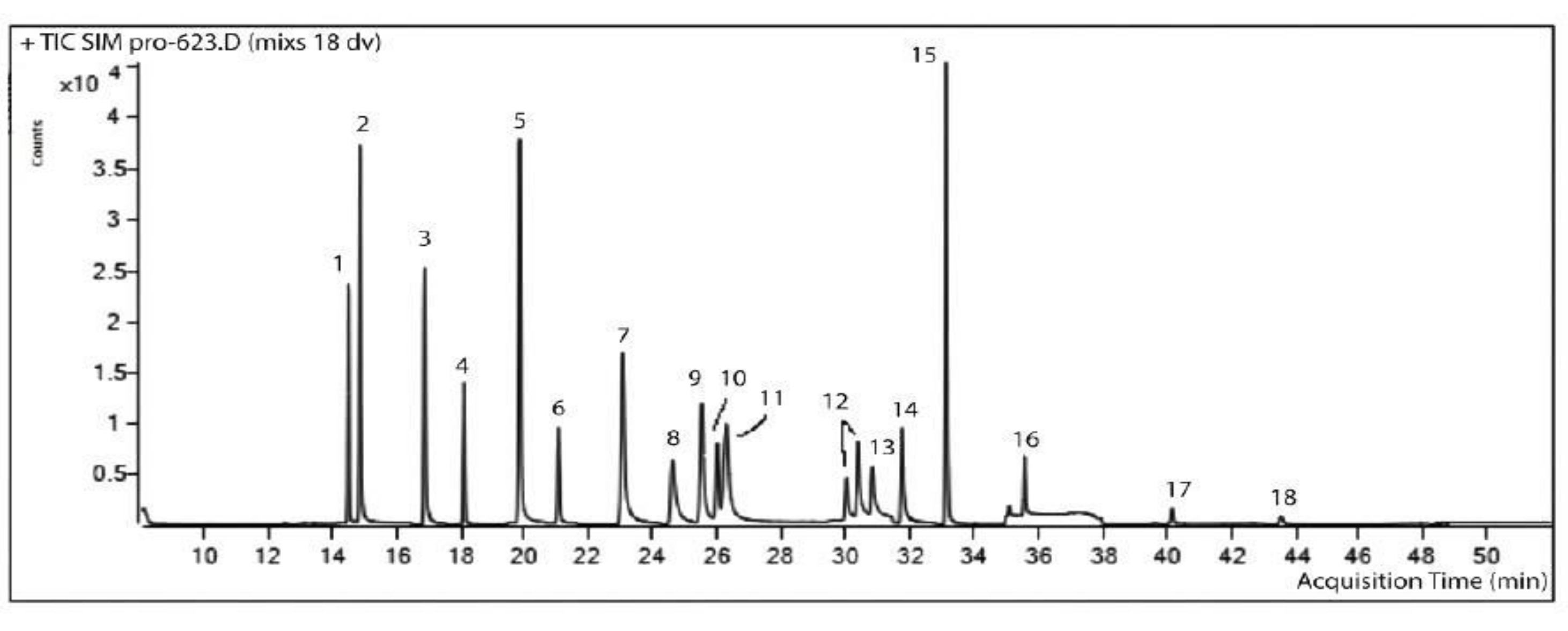
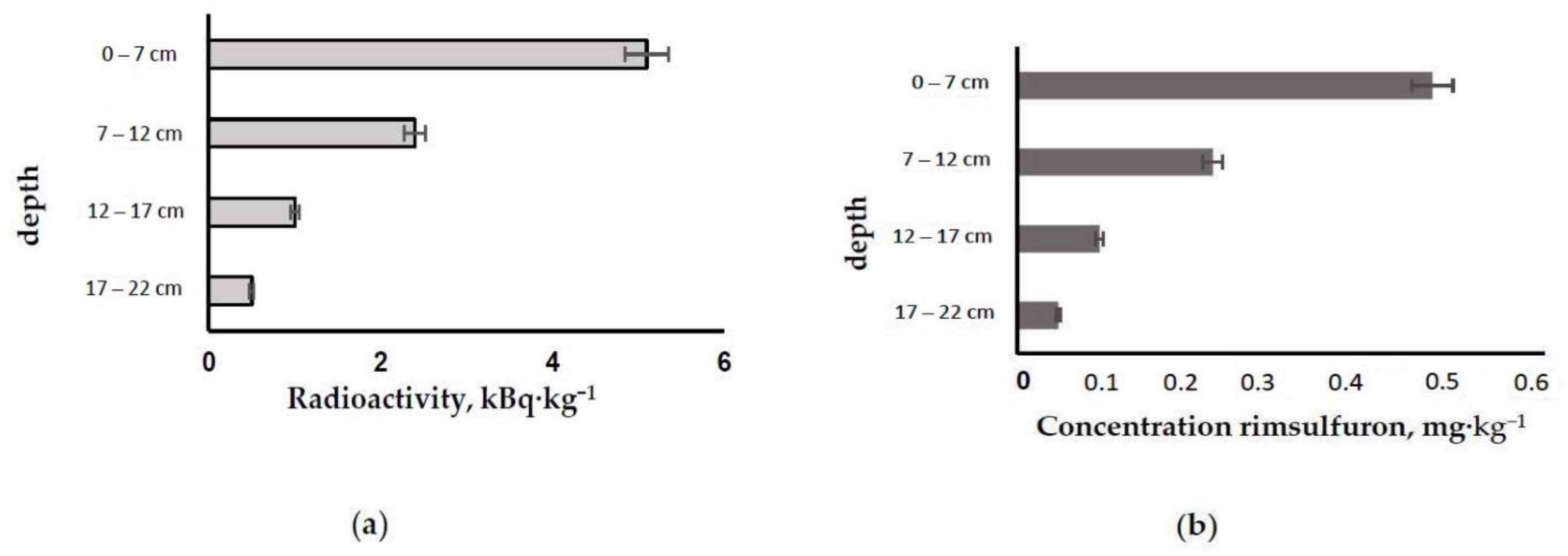
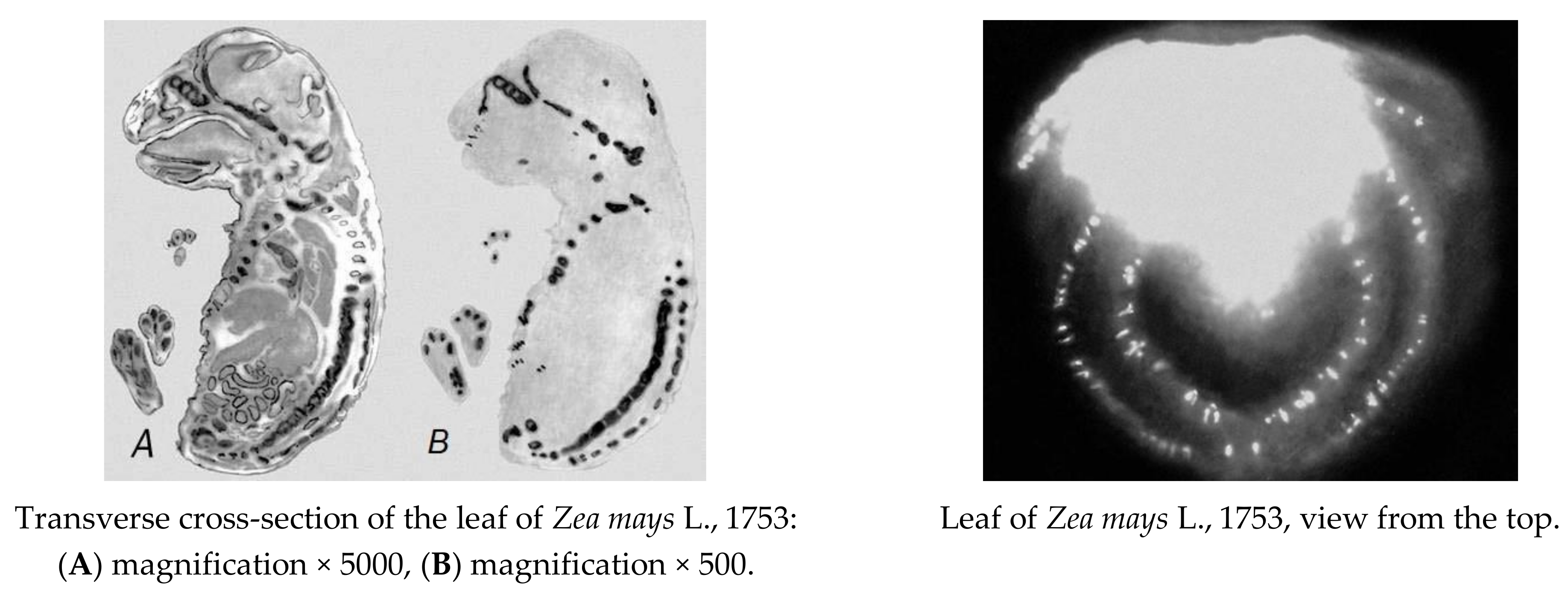
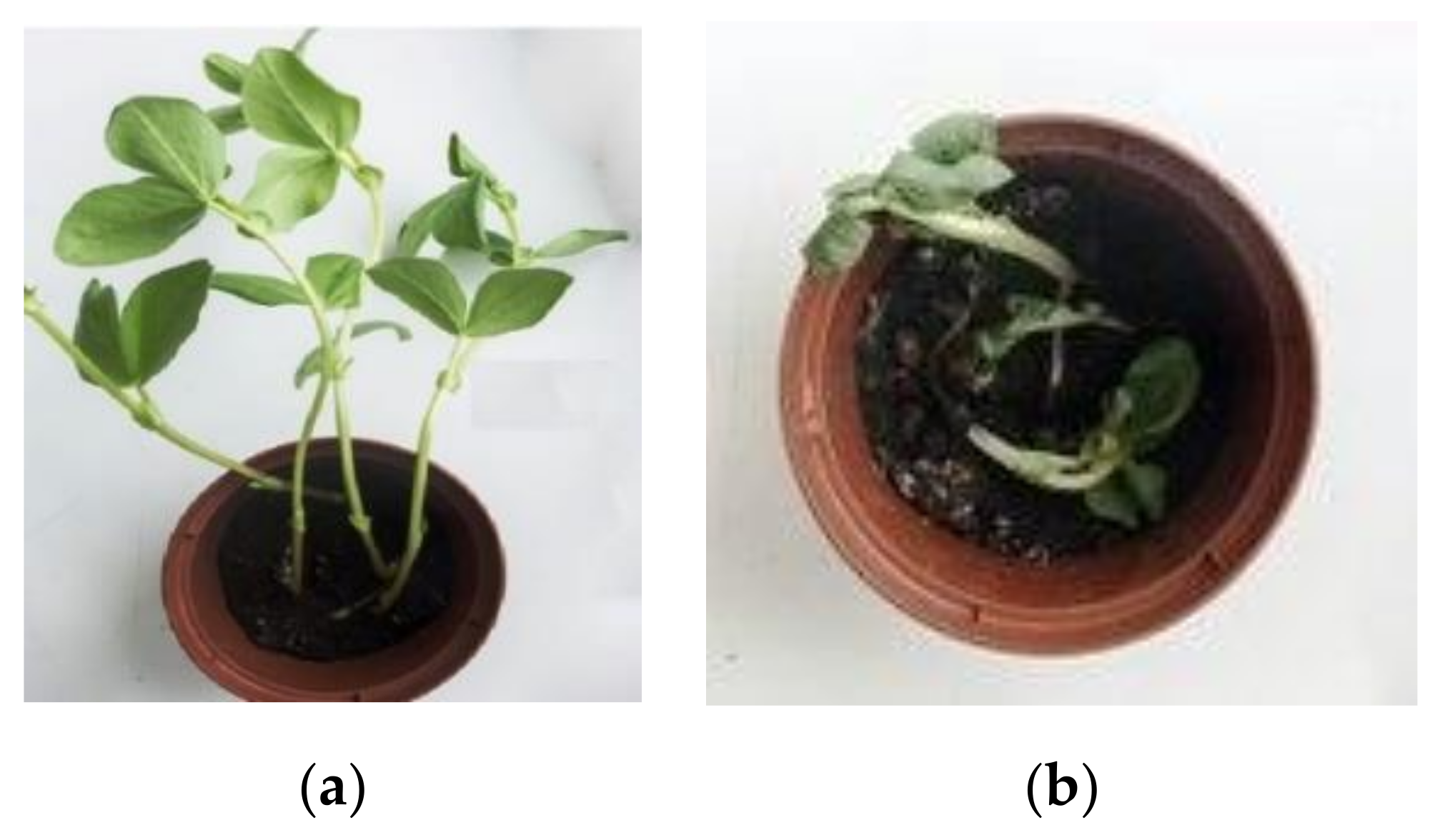

| System | Initial Parameters (n = 5) | Parameters at the End of the Experiment (n = 5) | ||||
|---|---|---|---|---|---|---|
| Length, cm | Weight, g | Length, cm | Weight, g | |||
| Wet | Dry | Dry/Wet, % | ||||
| Sinapis arvensis L., | 1.5 ± 0.2 | 0.7 ± 0.4 | 1.6 ± 0.4 | 0.6 ± 0.2 | 0.03 ± 0.01 | 5.0 |
| Zea mays L., 1753 | 5.4 ± 1.2 | 4.7 ± 1.7 | 8.3 ± 2.1 | 10.5 ± 3.0 | 3.2 ± 1.4 | 30.5 |
| System | Content 14C, kBq (% from the Introduced One) | ||||
|---|---|---|---|---|---|
| Soil (the Whole Amount) | Rhizosphere | Stems (the Whole Amount) | Leaves (the Whole Amount) | Tube for 14C-CO2 Capture | |
