Starve to Sustain—An Ancient Syrian Landrace of Sorghum as Tool for Phosphorous Bio-Economy?
Abstract
:1. Introduction
2. Results and Discussion
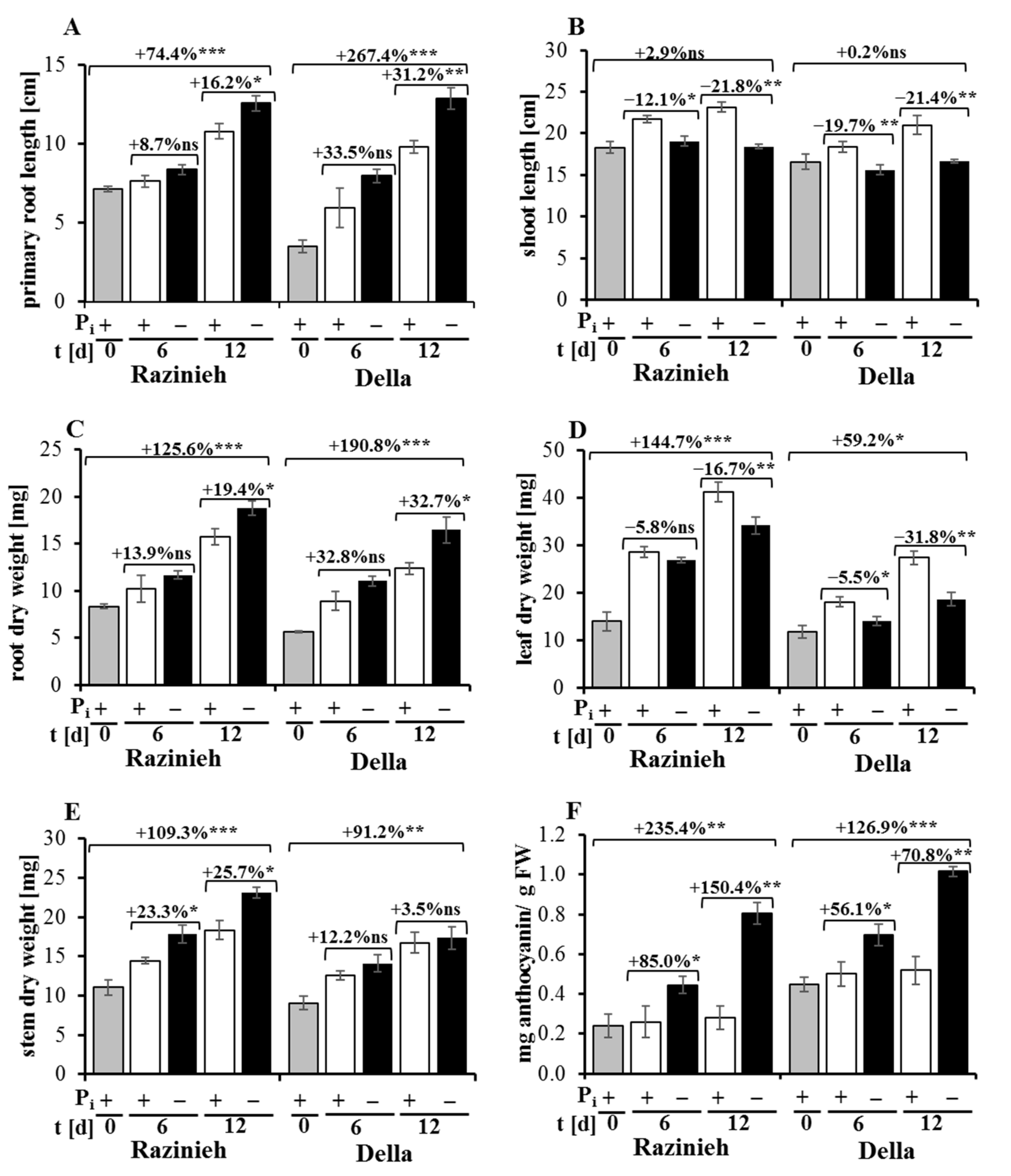
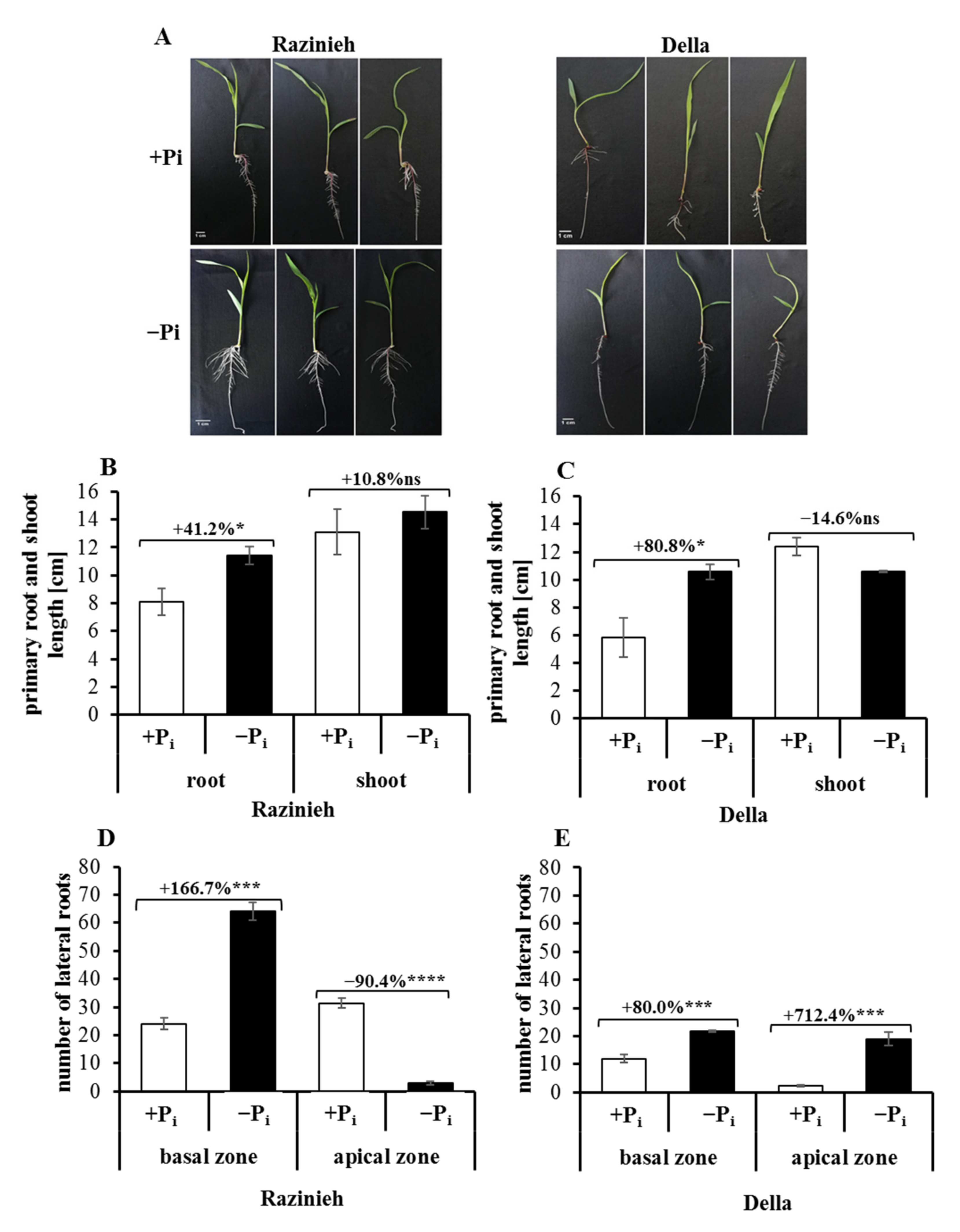
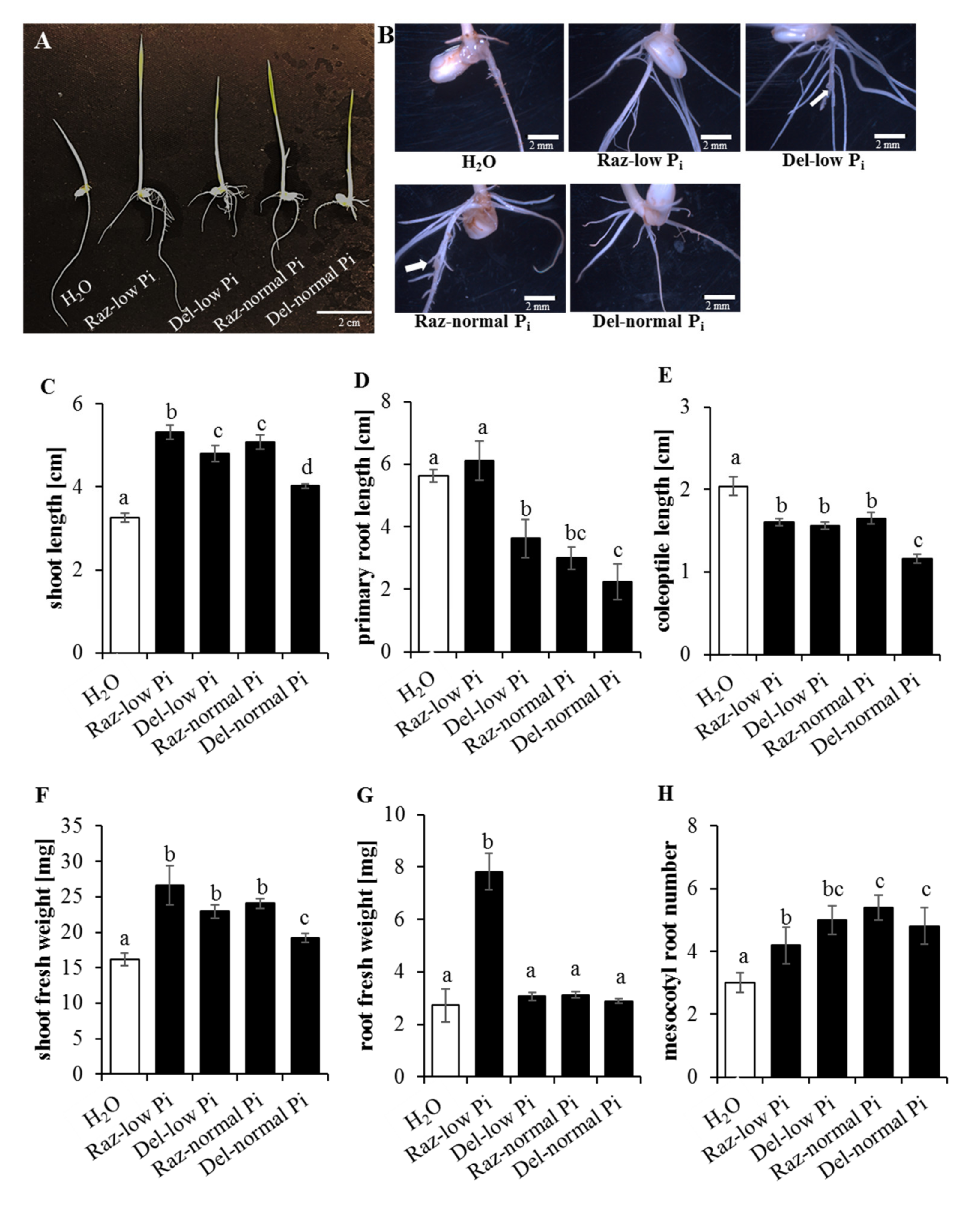
2.1. Phosphorus Starvation Enhances Primary Root Elongation and Inhibits Shoot Growth
2.2. Lateral Root Formation Is Induced by Phosphate Starvation
2.3. Razinieh Retains More P and Fe in Roots and Shoots under Pi Starvation
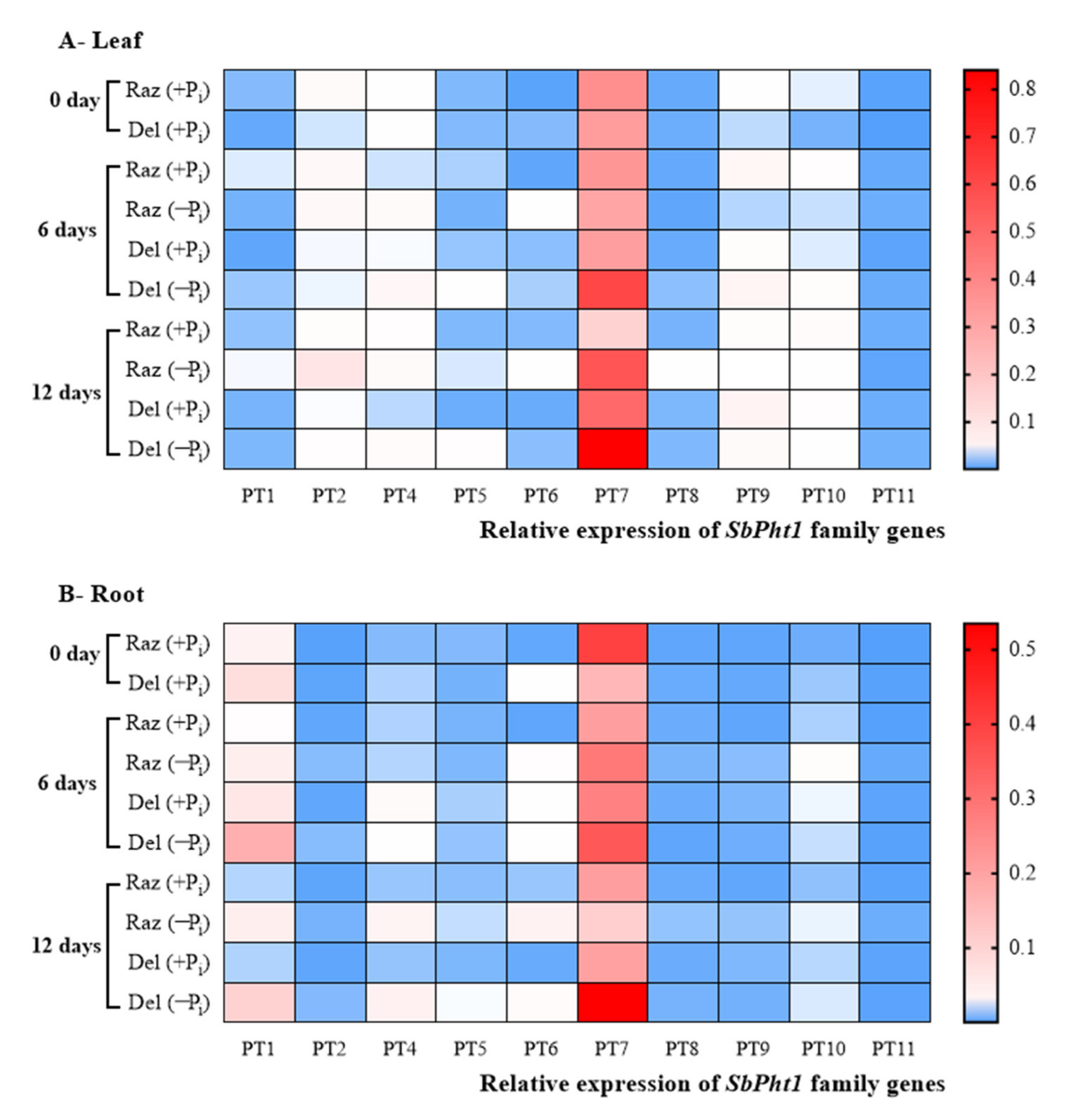
2.4. The Phosphate Transporter SbPT7 Is Induced under Pi Starvation
2.5. Composition of Straw and Ash Depends on Pi Availability and on Genotype
2.5.1. Razinieh Accumulates and Retains P More Efficiently in the Straw
2.5.2. Quantitative Analysis of Mineral Phases in the Ash
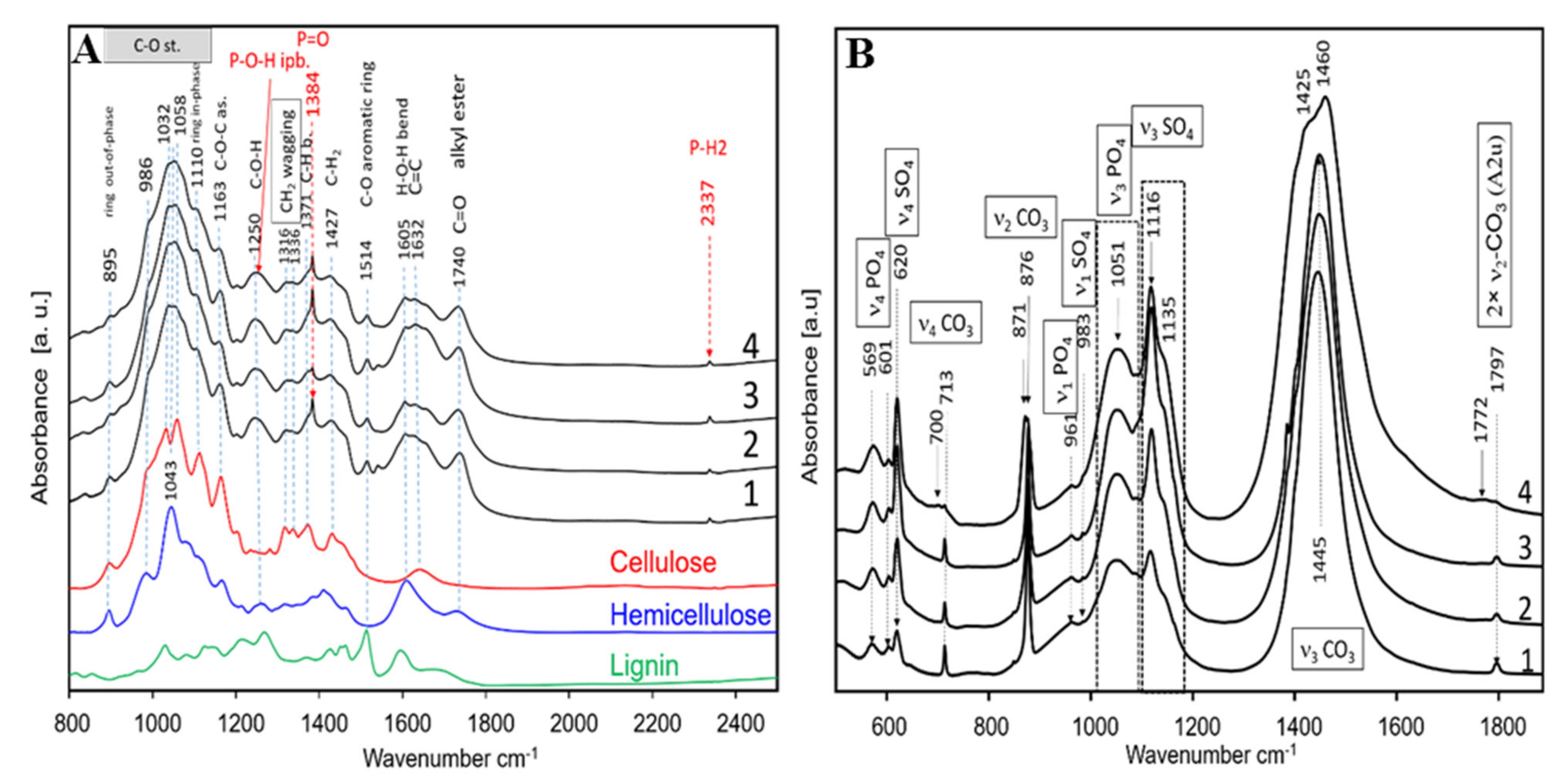
2.5.3. Physicochemical Characterisation of Mature Sorghum Straw and Ash
2.6. Sorghum Ash Accelerates Seedling Development in Rice
3. Materials and Methods
3.1. Plant Material
3.2. Hydroponic Culture of Sorghum under Pi Starvation
3.3. Measuring Lateral Root Growth under Pi Starvation
3.4. Quantification of SbPht1 Transcripts
3.5. Chemical and Mineralogical Analysis of Sorghum Straw and Ash from Pi-Rich and -Poor Soils
3.5.1. Chemical Analysis
3.5.2. X-ray Diffraction
3.5.3. IR Spectroscopy
3.6. Sorghum Ash Fertilisation Effects on the Growth of Rice Seedlings
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; p. 889. [Google Scholar]
- López-Bucio, J.; Martínez de la Vega, O.; Guevara-García, A.; Herrera-Estrella, L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000, 18, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Haarr, A. The reuse of phosphorus. In Eureau Position Paper EU2-04-SL09; European Union of National Associations of Water Suppliers and Waste Water Services: Brussels, Belgium, 2005. [Google Scholar]
- Holford, I.C.R. Soil phosphorus, its measurement, and its uptake by plants. Soil Res. 1997, 35, 227–240. [Google Scholar] [CrossRef]
- Dai, L.; Li, H.; Tan, F.; Zhu, N.; He, M.; Hum, G. Biochar, a potential route for recycling of phosphorus in agricultural residues. Gcb Bioenergy 2016, 8, 852–858. [Google Scholar] [CrossRef]
- Somaa, D.M.; Kibaa, D.I.; Ewusi-Mensahb, N.; Gnankambarya, Z.; Lompoa, F.; Sedogoa, M.P.; Abaidoob, R.C. Changes in sorghum production, soil P forms and P use efficiency following long-term application of manure, compost and straw in a Ferric Lixisol. Soil Plant Sci. 2017, 68, 401–411. [Google Scholar] [CrossRef]
