Abstract
This is the first report to identify the presence of 3-O-caffeoyl quinic acid (1), 4-O-caffeoyl quinic acid (2), 5-O-caffeoyl quinic acid (3), epi-catechin (4), and procyanidin B2 (5) in the young propagules of Rhizophora mucronata. Compounds 2–5 were purified and then treated against breast, colorectal, and ovarian cancer cell lines for 72 h and the results of the Sulphorhodomine-B (SRB) assay were evaluated for percent cell viability and IC50 values. Epi-catechin, 4-O-caffeoyl quinic acid, 5-O-caffeoyl quinic acid and procyanidin B2 showed strong to moderate inhibitory effects when treated on breast (T47D), colorectal (HT29), and ovarian (A2780, SKOV3) cancer cell lines with IC50 values ranging from 16.77 ± 0.58 to 28.28 ± 0.89 μg/mL. In silico evaluation was performed to evaluate the drug-likeness and toxicological effects of these compounds using Molinspiration calculation and OSIRIS program. It was found that compounds 2, 3, and 4 have the potential to be orally active and have a low risk in exerting the mutagenic, tumorigenic, irritant, and reproductive effects.
1. Introduction
Rhizophora mucronata (Rhizophoracea) is one of the mangrove plant species that can be found in the tropical and subtropical coastal areas and river estuaries from the east coast of Africa to Asia and Australia, and its hardy wood has been popularly used as fuel and charcoal [1]. The bark, leaves, and fruits consist of tannin [2] along with other phytochemicals that were later scientifically discovered. Mangrove plants are not only ecologically important in protecting the coastal area from soil erosion and strong tidal waves, but they also have various medicinal uses. The bark and leaves of R. mucronata are used to treat diarrhea, dysentery, fever, malaria, leprosy inflammation, and angina disorders [1,3]. The plant has also been reported to be used to treat tonsillitis, pharyngeatis, hemorrhoids, and burns [4]. The bark is claimed to have astringent and anti-diabetic properties [2].
Secondary plant metabolites are known to be the main source for the development of new therapeutics. Alkaloids, triterpenoids, flavonoids, and steroid compounds have reportedly been isolated and characterized from various plant parts of R. mucronata. The leaves contain sterol compounds, namely sitosterol, campesterol, stigmasterol, and 28-isofucosterol and stigmast-7-en-3β-ol [5], 1-hydroxy-5-oxobicyclo [6.4.0] dodecane along with lupeol, betulin, and palmitic acid [6], alkaloids such as amalicine, vindoline, catharanthine, and serpentine [7], and luteolin [8]. The lupeol and betulin have been reported to have antimalarial and antiviral effects [9]. Other studies have shown the presence of endophytic fungi belonging to Pestalotiopsis sp. that grows on the leaves of R. mucronata and was identified by Hemberger et al. in 2013 [10]. Chemicals that were detected in this fungus were novel hybrid sesquiterpene–cyclopaldic acid metabolites with an unusual carbon skeleton, named pestalotiopensA and B, and also phytotoxin altiloxinB. Two new coumarins ((Z)-apetatolide and mixture of isomers E and Z of new coumarin) and a new xanthone (2,4,5-trihydroxy3-(2-hydroxy-3-methylbut-3-enyl)-xanthone), together with 14 known compounds that include eight coumarins (methoxyinophyllum P, calocoumarin B, calophyllolide, brasimarin C, inophyllum C, isocalophyllic acid, inophyllum E, and calophyllic acid), three xanthones (6-deoxy-jacareubin, jacareubin, and 1,3,5-trihydroxy-2-(3-methylbut-2-enyl)xanthone), a benzoic acid, and two flavonones (amentoflavone and naringenin) have been isolated from the methanol extract of the leaves of R. mucronata [11].
The ethanol and methanol extracts from the leaves of R. mucronata have been found to have anti-microbial properties in which these extracts inhibited the growth of Escherichia coli, Staphylococcus aureus, and Bacillus cereus [12], while the ethyl acetate extract inhibited the growth of E. coli, S. aureus, Klebsiella pneumonia, Proteus vulgaris, Pseudomonas aeruginosa, Pseudomonas fluorescens, Salmonella typhii, and Bacillus subtilis [13]. The leaves have also been reported to have anti-oxidative [14], antinociceptive [8,15], and anti-HIV activities [16]. A compound from the leaves of R. mucronata identified as quinizarin showed anti-microbial activity against Bacillus cereus, Klebsiella aerogenes, anti-oxidant activity via the DPPH radical scavenging assay, and cytotoxic activity against cervical HeLa and MDA-MB231 breast cancer cell lines [17]. Taniguchi et al. 2018 [11] reported that a xanthone (jacareubin) compound isolated from the leaves showed cytotoxic potential against HeLa and HL60 cancer cell lines.
