Biodegradable Zinc Oxide Nanoparticles Doped with Iron as Carriers of Exogenous Iron in the Living Organism
Abstract
:1. Introduction
2. Results
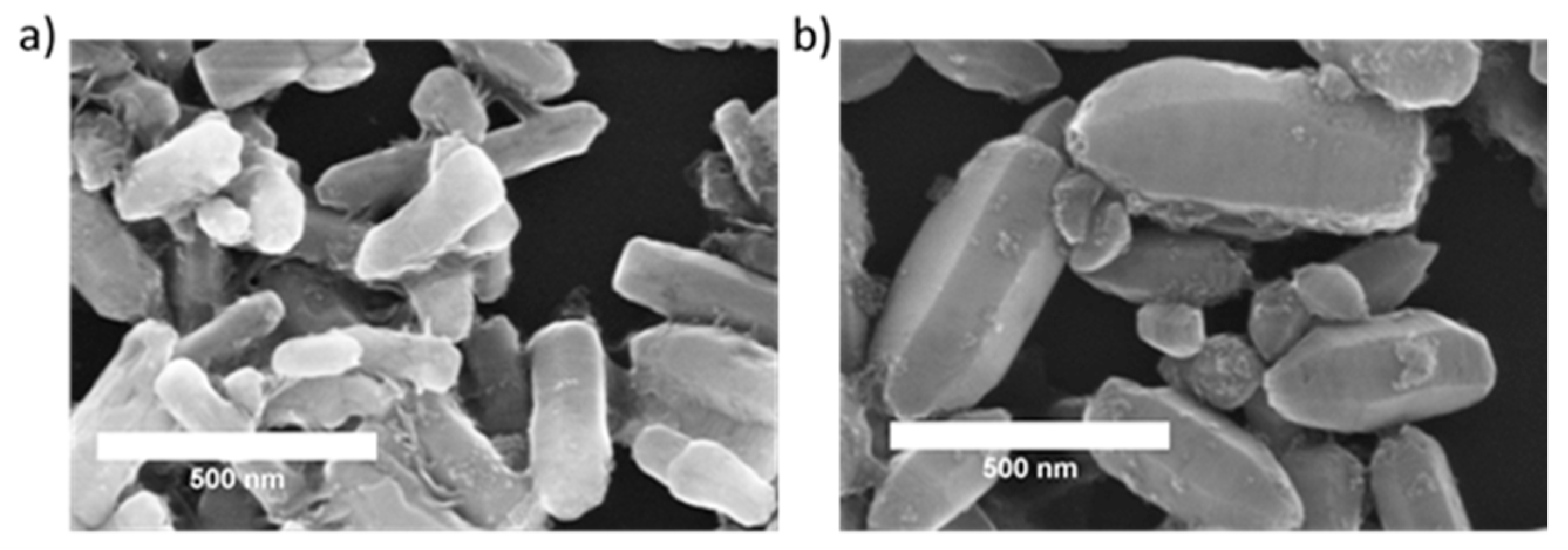
2.1. Characterization of Nanoparticles