- Tesfaye, K. Genetic diversity study of sorghum [Sorghum bicolor (L.) Moench] genotypes, Ethiopia. Agric. Environ. 2017, 9, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Irving, L.J. Carbon assimilation, biomass partitioning and productivity in grasses. Agriculture 2015, 5, 1116–1134. [Google Scholar] [CrossRef] [Green Version]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Thibaud, M.C.; Arrighi, J.F.; Bayle, V.; Chiarenza, S.; Creff, A.; Bustos, R.; Paz-Ares, J.; Poirier, Y.; Nussaume, L. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J. 2010, 64, 775–789. [Google Scholar] [CrossRef]
- Walder, F.; Brulé, D.; Koegel, S.; Wiemken, A.; Boller, T.; Courty, P.E. Plant phosphorus acquisition in a common mycorrhizal network: Regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol. 2015, 205, 1632–1645. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, X.; Deng, Y.; Wu, H.; Liu, P.; Li, X. AtOPR3 specifically inhibits primary root growth in Arabidopsis under phosphate deficiency. Sci Rep. 2016, 6, 24778. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Poling, M.D.; Karthikeyan, A.S.; Blakeslee, J.J.; Peer, W.A.; Titapiwatanakun, B.; Murphy, A.S.; Raghothama, K.G. Differential effects of sucrose and auxin on localized phosphate deficiency induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol. 2007, 144, 232–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jiang, F.; Shen, Y.; Zhan, Q.; Bai, B.; Chen, W.; Chi, Y. Transcriptome analysis reveals candidate genes related to phosphorus starvation tolerance in sorghum. BMC Plant Biol. 2019, 19, 306. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, J.; Han, Z.; Yang, J.; Ge, C.; Wu, Q. Revealing new insights into different phosphorus starving responses between two maize inbred lines by transcriptomic and proteomic studies. Sci. Rep. 2017, 7, 44294. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Wang, Y.; Yang, A.; Zhang, W.H. OsMYB2P-1, an R2R3MYB transcription factor, is involved in the regulation of phosphate starvation responses and root architecture in rice. Plant Physiol. 2012, 159, 169–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, A.; Reddy, B.K. Effect of phosphorus deficiency on the form of plant root system. In Plant Nutrition, 1st ed.; Scaife, A., Ed.; Commonwealth Agricultural Bureau: Slough, UK, 1982; Volume 2, pp. 412–417. [Google Scholar]
- Lynch, J.P.; Brown, K.M. Topsoil foraging an architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Sega, P.; Pacak, A. Plant PHR Transcription Factors: Put on A Map. Genes 2019, 6, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casieri, L.; Ait Lahmidi, N.; Doidy, J.; Veneault Fourrey, C.; Migeon, A.; Bonneau, L.; Courty, P.E.; Garcia, K.; Charbonnier, M.; Delteil, A. Biotrophic transportome in mutualistic plant fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef]