Screening of the phytochemical components in the ethanolic extract of R. mucronata bark revealed the presence of alkaloids, triterpenes, flavonoids, tannins, catachin, anthraquinone, phenols, sugars, and proteins [18]. Triterpenoids (3β-O-(E)-(4-methoxy) cinnamoyl-15 α-hydroxyl β-amyrin, adian-5-en 3-ol and lupeol) have been isolated from the chloroform extract of the stem bark [19]. Diterpenoids (rhizomucronol A and B) have been isolated from the acetone extract of the stem [20]. Extracts from the bark of R. mucronata have anti-diabetic (inhibition of the α-glucosidase enzyme) effect [21], moderate antibacterial effects against Escherichia coli and Staphylococus epidermidis [22], and antiplasmodial activities [18]. Alkaloids such as ajmalicine and serpentine, which have been investigated in silico for their binding effects on the cyclooxygenase 2 receptor, have shown strong anti-inflammatory activity [23], while the bark polysaccharide has shown anti-HIV activity [24].
Fewer phytochemical and bioactivity studies have been reported for other parts of the plant, including roots and fruits or propagules. Beyerane diterpenoid (Rhizophorin B) and isopimarane diterpenoids have been isolated from the ethyl acetate extract of R. mucronata roots [25], while sesquiterpene (mucronatone) and pentacyclic triterpenoid esters (3β-E-caffeoyltaraxerol and 3β-Z-caffeoyltaraxerol) have been isolated from R. mucronate fruits [26]. The extract from R. mucronata stilt root has in vitro antioxidant properties [27] and larvicidal activity [28]. To our knowledge, however, scientific studies on the medicinal properties of fruits and propagules of R. Mucronata are still lacking. Therefore, the aim of this study was to isolate and identify the chemical constituents present in the propagules of R. mucronata and to evaluate their anti-cancer activities in vitro against some cancer cell lines with their drug-likeness potential.
2. Materials and Methods
Chemicals and general methods: All reagents and solvents used were of analytical or gradient grade for liquid chromatography.
1H and 13C NMR spectra were recorded in ppm (δ) in CD3OD and acetone-d6 employing a Bruker DRX 300 spectrometer operating at 300 MHz for 1H and 75 MHz for 13C, respectively. Column chromatography was performed with MCI gel CHP 20P (75–150 µm, Supelco, Sigma-Aldrich, St. Louis, MO, USA), Chromatorex ODS (100–200 mesh, Fuji Silysia Chemical Ltd., Aichi, Japan) and Sephadex LH-20 (GE Healthcare Bio-Science AB, Uppsala, Sweden). HPLC analysis was carried out on a Waters Delta 600 system, equipped with an in-line degasser, a quaternary pump, an auto sampler, and a diode array detection (DAD) system, connected to an analytical column (Phenomenex LUNA PFP(2)). Data collection was performed using Empower software (Waters). The water used for all the solutions and dilutions was prepared with an ELGA Purelab Classic water purification system. Bransonic Series ultrasonic bath was used for extraction. TLC was performed on precoated silica gel 60 F254 plates (0.2 mm thick, Merck) with CHCl3−MeOH−H2O (9:1:0.1 or 8:2:0.1 or 7:3:0.5 or 6:4:1), and spots were detected by UV illumination and by spraying with 10% H2SO4 solution followed by heating or spraying with 2% FeCl3 solution followed by drying with a stream of cold air.
Plant material: Young and old propagules of R. mucronata were collected from Carey Island, Selangor, in Malaysia and the samples were coded as BK(A), BK(B), and BK(C) (Figure 1). The voucher specimen was prepared and deposited with number NMY1-KEP 245125 in the herbarium of FRIM, Kepong.
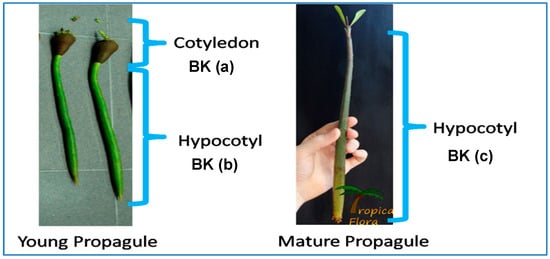
Figure 1.
Plant parts of young and mature propagules of R. mucronata: (a) cotyledon, (b) young hypocotyl, (c) mature hypocotyl.
Preparation of the crude plant extracts: The sliced fresh samples were immediately extracted by soaking in methanol (fresh weight/volume, 1 g/10 mL) for 3 days at room temperature. After that, the solvent was filtered, and the residue was extracted again using methanol with the same weight/volume ratio for another 3 days at room temperature. The solvent was evaporated under reduced pressure using a rotary evaporator with a bath temperature of 45 °C. The extraction process was repeated for the third time. The combined extracts were then stored at −20 °C for further analysis.
Isolation, purification, and characterization of pure compounds: The concentrated methanol extract (150 g) of the hypocotyl of young propagule coded as BK(B) was fractionated through liquid column chromatography employing MCI gel CHP 20P. The resulting fractions of eluent were combined to three fractions based on the thin layer chromatographic profiles. Fraction 2, encoded as BK(2), which had the most visible spots on thin layer chromatography, was further fractionated and purified, employing a combination of Sephadex LH-20, Chromatorex ODS, and MCI gel CHP 20P to yield four thin layer chromatographically homogeneous compounds (compounds 2–5) as shown in Figure 2. The identities and structures of these compounds were deterimined by 1H and 13C NMR spectroscopic analyses and by comparison with values in the literature or standard compounds to be 4-O-caffeoyl quinic acid (2), 5-O-caffeoyl quinic acid (3), Epi-catechin (4), and procyanidin B2 (5).