| Sinapis arvensis L., | 53.8 ± 0.9 (56.6) | 21.4 ± 0.6 (22.5) | 7.0 ± 1.3 (7.4) | 10.8 ± 0.8 (11.4) | 2.0 ± 0.7 (2.1) |
| Zea mays L., 1753 | 57.1 ± 0.7 (60.1) | 19.5 ± 0.7 (20.5) | 6.1 ± 0.9 (6.4) | 8.8 ± 1.1 (9.3) | 3.5 ± 1.0 (3.6) |
| Sinapis arvensis L. + Zea mays L., 1753 | 52.6 ± 0.8 (55.4) | 20.9 ± 1.1 (22.0) | 7.0 ± 1.1 (7.4) | 10.5 ± 1.2 (11.1) | 4.0 ± 0.9 (4.2) |
| Soil (sod-podzolic with sandy-clay texture) | 94.5 ± 0.7 (99.5) | - | - | - | 0.5 ± 0.2 (0.5) |
| Pesticide Type | Toxic Effects | |
|---|---|---|
| Soil | Plants | |
| Insecticides | Destruction of microbial structural proteins, symbiotic attributes reduction, change in soil chemistry and enzymatic activity | Reduction in grain protein content, blockage of stomatal conductance and alterations in the photosynthetic process |
| Herbicides | Reduction in the soil nutrient availability and suppression of phosphatase and nitrogenase activities | Alteration of the physiological and biochemical plant efficiency, increasing the susceptibility of plants to diseases |
| Fungicides | Interruption of phosphatase, urease and dehydrogenase activities and inhibition of the nitrifying bacterial growth | Reduction in chlorophyll and carotenoid concentration, destruction of chloroplasts, stomatal closure and electron transfer suppression |
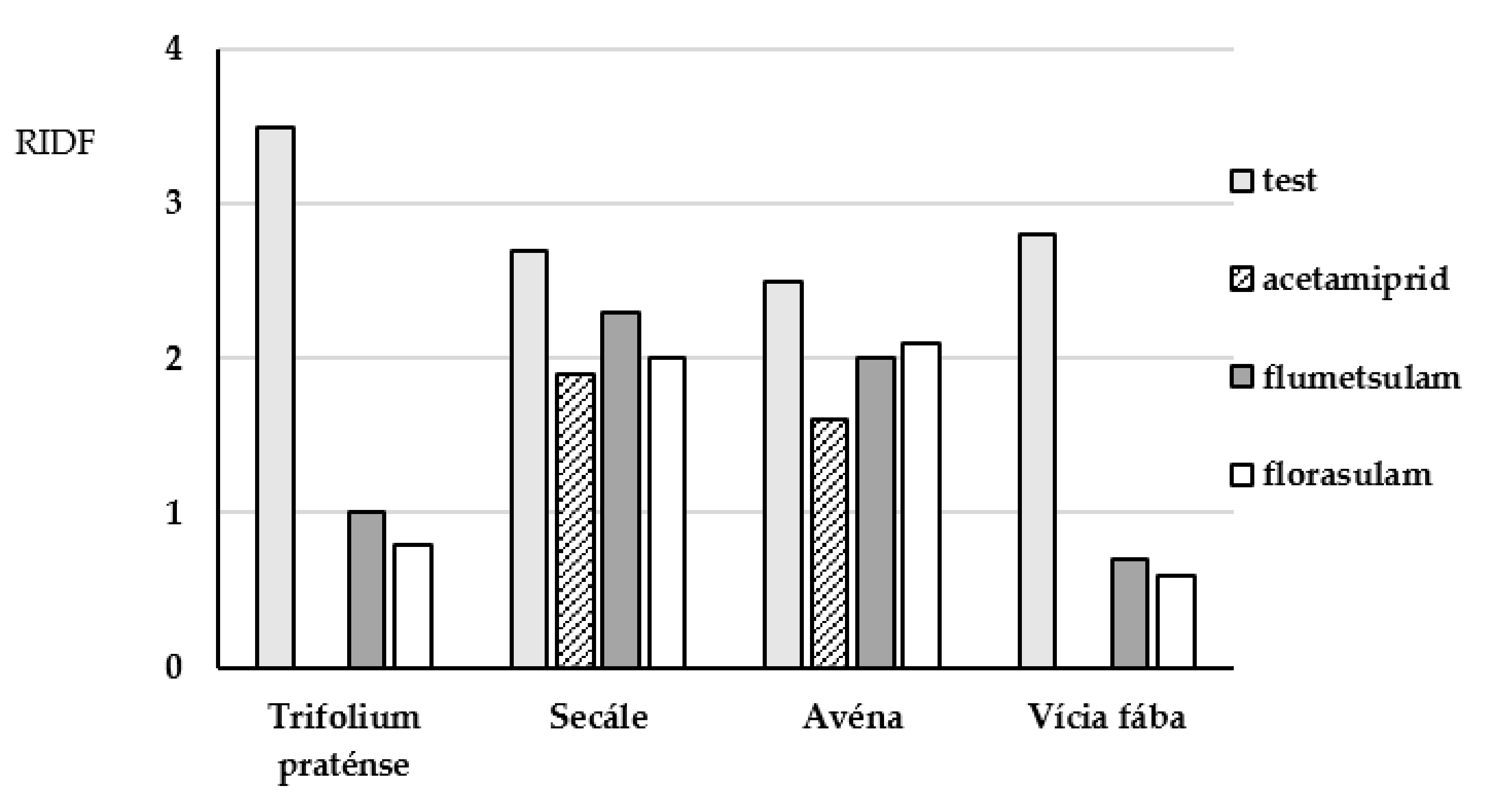
| Pesticide | Plant | Size of the Plant, Length, cm | |||
|---|---|---|---|---|---|