- Gu, M.; Chen, A.; Sun, S.; Xu, G. Complex regulation of plant phosphate transporters and the gap between molecular mechanisms and practical application. Mol. Plant 2016, 9, 396–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, P.H.; Sun, S.B.; Zhao, J.N.; Fan, X.R.; Xin, W.J.; Guo, Q.; Yu, L.; Shen, Q.R.; Miller, W.P. Two rice phosphate transporters, OsPht1, 2 and OsPht1, 6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.J.; Acharya, S.N.; Thomas, J.E. Integrated soil and crop management. Barley biomass and grain yield and canola seed yield response to land application of wood ash. Agron. J. 2004, 96, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Perucci, P.; Monaci, E.; Casucci, C.; Vischetti, C. Effect of recycling wood ash on microbiological and biochemical properties of soils. Agron. Sustain. Dev. 2006, 26, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Bergfeldt, B.; Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D. Recovery of phosphorus and other nutrients during pyrolysis of Chicken manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Kanbar, A.; Shakeri, E.; Alhajturki, D.; Thomas, H.; Emam, Y.; Tabatabaei, S.A.; Nick, P. Morphological and molecular characterization of sweet, grain and forage sorghum (Sorghum bicolor L.) genotypes grown under temperate climatic conditions. Plant Biosyst. 2019, 154, 49–58. [Google Scholar] [CrossRef]
- Kanbar, A.; Flubacher, N.; Hermuth, J.; Kosová, K.; Horn, T.; Nick, P. Mining sorghum biodiversity-potential of dual purpose hybrids for bio-economy. Diversity 2021, 13, 192. [Google Scholar] [CrossRef]
- Kanbar, A.; Shakeri, E.; Alhajturki, D.; Riemann, M.; Bunzel, M.; Morgano, M.; Stapf, D.; Nick, P. Sweet versus grain sorghum: Differential sugar transport and accumulation are linked with vascular bundle architecture. Ind. Crop. Prod. 2021, 167, 113550. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Brandt, M.; Ren, S.; Kering, M.K. Juice, ethanol, and grain yield potential of five sweet sorghum cultivars. J. Sustain. Bioenergy Syst. 2013, 3, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Ma, X.; Ye, L.; Zeng, J.; Chen, G.; Zhang, G. Root plasticity and Pi recycling within plants contribute to low-P tolerance in Tibetan wild barley. BMC Plant Biol. 2019, 19, 341. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negi, M.; Sanagala, R.; Rai, V.; Jain, A. Deciphering phosphate deficiency-mediated temporal effects on different root traits in rice grown in a modified hydroponic system. Front. Plant Sci. J. 2016, 7, 550. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Li, D.; Wang, P.; Li, J.; Lu, X. Transcriptome and ionome analysis of nitrogen, phosphorus and potassium interactions in sorghum seedlings. Theor. Exp. Plant Physiol. 2020, 32, 271–285. [Google Scholar] [CrossRef]