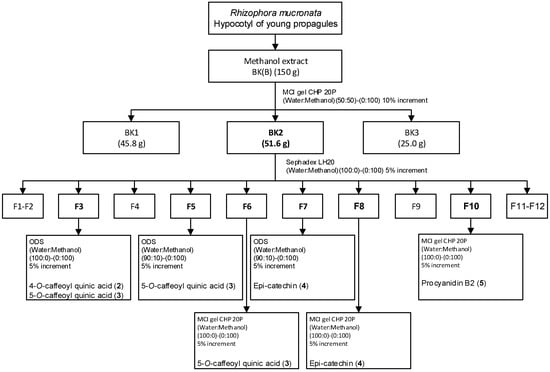
Figure 2.
Isolation of compounds from the hypocotyl of young propagules.
HPLC analysis: The chromatographic separation of the compounds in the extract was carried out with a Phenomenex Luna PFP(2) column (250 × 4.6 mm, 5 µm, Phenomenex, USA) at room temperature. The solvent system consisted of mixtures of water with 0.1% formic acid (solvent A), acetonitrile (solvent B), and methanol (solvent C). Samples for HPLC analysis were filtered through a 0.45 µm membrane filter. The optimized HPLC condition was as follows: 95–70% A, 5–20% B, and 0–10% C (0–50 min), 70% A, 20% B, and 10% C (50–55 min), 70–90% A, 20–10% B, and 10-0% C (55–60 min), 90% A and 10% B (60–65 min). The flow rate was 1.0 mL. The injection volume was 10 µL with the extract dissolved in methanol at 20 mg/mL. Signal was monitored at 280 and 330 nm. Standards of 3-O-caffeoyl quinic acid (CAS 202650-88-2), 4-O-caffeoyl quinic acid (CAS 905-99-7), 5-O-caffeoyl quinic acid (CAS 906-33-2), and epi-catechin (CAS 490-46-0) were used for the identification of major peaks in the chromatogram.
Cancer cell lines, cell culture maintenance, and treatments: Ovarian (SKOV-3), breast (T47D), and colorectal (HT-29) cell lines were purchased from American Type Culture Collections, USA, and A2780 ovarian cancer cell line was purchased from European Collection of Cell Cultures (ECACC), UK. The cell lines were cultured and sub-cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 1% penicillin-streptomycin, 0.25% amphotericin B, and 1% gentamycin. Approximately 4000 to 6000 cells were seeded in each well of the 96 well plates and incubated in a humidified incubator at 37 °C and 5% carbon dioxide in air for 24 h. Each cell line was then treated with the extracts and compounds obtained from R. mucronata at five different concentrations (0.1, 1, 10, 20, and 100 μg/mL) in triplicate. Cisplatin, a known chemo-drug, was also treated on these cell lines in different concentrations (0.25, 1, 5, 25, and 50 μg/mL) as in the comparative studies. The treated cells were then incubated in the same incubator with the mentioned conditions for 72 h. The experiment was repeated at least three times.
In vitro anti-cancer activities assessed via Sulphorhodamine B (SRB) assay: The 72 h treatments were stopped by performing Sulphorhodamine B (SRB) assay [29,30]. Briefly, 50 μL of ice cold tricholoroacetic acid (TCA) was added to each well and allowed to stand for 30 min at room temperature, followed by rinsing each well with tap water. Then, 100 μL of 0.4% SRB was added to each well to stain living cells for 30 min followed by a rinse with 1% acetic acid. Finally, 100 μL of Tris buffer was added to each well and the optical density (OD) of the treated and non-treated cells were read at 492 nm with a Magellan V.4 microtiter plate reader (Tecan, Switzerland). The percentage of cell viability was calculated based on (OD492nm of the treated cells/ OD492nm of the non-treated cells) × 100. The IC50 values were determined from the dose–response curve of percentage of cell viability versus the concentration of the extracts (μg/mL). Cell viability assays for each treatment were performed in triplicate in at least three independent experiments, and the IC50 values are given as the mean ± S.D.
In silico analysis: In silico analysis of drug-likeness and ADMET properties were performed based on Lipinski’s Rule of Five (ROF) using a free online server. Prediction of drug-likeness and bioactivity score was conducted using Molinspiration (Molinspiration Chemiformatics, Nova ulica 61, SK-900 26 Slovensky Grob, Slovak Republic) and OSIRIS property explorer for calculation of the physicochemical properties (www.organicchemistry.org/prog/peo/, accessed on 9 May 2019). Prediction on ADMET of the compounds was performed using vNN-ADMET [31] and OCHEM [32].