| Above-Ground Part Lcp. ± ∆ | Root Part Lcp. ± ∆ | ||||
| Test | 0.001 mg·kg−1 | Test | 0.001 mg·kg−1 | ||
| acetamiprid | clover (Trifolium praténse) | 4.8 ± 0.4 | 0 | 1.3 ± 0.3 | 0 |
| flumetsulam | 4.8 ± 0.4 | 3.3 ± 0.5 | 1.3 ± 0.3 | 0.9 ± 0.3 | |
| florasulam | 4.8 ± 0.4 | 2.6 ± 0.4 | 1.3 ± 0.3 | 0.5 ± 0.3 | |
| acetamiprid | rye (Secále) | 26.0 ± 1.0 | 20.0 ± 2.0 | 9.5 ± 0.6 | 7.3 ± 0.7 |
| flumetsulam | 26.0 ± 1.0 | 15.0 ± 1.0 | 9.5 ± 0.6 | 10.0 ± 1.0 | |
| florasulam | 26.0 ± 1.0 | 11.3 ± 0.7 | 9.5 ± 0.6 | 5.1 ± 0.9 | |
| acetamiprid | oats (Avéna) | 21.0 ± 1.0 | 20.0 ± 0.8 | 7.0 ± 0.4 | 5.5 ± 0.6 |
| flumetsulam | 21.0 ± 1.0 | 18.7 ± 0.7 | 7.0 ± 0.4 | 4.8 ± 0.5 | |
| florasulam | 21.0 ± 1.0 | 18.1 ± 0.8 | 7.0 ± 0.4 | 5.7 ± 0.3 | |
| acetamiprid | bean (Vícia fába) | 3.1 ± 0.6 | 0 | 0.7 ± 0.2 | 0 |
| flumetsulam | 3.1 ± 0.6 | 0 | 0.7 ± 0.2 | 0 | |
| florasulam | 3.1 ± 0.6 | 0 | 0.7 ± 0.2 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondareva, L.; Fedorova, N. Pesticides: Behavior in Agricultural Soil and Plants. Molecules 2021, 26, 5370. https://doi.org/10.3390/molecules26175370
Bondareva L, Fedorova N. Pesticides: Behavior in Agricultural Soil and Plants. Molecules. 2021; 26(17):5370. https://doi.org/10.3390/molecules26175370
Chicago/Turabian StyleBondareva, Lydia, and Nataliia Fedorova. 2021. "Pesticides: Behavior in Agricultural Soil and Plants" Molecules 26, no. 17: 5370. https://doi.org/10.3390/molecules26175370