- Kliebenstein, D.J. Secondary metabolites and plant/environment interactions, a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- Leong, S.J.; Lu, W.C.; Chiou, T.J. Phosphite-mediated suppression of anthocyanin accumulation regulated by mitochondrial ATP synthesis and sugar in Arabidopsis. Plant Cell Physiol. 2018, 59, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Pant, P.; Erban, A.; Huhman, D.; Kopka, J.; Scheible, W.R. Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ. 2015, 38, 172–187. [Google Scholar] [CrossRef]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.S.; Xu, M.K.; Wan, W.; Yu, F.; Li, C.; Wang, J.Y.; Wei, Z.Q.; Lv, M.J.; Cao, X.Y.; Li, Z.Y.; et al. Sucrose Signaling Regulates Anthocyanin Biosynthesis Through a MAPK Cascade in Arabidopsis thaliana. Genetics 2018, 210, 607–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouached, H.; Arpat, A.B.; Poirier, Y. Regulation of phosphate starvation responses in plants: Signaling players and cross-talks. Mol. Plant 2010, 3, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Edwards, M.; Elser, J.J. A transgenic approach to enhance phosphorus use efficiency in crops as part of a comprehensive strategy for sustainable agriculture. Chemosphere 2011, 84, 840–845. [Google Scholar] [CrossRef]
- Yi, K.; Wu, Z.; Zhou, J.; Du, L.; Guo, L.; Wu, Y.; Wu, P. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol. 2005, 138, 2087–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gao, Q.; Liu, Y.; He, C.; Zhang, X.; Zhang, J. Overexpression of transcription factor ZmPTF1 improves low phosphate tolerance of maize by regulating carbon metabolism and root growth. Planta 2011, 233, 1129–1143. [Google Scholar] [CrossRef]
- Tsai, H.H.; Schmidt, W. Mobilization of iron by plant-borne coumarins. Trends Plant Sci. 2017, 22, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.F.; Ren, H.Y.; Gu, M.; Zhao, J.N.; Sun, S.B.; Zhang, X.; Chen, J.Y.; Wu, P.; Xu, G.H. The phosphate transporter gene OsPht18 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Phosphorus. In Principles of Plant Nutrition; Mengel, K., Kirkby, E.A., Kosegarten, H., Appel, T., Eds.; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- De Oliveira, J.S.; Inda, A.V.; Barrón, V.; Torrent, J.; Tiecher, T.; Camargo, F.A. Soil properties governing phosorous adsorption in soils of Southern Brazil. Geodermal Reg. 2020, 22, e00318. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorous adsorption and desorption in black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Hu, A.Y.; Che, J.; Shao, J.F.; Yokosho, K.; Zhao, X.Q.; Shen, R.F.; Ma, J.F. Silicon accumulated in the shoots results in down-regulation of phosphorus transporter gene expression and decrease of phosphorus uptake in rice. Plant Soil. 2018, 423, 317–332. [Google Scholar] [CrossRef]