3. Results
3.1. Isolation, Purification, and Identification of Compound
The methanol extract, BK(B), exhibited IC50 values of ≤20 μg/mL for three of the cancer cell lines used in the in vitro anti-cancer study, indicating very potent anti-cancer effects [33,34]. The isolation and subsequent purification activities of the BK(B) extract identified four compounds known as 4-O-caffeoyl quinic acid (2) [35], 5-O-caffeoyl quinic acid (chlorogenic acid) (3) [36,37], epi-catechin (4) [38], and procyanidin B2 (5) [39,40,41] by 1H and 13C spectroscopic analyses and comparison with the values in the literature or standard compounds (Figure 3).
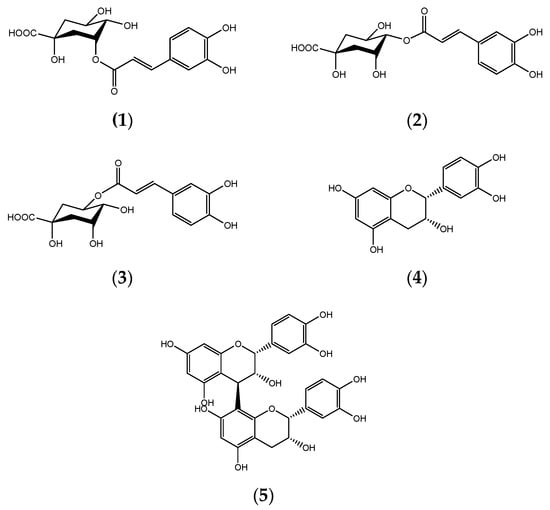
Figure 3.
Chemical structures of 3-O-caffeoyl quinic acid (1), 4-O-caffeoyl quinic acid (2), 5-O-caffeoyl quinic acid (chlorogenic acid) (3), epi-catechin (4), and procyanidin B2 (5).
3.2. Separation and Identification of Chromatographic Peaks in Extract
Figure 4 represents the analytical HPLC chromatogram of BK(B) extract with the identification of 3-O-caffeoyl quinic acid, 4-O-caffeyl quinic acid, 5-O-caffeoyl quinic acid, and epi-catechin. The main peaks were dominated by caffeoyl quinic acids.
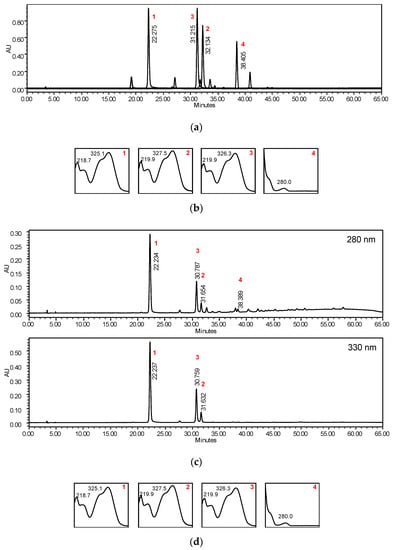
Figure 4.
HPLC chromatogram of standard compounds, 3-O-caffeoyl quinic acid (1), 4-O-caffeyl quinic acid (2), 5-O-caffeoyl quinic acid (3), and epi-catechin (4); 1-3 extracted at 330 nm, 4 extracted at 280 nm (a), UV spectra of standard compounds 1-4 (b), HPLC chromatograms of BK(B) extract (c), and UV spectra of compounds 1-4 in BK(B) extract (d).
3.3. In Vitro Anti-Cancer Studies
The in vitro anti-cancer activities of extracts, fractions, and compounds of Rhizophora mucronata were evaluated on the basis of theirIC50 values (Table 1). Phytochemicals that give an IC50 value ≤ 20 μg/mL indicate very potent anti-cancer activities [33,34]. The results indicate that the four compounds have inhibitory effects with IC50 values of less than 100 μg/mL. 4-O-caffeoyl quinic acid was found to be the most active of the four compounds in the treatment of ovarian (SKOV3) and colorectal (HT29) cancer cell lines, followed by epicatechin and 5-O-caffeoyl quinic acid, which are also active against ovarian (A2780) cancer cell lines. The compound procyanidin-B2 showed moderate to almost very potent anti-cancer activities in all cancer cell lines tested.

Table 1.
IC50 values (μg/mL) of four compounds isolated from the hypocotyl of young propagules of R. mucronata.
3.4. In Silico Analysis on Drug-Likeness and Toxicity of Selected Compounds
The evaluation of the drug-likeness properties of four compounds isolated from BK(B) showed that all compounds, except procyanidin B2, fulfilled Lipinski’s Rule of Five (RoF) (Table 2). Procyanidin B2 violated three of the RoF, namely molecular weight (578.5 > 500 Da), number of hydrogen acceptors (12 > 10), and number of hydrogen donors (10 > 5). The bioactivity score indicated that all compounds were active and moderately active against normal human receptors (Table 3). Interestingly, all of the compounds were active against nuclear receptor ligands, GPCR ligands, protease inhibitors, and enzyme inhibitors. Epicatechin is the only compound that was active against all of the predicted receptors, with the highest score for the nuclear receptor ligand (0.60), but the highest score for this receptor was 5-O-caffeoyl quinic acid (0.74). Prediction of toxicity showed that not all compounds have mutagenic, tumorigenic, reproductive, and irritant potential, except procyanidin B2, which displayed a high risk of reproductive effects (Table 4). However, as shown in Table 5, not all compounds were cytotoxic. The results of ADMET prediction are summarized in Table 5. All compounds showed no inhibition of cytochrome P450 metabolism (CYP3A4, CYP2D6, CYP2C19, CYP2C6, and CYP1A2), as predicted by OCHEM and vNN-ADMET.

Table 2.
Physicochemical properties of compounds isolated from BK(B).

Table 3.
Bioactivity score of compounds isolated from BK(B).

Table 4.
Toxicity prediction of compounds isolated from BK(B).

Table 5.
ADMET prediction using OCHEM and vNN-ADMET web servers.
4. Discussion
To the best of our knowledge, these are the first reported results for all four compounds, namely epi-catechin, 4-O-caffeoyl quinic acid, 5-O-caffeoyl quinic acid, and procyanidin B2, which were isolated from the propagules of R. mucronata. Although these compounds have newly been isolated and identified in the species, they can be found in other plant species, including many types of fruits and vegetables. The 4-O-caffeoyl quinic acid and 5-O-caffeoyl quinic acid are chlorogenic acids (CGA) that are found in many species of plants, and the most notable one that is rich in CGAs is the green coffee bean [42]. CGAs are phenolic acids which are obtained from the esterification of cinnamic acids with quinic acid. CGAs exhibit several biological properties, including antioxidant, anti-inflammatory, antidiabetic, anticarcinogenic, and antibacterial activities. 5-O-caffeoyl quinic acid is the main CGA of arabica and robusta green coffee beans [42]. Chlorogenic acids, which include 4-O-caffeoyl quinic acid and 5-O-caffeoyl quinic acid, and caffeic acid have been shown to have an additive anti-proliferative effect with cisplatin on ovarian cancer cells [43].
Epi-catechin is one of the catechin polyphenols, which is one of the main compounds in green tea and cocoa [44,45]. Cocoa powder, which is rich in epi-catechin, has been found to be active and kill pancreatic cancer cells through apoptosis [46]. However, another in vitro anti-cancer study suggested that epi-catechin was less active than other catechins, such as epigallocatechin-3-gallate, when tested in prostate (DU-145) and ovarian cancer (HH639) cells [44].
Assessment of the drug-likeness properties of four compounds isolated from young propagules of R. mucronata uses the criteria of Lipinski’s Rule of Five (RoF). The criteria are often used in drug design and development to predict the oral bioavailability of potential lead or drug molecules. According to Lipinski’s RoF, a compound is more likely to be orally active if it has no more than one violation of the following criteria: Log P is less than 5; molecular weight is less than 500 Da; hydrogen bond donor is less than 5; and hydrogen bond acceptor is less than 10 [47]. Among the four compounds, epi-catechin, 5-O-caffeoyl quinic acid, and 4-O-caffeoyl quinic acid fulfilled Lipinski’s RoF, with only one violation being observed for the latter two compounds. Epi-catechin was found to have no violation to the rule, with a TPSA below 160Å2, molecular weight < 500 Da, number of hydrogen donors < 5, number of hydrogen acceptors < 10, and number of rotatable bonds < 10.
Bioactivity score, as shown in Table 3, indicated that all compounds were active and moderately active against normal human receptors. A molecule with a bioactivity score greater than 0.00 is considerably active, while values from −5.00 to 0.00 are moderately active, and if the score is less than −5.00 it is considered to be inactive. Epi-catechin was active against all receptors, i.e., GPCR ligands, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor, and enzyme inhibitor. 5-O caffeoyl quinic acid and 4-O caffeoyl quinic acid were also active against these biological targets, but were moderately effective against the kinase inhibitor. Procyanidin B2 was only active against three biological targets, namely GPCR ligand, nuclear receptor, protease inhibitor, and enzyme inhibitor, and moderately active against the other two receptors.
The ADME assessment was conducted using OCHEM and vNN-ADMET webservers. The results are summarized in Table 5. All compounds showed no inhibition of cytochrome P450 metabolism, which was predicted using both servers, with the exception of procyanidin B2, which showed conflicting results for the CYP1A2 inhibitor. Cytochrome P450 enzymes are essential for the metabolism of drugs. While there are more than 50 CYP450 enzymes, CYP3A4, CYP2D6, CYP2C19, CYP2C9, CYP1A2, and CYP3A5 enzymes metabolize 90 percent of drugs [48,49]. The human liver microsomal (HLM) stability assay is commonly used to identify and exclude compounds that are too rapidly metabolized. In order to achieve the effective therapeutic concentrations in the body, he molecule or drug cannot be metabolized too rapidly by the liver. Compounds with half-life of 30 min or longer in an HLM assay are considered to be stable; otherwise, they are considered unstable. All compounds have been classified as stable in the liver.