- Kostic, L.; Nikolic, N.; Bosnic, D.; Samardzic, J.; Nikolic, M. Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 2017, 419, 447–455. [Google Scholar] [CrossRef]
- Higgins, H.G.; Stewart, C.M.; Harrington, K.J. Infrared spectra of cellulose and related polysaccharides. J. Polym. Sci. 1961, 51, 59–84. [Google Scholar] [CrossRef]
- Marchessault, R.H. Application of infrared spectroscopy to cellulose and wood polysaccharides. Pure Appl. Chem. 1962, 5, 107–130. [Google Scholar] [CrossRef]
- Daash, L.W.; Smith, D.C. Infrared spectra of phosphorous compounds. Anal. Chem. 1951, 23, 853–868. [Google Scholar] [CrossRef]
- Durig, J.R.; Daeyaert, F.F.D.; van der Veken, B.J. Spectra and structure of organophosphorus compounds. L Raman and infrared spectra, conformational stability, ab initio calculations and vibrational assignment of fluoromethylphosphonic dichloride. J. Raman Spectrosc. 1994, 25, 869–886. [Google Scholar] [CrossRef]
- Nakayama, J. The infrared absorption spectra of organophosphorus compounds. J. Synth. Org. 1970, 28, 132–143. [Google Scholar] [CrossRef]
- Kwiatkowski, J.S.; Leszczynski, J. The quantitative prediction and interpretation of vibrational spectra of organophosphorus compounds. Part III. Comparative ab initio SCF studies of phosphine oxide, phosphine sulfide and their fluoroderivatives. J. Mol. Struct. 1992, 257, 85–103. [Google Scholar] [CrossRef]
- Rudolph, W.W. Raman and infrared-spectroscopic investigations of dilute aqueous phosphoric acid solutions. Dalton Trans. 2010, 39, 9642–9653. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.; Li, W.Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Bachmaier, H.; Kuptz, D.; Hartmann, H. Wood ashes from grate-fired heat and power plants: Evaluation of nutrient and heavy metal contents. Sustainability 2021, 13, 5482. [Google Scholar] [CrossRef]
- Nick, P. Phototropic stimulation can shift the gradient of crown-root emergence in maize. Bot. Acta 1997, 110, 291–297. [Google Scholar] [CrossRef]
- Riemann, M.; Riemann, M.; Takano, M. Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling. Plant Cell Environ. 2008, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.X.; Lv, B.S.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol. Biochem. 2015, 90, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hazman, M.; Hause, B.; Eiche, E.; Riemann, M.; Nick, P. Different forms of osmotic stress evoke qualitatively different responses in rice. J. Plant Physiol. 2016, 202, 45–56. [Google Scholar] [CrossRef]
- Augusto, L.; Bakker, M.R.; Meredieu, C. Wood ash applications to temperate forest ecosystems potential benefits and drawbacks. Plant Soil 2008, 306, 181–198. [Google Scholar] [CrossRef]
- Lv, B.S.; Li, X.W.; Ma, H.Y.; Sun, Y.; Wie, L.X.; Jiang, C.J. Differences in growth and physiology of rice in response to different saline alkaline stress factors. Agron. J. 2013, 105, 1119–1128. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics, a Ready Reference Book of Chemical and Physical Data, 63rd ed.; The CRC Press: Boca Raton, FL, USA, 1982; p. 2661. [Google Scholar]
- Milton, C.; Axelrod, J. Fused wood-ash stones: Fairchildite (n. sp.) K2CO3·CaCO3, Buetschliite (n. sp.) 3K2CO3·2CaCO3·6H2O and Calcite, CaCO3, Their Essential Components. Amer. Mineral 1947, 32, 607–624. [Google Scholar]
- IFA. GESTIS Substance Database; Institute for Occupational Safety and Health of the German Social Accident Insurance: Sankt Augustin, Germany, 2020. [Google Scholar]
- Adeleke, R.; Nwangburuk, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef]
- Sonobe, K.; Hattori, T.; An, P.; Tsuji, W.; Eneji, A.E.; Tanaka, K.; Inanaga, S. Diurnal variations in photosynthesis, stomatal conductance and leaf water relation in sorghum grown with or without silicon under water stress. J. Plant Nutr. 2009, 32, 433–442. [Google Scholar] [CrossRef]
- Becker, M.; Ngo, N.S.; Schenk, M.K.A. Silicon reduces the iron uptake in rice and induces iron homeostasis related genes. Sci. Rep. 2020, 10, 5079. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.D.; Morison, C.G.T. On the function of silica in the nutrtion of cereals, Part I. Proc. Roy. Soc. 1906, 77, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Brenchley, W.E.; Maskell, E.J.; Warington, K. The inter-relation between silicon and other elements in plant nutrition. Ann. Appl. Biol. 1927, 14, 45–82. [Google Scholar] [CrossRef]
- Ma, J.; Takahashi, E. Effect of silicon on the growth and phosphorus uptake of rice. Plant Soil 1990, 126, 115–119. [Google Scholar] [CrossRef]
- McKinley, B.; Rooney, W.; Wilkerson, C.; Mullet, J. Dynamics of biomass partitioning, stem gene expression, cell wall biosynthesis, and sucrose accumulation during development of Sorghum bicolor. Plant Sci. J. 2016, 88, 662–680. [Google Scholar] [CrossRef]
- Lange, H.; Shropshire, W., Jr.; Mohr, H. Analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol. 1970, 47, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svyatyna, K.; Jikumaru, Y.; Brendel, R.; Reichelt, M.; Mithöfer, A.; Takano, M.; Kamiya, Y.; Nick, P.; Riemann, M. Light induces jasmonate-isoleucine conjugation via OsJAR1-dependent and -independent pathways in rice. Plant Cell Environ. 2014, 37, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2∆∆C(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Farmer, V.C. The Infrared Spectra of Minerals, 1st ed.; Mineralogical Society: London, UK, 1974; p. 539. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Minerals, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–49. [Google Scholar] [CrossRef]