All compounds were classified as BBB non-permeable. BBB is a highly selective barrier that separates the circulating blood from the central nervous system. Recently, many methods have been explored to improve the permeability of BBB, including strategies in the field of physical, chemical, biological, and various nanoparticle systems [50].
P-glycoprotein (P-gp) is an essential cell membrane protein and plays an important role in the absorption and disposition of drugs. This drug transporter is also involved in the metabolism, distribution, and interaction of drugs. In this study, it was shown that all compounds are not P-gp inhibitors, however epi-catechin and procyanidin B2 were classified as substrates of P-gp. P-gp is not only important in ADMET issues, but is also extended to a field of multidrug resistance cancer [51]. Identification of compounds that are either transported out of the cell by P-gp (substrates) or impair the function of P-gp (inhibitors) is of great interest.
In this study, several toxicity parameters were predicted with OSIRIS, OCHEM, and vNN-ADMET. The prediction of the toxicity of compounds with the OSIRIS software revealed that all compounds have no mutagenic, tumorigenic, reproductive, or irritant potential, however procyanidin B2 has shown a high risk of reproductive effects (Table 4). In terms of cytotoxicity, mitochondrial toxicity (MP), and mutagenicity (AMES test), all compounds were found to be non-toxic, except for procyanidin B2, which was predicted by OCHEM to be active (Table 5). Epi-catechin and procyanidin B2 were classified as hERG blockers, while the other two compounds were classified as non-blockers. In the vNN-ADMET prediction program, compounds with an IC50 cutoff value of 10 µM or less were classified as blockers, while compounds with an IC50 greater than 10 µM were classified as non-blockers. It was predicted that only procyanidin B2 did not cause drug-induced liver injury damage (DILI), while the other three compounds were at high risk of causing DILI.
Overall, the drug score for epi-catechin (0.87) is the highest of all, followed by 4-O-caffeoyl quinic acid (0.697), 5-O-quinic acid (0.437) and the lowest is procyanidin B2 (0.33) suggesting that epi-catechin could be developed as a potential cancer drug (Table 4).
5. Conclusions
Propagules of R. mucronata have anti-cancer properties and four compounds have been identified. 4-O-caffeoyl quinic acid, epi-catechin and 5-O-caffeoyl quinic acid were shown to be active, but procyanidin B2 showed moderate inhibition of ovarian cancer cell proliferation. Of the four compounds, epi-catechin met the ADMET criteria and showed the highest drug score (0.871). Toxicological risk and metabolic prediction show that all four compounds were non–corrosive, with the exception of epi-catechin, which was believed to be irritating or corrosive to the skin. Epi-catechin can be seen as one of the most important potential compounds for the development of new drugs, e.g., as an anti-cancer agent, but efficacy, mechanism of action, and toxicity studies need further validation at the in vitro and in vivo levels.
Author Contributions
Conceptualization, N.M.Y. and S.K.L.; methodology, N.M.Y., S.K.L. and A.O.; software, A.O.; validation, N.M.Y. and S.K.L.; formal analysis, S.K.L., A.O. and Z.A.; investigation, N.M.Y. and N.J.S.; writing—original draft preparation, N.M.Y.; writing—review and editing, N.M.Y., S.K.L. and A.O.; project administration, N.M.Y. and S.K.L.; funding acquisition, S.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by the Development Fund under 11th and 12th Malaysian Plans (23-41-01-08-006 and 24-01-070-1001) by the government of Malaysia.
Data Availability Statement
All relevant data already included in the text.
Acknowledgments
The authors are grateful to Nor Afiedatul Akmal Mohd Yousof and Ruzana Rabuzin for assisting in the cell culture activities, and Muhd Haffiz Jauri and Muhd Hafidz Hadi for sample collections and processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duke, N.C. Rhizophora apiculata, R. mucronata, R. stylosa, R. x annamalai, R. x lamarckii (Indo-West Pacific stilt mangrove). In Traditional Trees of Pacific Islands: Their Culture, Environment, and Use; Elevith, C.R., Ed.; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2006; pp. 641–660. [Google Scholar]
- Quisumbing, E.A. Medicinal Plants of the Philippines; Katha Publishing Company: Quezon City, Philippines, 1978. [Google Scholar]
- Rohini, R.M.; Das, A.K. A comparative evaluation of analgesic and anti-inflammatory activities of Rhizophora mucronata bark extracts. Pharmacologyonline 2009, 1, 780–791. [Google Scholar]
- Govindasamy, C.; Kannan, R. Pharmacognosy of mangrove plants in the system of unani medicine. Asian Pac. J. Trop. Dis. 2012, 2, S38–S41. [Google Scholar] [CrossRef]