| Razinieh | Della | |||
|---|---|---|---|---|
| Normal Pi | Low Pi | Normal Pi | Low Pi | |
| Ash content at 550 °C (%) | 6.46 ± 0.24 | 5.93 ± 0.15 | 4.11 ± 0.17 | 3.47 ± 0.04 |
| C (%) | 43.10 ± 0.52 | 43.20 ± 0.39 | 43.90 ± 0.36 | 44.40 ± 0.52 |
| H (%) | 5.63 ± 0.10 | 5.76 ± 0.03 | 5.77 ± 0.08 | 5.78 ± 0.04 |
| N (%) | 1.76 ± 0.02 | 1.25 ± 0.07 | 1.11 ± 0.01 | 0.99 ± 0.02 |
| S (%) | 0.29 ± 0.05 | 0.18 ± 0.03 | 0.17 ± 0.05 | 0.19 ± 0.05 |
| Cl (%) | 1.01 | 0.53 | 0.61 | 0.51 |
| Na (mg/kg) | 76.00 ± 0.58 | 30.0 ± 3.21 | 23.00 ± 0.58 | 14.00 ± 3.06 |
| Mg (mg/kg) | 3580 ± 10.0 | 2530 ± 15.3 | 1850 ± 10.0 | 1900 ± 17.3 |
| Al (mg/kg) | 98.00 ± 1.73 | 125.00 ± 0.71 | 41.00 ± 4.04 | 34.00 ± 0.71 |
| Si (mg/kg) | 1600 ± 10.0 | 3890 ± 14.1 | 707 ± 10.4 | 1580 ± 14.1 |
| P (mg/kg) | 1710 ± 5.77 | 1300 ± 5.77 | 924 ± 7.23 | 772 ± 16.0 |
| K (mg/kg) | 16100 ± 100 | 9210 ± 68.1 | 13000 ± 57.7 | 7990 ± 92.4 |
| Ca (mg/kg) | 9720 ± 20.8 | 11000 ± 57.7 | 4720 ± 17.3 | 5050 ± 78.1 |
| Ti (mg/kg) | 7.00 ± 0.58 | 7.00 ± 0.58 | <4.00 | <4.00 |
| Fe (mg/kg) | 109 ± 3.46 | 96.00 ± 1.73 | 55 ± 0.71 | 44.0 ± 1.00 |
| Zn (mg/kg) | 54.00 ± 0.00 | 78.00 ± 0.71 | 44.00 ± 1.00 | 37.00 ± 0.58 |
| Pb (mg/kg) | <4.00 | <4.00 | <4.00 | <4.00 |
| Razinieh | Della | |||
|---|---|---|---|---|
| Normal-Pi | Low-Pi | Normal-Pi | Low-Pi | |
| C (%) | 3.36 ± 0.07 | 4.61 ± 0.04 | 4.17 ± 0.03 | 4.09 ± 0.16 |
| S (%) | 2.16 ± 0.28 | 1.11 ± 0.16 | 1.59 ± 0.24 | 1.48 ± 0.17 |
| Cl (%) | 10.70 | 10.90 | 9.62 | 8.25 |
| Mg (%) | 5.49 ± 0.01 | 4.23 ± 0.01 | 4.50 ± 0.02 | 5.55 ± 0.00 |
| Si (%) | 2.14 ± 0.02 | 6.54 ± 0.04 | 1.68 ± 0.02 | 4.44 ± 0.00 |
| P (%) | 2.63 ± 0.00 | 2.12 ± 0.00 | 2.24 ± 0.03 | 2.20 ± 0.00 |
| K (%) | 25.60 ± 0.08 | 15.80 ± 0.07 | 32.70 ± 0.40 | 24.20 ± 0.00 |
| Ca (%) | 14.70 ± 0.01 | 18.90 ± 0.08 | 11.70 ± 0.01 | 14.90 ± 0.01 |
| Fe (%) | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 |
| Zn (%) | <0.20 | <0.20 | <0.20 | <0.20 |
| Phase | Formula | Razinieh | Della | ||
|---|---|---|---|---|---|
| Normal-Pi | Low-Pi | Normal-Pi | Low-Pi | ||
| Amorphous | 27.40 ± 0.8 | 31.80 ± 0.9 | 31.20 ± 1.0 | 34.00 ± 0.8 | |
| Hydroxylapatite | Ca5(PO4)3OH | 6.90 ± 0.19 | 5.60 ± 0.2 | 4.00 ± 0.2 | 6.26 ± 0.18 |
| Chlorapatite | Ca5(PO4)3Cl | 1.69 ± 0.19 | 2.12 ± 0.18 | 5.40 ± 0.3 | 1.66 ± 0.18 |
| Apatite | Ca5(PO4)3F | 0.31 ± 0.05 | 0.22 ± 0.05 | 0.22 ± 0.05 | 0.25 ± 0.04 |
| Na-Al-Phosphate | NaAl(P2O7) | 0.30 ± 0.11 | 1.30 ± 0.2 | 0.30 ± 0.12 | 0.10 ± 0.10 |