- Misra, S.; Choudhury, A.; Dutta, A.K.; Ghosh, A. Sterols and fatty acids from three species of mangrove. Phytochemistry 1984, 23, 2823–2827. [Google Scholar] [CrossRef]
- Lakshmi, V.; Misra, A. The novel 1-hydroxy-5-oxobicyclo [6.4.0] dodecane from Rhizophora mucronata. Planta Med. 1995, 61, 382–383. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Ramanathan, T.; Satyavani, K. Antimicrobial and radical scavenging effects of alkaloid extracts from Rhizophora mucronata. Pharm. Chem. J. 2015, 49, 34–37. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Kaliamurthi, S.; Sheik, H.S.; Thiruganasambandam, R. Molecular docking, isolation and biological evaluation of Rhizophora mucronata flavonoids as anti-nociceptive agents. Biomed. Prev. Nutr. 2014, 4, 555–560. [Google Scholar] [CrossRef]
- Hridya, V.K.; Godson, P.S.; Chandrasekar, N. Chromatographic identification of two biologically important triterpenoids from the chloroform extract of Rhizophora mucronata. Acta Chromatogr. 2012, 24, 123–129. [Google Scholar] [CrossRef]
- Hemberger, Y.; Xu, J.; Wray, V.; Proksch, P.; Wu, J.; Bringmann, G. Pestalotiopens A and B: Stereochemically challenging flexible sesquiterpene-cyclopaldic acid hybrids from Pestalotiopsis sp. Chem. A Eur. J. 2013, 19, 15556–15564. [Google Scholar] [CrossRef]
- Taniguchi, K.; Funasaki, M.; Kishida, A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M.; Ohsaki, A. Two new coumarins and a new xanthone from the leaves of Rhizophora mucronata. Bioorganic Med. Chem. Lett. 2018, 28, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Imdadul, H.; Wirakarnain, S.; Koshy, P.; Arash, R.; Shariff, H.A.B.M.; Mat, T.R. Valuable antioxidant and antimicrobial extracts from Rhizophora mucronata of Asiatic Mangrove forests. Res. J. Biotechnol. 2011, 6, 10–14. [Google Scholar]
- Joel, E.L.; Bhimba, V. Isolation and characterization of secondary metabolites from the mangrove plant Rhizophora mucronata. Asian Pac. J. Trop. Med. 2010, 3, 602–604. [Google Scholar] [CrossRef] [Green Version]
- Suganthy, N.; Kesika, P.; Pandian, S.K.; Devi, K.P. Mangrove plant extracts: Radical scavenging activity and the battle against food-borne pathogens. Complement. Med. Res. 2009, 16, 41–48. [Google Scholar] [CrossRef]
- Rahman, A.M.; Hasan, S.N.; Sampad, K.S.; Das, A.K. Atinociceptive, antidiarrhoeal and cytotoxic activities of Rhizophora mucronata lamk. Pharmacologyonline 2011, 1, 921–929. [Google Scholar]
- Rege, A.A.; Ambaye, R.Y.; Deshmukh, R.A. In-vitro testing of anti-HIV activity of some medicinal plants. Indian J. Nat. Prod. Resour. 2010, 1, 193–199. [Google Scholar]
- Sachithanandam, V.; Lalitha, P.; Parthiban, A.; Muthukumaran, J.; Jain, M.; Misra, R.; Mageswaran, T.; Sridhar, R.; Purvaja, R.; Ramesh, R. A comprehensive in silico and in vitro studies on quinizarin: A promising phytochemical derived from Rhizophora mucronata Lam. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef]
- Ravikumar, S.; Jacob Inbaneson, S.; Suganthi, P.; Gnanadesigan, M. In vitro antiplasmodial activity of ethanolic extracts of mangrove plants from South East coast of India against chloroquine-sensitive Plasmodium falciparum. Parasitol. Res. 2011, 108, 873–878. [Google Scholar] [CrossRef]
- Rohini, R.M.; Das, A.K. Triterpenoids from the stem bark of Rhizophora mucronata. Nat. Prod. Res. 2010, 24, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Madhu, A.; Suresh Chandra Venkata Appa Rao, A.; Sushma, S.; Gowri, P.M.; Raju, T.V. Isomeric ent-labdane-type diterpenoids from the stems of Rhizophora mucronata. Helv. Chim. Acta 2014, 97, 1531–1538. [Google Scholar] [CrossRef]
- Lawag, I.L.; Aguinaldo, A.M.; Naheed, S.; Mosihuzzaman, M. α-Glucosidase inhibitory activity of selected Philippine plants. J. Ethnopharmacol. 2012, 144, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Islam Howlader, M.S.; Jamil Ahmed, M.; Hamidul Kabir, A.N.M.; Gias Uddin, M.; Khalid Hossain, M. Antibacterial, cytotoxic, analgesic and diuretic activities of Rhizophora mucronata Lam. bark. Indian J. Nat. Prod. Resour. 2013, 4, 229–232. [Google Scholar]
- Manigandan, V.; Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Molecular docking studies of Rhizhopora mucronata alkaloids against neuroinflammatory marker cyclooxygenase 2. Int. J. Biol. Chem. 2014, 8, 91–99. [Google Scholar]
- Premanathan, M.; Kathiresan, K.; Yamamoto, N.; Nakashima, H. In vitro anti-human immunodeficiency virus activity of polysaccharide from Rhizophora mucronata poir. Biosci. Biotechnol. Biochem. 1999, 63, 1187–1191. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Anjaneyulu, V.; Rao, V.L. Rhizophorin B: A novel beyerane diterpenoid from the Indian mangrove plant Rhizophora mucronata. J. Chem. 2000, 39B, 803–807. [Google Scholar]
- Laphookhieo, S.; Karalai, C.; Ponglimanont, C. New sesquiterpenoid and triterpenoids from the fruits of Rhizophora mucronata. Chem. Pharm. Bull. 2004, 52, 883–885. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, S.; Gnanadesigan, M. Hepatoprotective and antioxidant properties of Rhizophora mucronata mangrove plant in CCl 4 intoxicated rats. J. Exp. Clin. Med. 2012, 4, 66–72. [Google Scholar] [CrossRef]
- Syed Ali, M.; Ravikumar, S.; Margaret Beula, J.; Anuradha, V.; Yogananth, N. Insecticidal compounds from Rhizophoraceae mangrove plants for the management of dengue vector Aedes aegypti. J. Vector Borne Dis. 2014, 51, 106–114. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Nurhanan, M.Y.; Nor Azah, M.A.; Zunoliza, A.; Siti Humeriah, A.G.; Siti Syarifah, M.M.; Nor Hayati, A. In vitro anticancer activity and high-performance liquid chromatography profiles of Aquilaria subintegra fruit and seed extracts. J. Trop. For. Sci. 2017, 29, 208–214. [Google Scholar]
- Schyman, P.; Liu, R.; Desai, V.; Wallqvist, A. VNN web server for ADMET predictions. Front. Pharmacol. 2017, 8, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online chemical modeling environment (OCHEM): Web platform for data storage, model development and publishing of chemical information. J. Comput. Aided. Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, M.E.; Taylor, H.; Wani, M.C. Plant antitumor agents, 24. Rapid 9-KB assay. J. Nat. Prod. 1987, 50, 764–766. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI in vitro anticancer drug discovery screen, concept, implementation, and operation 1985–1995. In Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval; Teicher, B., Ed.; Humana Press: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Wong, S.K.; Lim, Y.Y.; Ling, S.K.; Chan, E.W.C. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacogn. Res. 2014, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Pauli, G.F.; Poetsch, F.; Nahrstedt, A. Structure assignment of natural quinic acid derivatives using proton nuclear magnetic resonance techniques. Phytochem. Anal. 1998, 9, 177–185. [Google Scholar] [CrossRef]
- Pauli, G.F.; Kuczkowiak, U.; Nahrstedt, A. Solvent effects in the structure dereplication of caffeoyl quinic acids. Magn. Reson. Chem. 1999, 37, 827–836. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. The polyphenol constituents of grape pomace. Food Chem. 1999, 65, 1–8. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Wu, Y.; Tan, H.; Meng, F.; Wang, Y.S.; Li, M.; Zhao, L.; Liu, L.; Qian, Y.; et al. Analysis of accumulation patterns and preliminary study on the condensation mechanism of proanthocyanidins in the tea plant [Camellia sinensis]. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Resende, F.O.; Rodrigues-Filho, E.; Luftmann, H.; Petereit, F.; De Mello, J.C.P. Phenylpropanoid substituted flavan-3-ols from trichilia catigua and their in vitro antioxidative activity. J. Braz. Chem. Soc. 2011, 22, 2087–2093. [Google Scholar] [CrossRef] [Green Version]
- Orisakeye, O.T.; Olugbade, T.A. Epicatechin and procyanidin B2 in the stem and root bark of Sterculia tragacantha Lindl (Sterculiaceae). Med. Chem. 2014, 4, 334–337. [Google Scholar] [CrossRef] [Green Version]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-O-caffeoylquinic acid: A spectroscopic study and biological screening for antimicrobial activity. LWT-Food Sci. Technol. 2016, 65, 471–479. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Chan, E.; Hasman, D. Antiproliferation effect of commercially brewed coffees on human ovarian cancer cells in vitro. Nutr. Cancer 2010, 62, 1044–1057. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Saravanan, T.S.; Monteclaro, C.C.; Presser, N.; Ye, X.; Selvan, S.R.; Brosman, S. Epicatechins purified from green tea (Camellia sinensis) differentially suppress growth of gender-dependent human cancer cell lines. Evid.-Based Complement. Altern. Med. 2006, 3, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddique, H.R.; Liao, D.J.; Mishra, S.K.; Schuster, T.; Wang, L.; Matter, B.; Campbell, P.M.; Villalta, P.; Nanda, S.; Deng, Y.; et al. Epicatechin-rich cocoa polyphenol inhibits Kras-activated pancreatic ductal carcinoma cell growth in vitro and in a mouse model. Int. J. Cancer 2012, 131, 1720–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaughter, R.L.; Edwards, D.J. Recent advances: The cytochrome P450 enzymes. Ann. Pharmacother. 1995, 29, 619–624. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood-brain barrier-recent progress from a material and pharmaceutical perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, M.; Spiers, J.P. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacol. Res. 2007, 55, 1–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).