| Calcite | CaCO3 | 14.70 ± 0.14 | 25.50 ± 0.2 | 5.03 ± 0.15 | 13.56 ± 0.15 |
| Fairchildite | K2Ca(CO3)2 | 5.18 ± 0.10 | 2.43 ± 0.11 | 15.87 ± 0.17 | 7.25 ± 0.10 |
| Ankerite | CaFe(CO3)2 | 1.82 ± 0.15 | 3.00 ± 0.2 | 2.33 ± 0.17 | 1.77 ± 0.16 |
| Sylvite | KCl | 18.35 ± 0.16 | 8.61 ± 0.11 | 16.40 ± 0.2 | 14.11 ± 0.14 |
| Arcanite | K2(SO4) | 10.53 ± 0.12 | 4.70 ± 0.16 | 5.79 ± 0.13 | 6.81 ± 0.12 |
| Periclase | MgO | 7.57 ± 0.11 | 4.45 ± 0.11 | 7.10 ± 0.13 | 7.77 ± 0.12 |
| Wollastonite | CaSiO3 | 2.26 ± 0.13 | 6.10 ± 0.2 | 3.39 ± 0.14 | 3.54 ± 0.15 |
| Quartz | SiO2 | 0.71 ± 0.04 | 1.16 ± 0.05 | 0.38 ± 0.06 | 0.39 ± 0.04 |
| Huntite | CaMg3(CO3)4 | 1.30 ± 0.2 | 0.28 ± 0.11 | 1.20 ± 0.2 | 1.40 ± 0.2 |
| Mg-Sulphate | MgSO4 | 0.13 ± 0.04 | 0.83 ± 0.09 | 0.20 ± 0.06 | 0.20 ± 0.05 |
| Halite | NaCl | 0.37 ± 0.05 | 0.61 ± 0.05 | 0.44 ± 0.06 | 0.49 ± 0.05 |
| Ilmenite | FeTiO3 | 0.29 ± 0.05 | 0.63 ± 0.08 | 0.23 ± 0.05 | 0.20 ± 0.05 |
| Cristobalite low | SiO2 | 0.03 ± 0.02 | 0.17 ± 0.04 | 0.042 ± 0.02 | 0.04 ± 0.02 |
| Rutile | TiO2 | 0.16 ± 0.06 | 0.49 ± 0.06 | 0.37 ± 0.07 | 0.25 ± 0.06 |
| Elemental composition of the amorphous phase | |||||
| wt% of bulk sample | K | 9.53 ± 0.13 | 8.40 ± 0.12 | 16.25 ± 0.42 | 11.33 ± 0.10 |
| Cl | 1.60 ± 0.08 | 6.33 ± 0.06 | 1.19 ± 0.10 | 1.13 ± 0.07 | |
| Si | 1.24 ± 0.04 | 4.44 ± 0.07 | 0.67 ± 0.05 | 3.38 ± 0.04 | |
| Ca | 3.18 ± 0.14 | 2.44 ± 0.18 | 1.51 ± 0.17 | 3.31 ± 0.14 | |
| C | 0.71 ± 0.08 | 0.93 ± 0.05 | 1.54 ± 0.05 | 1.34 ± 0.16 | |
| Mg | 0.55 ± 0.08 | 1.19 ± 0.07 | 0 | 0.46 ± 0.08 | |
| P | 0.91 ± 0.06 | 0.31 ± 0.07 | 0.41 ± 0.08 | 0.67 ± 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanbar, A.; Mirzai, M.; Abuslima, E.; Flubacher, N.; Eghbalian, R.; Garbev, K.; Bergfeldt, B.; Ullrich, A.; Leibold, H.; Eiche, E.; et al. Starve to Sustain—An Ancient Syrian Landrace of Sorghum as Tool for Phosphorous Bio-Economy? Int. J. Mol. Sci. 2021, 22, 9312. https://doi.org/10.3390/ijms22179312
Kanbar A, Mirzai M, Abuslima E, Flubacher N, Eghbalian R, Garbev K, Bergfeldt B, Ullrich A, Leibold H, Eiche E, et al. Starve to Sustain—An Ancient Syrian Landrace of Sorghum as Tool for Phosphorous Bio-Economy? International Journal of Molecular Sciences. 2021; 22(17):9312. https://doi.org/10.3390/ijms22179312
Chicago/Turabian StyleKanbar, Adnan, Madeleine Mirzai, Eman Abuslima, Noemi Flubacher, Rose Eghbalian, Krassimir Garbev, Britta Bergfeldt, Angela Ullrich, Hans Leibold, Elisabeth Eiche, and et al. 2021. "Starve to Sustain—An Ancient Syrian Landrace of Sorghum as Tool for Phosphorous Bio-Economy?" International Journal of Molecular Sciences 22, no. 17: 9312. https://doi.org/10.3390/ijms22179312