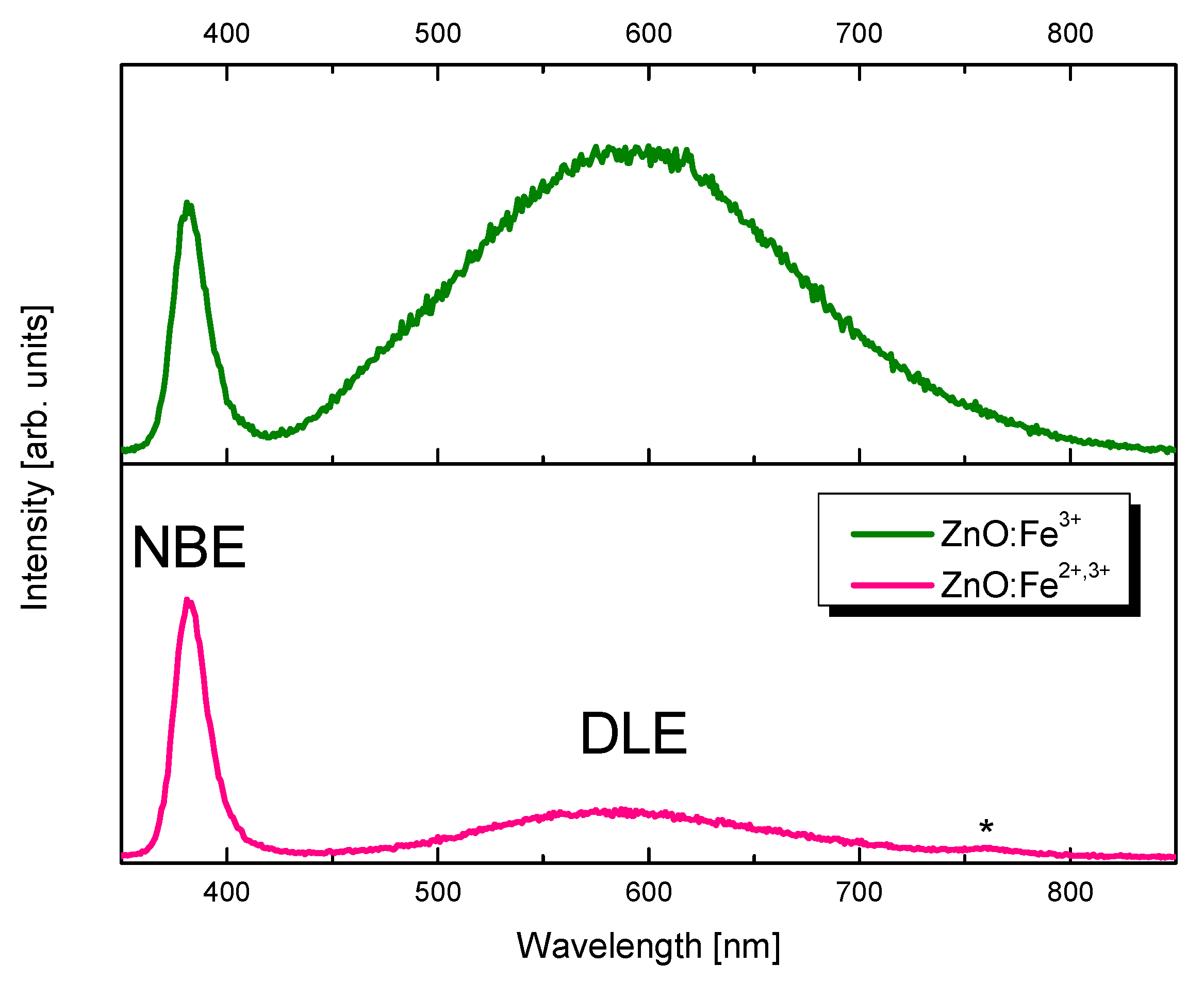
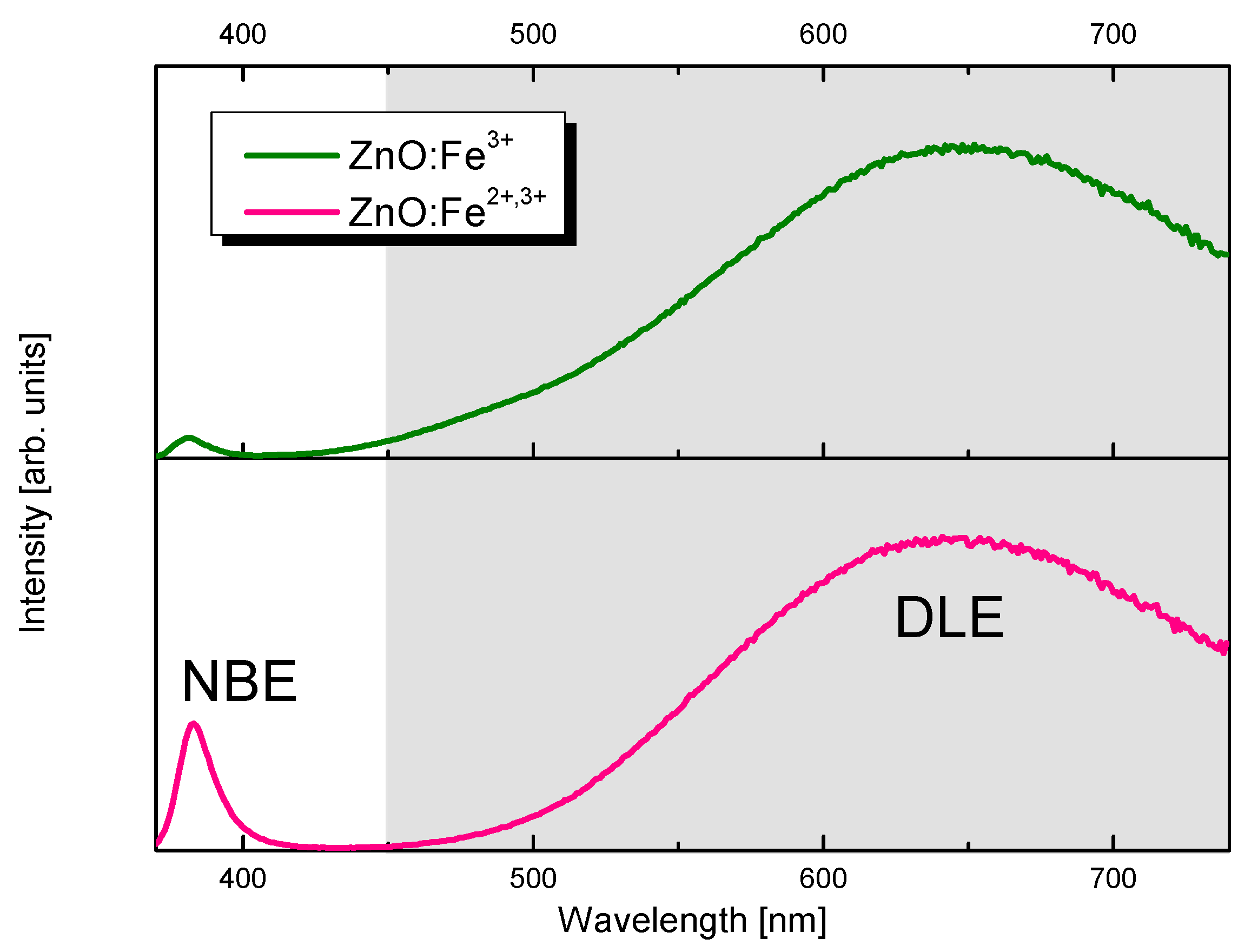
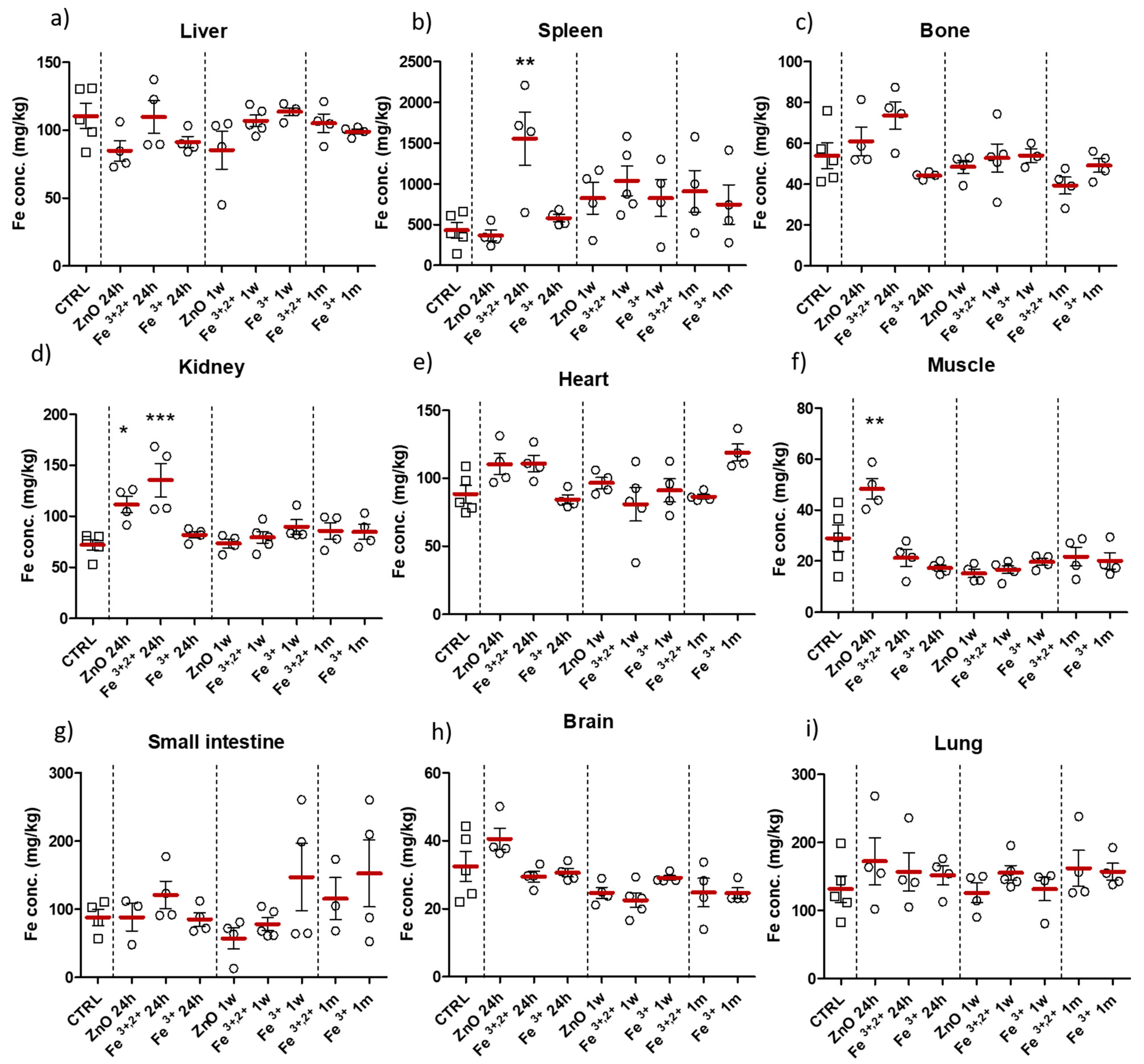
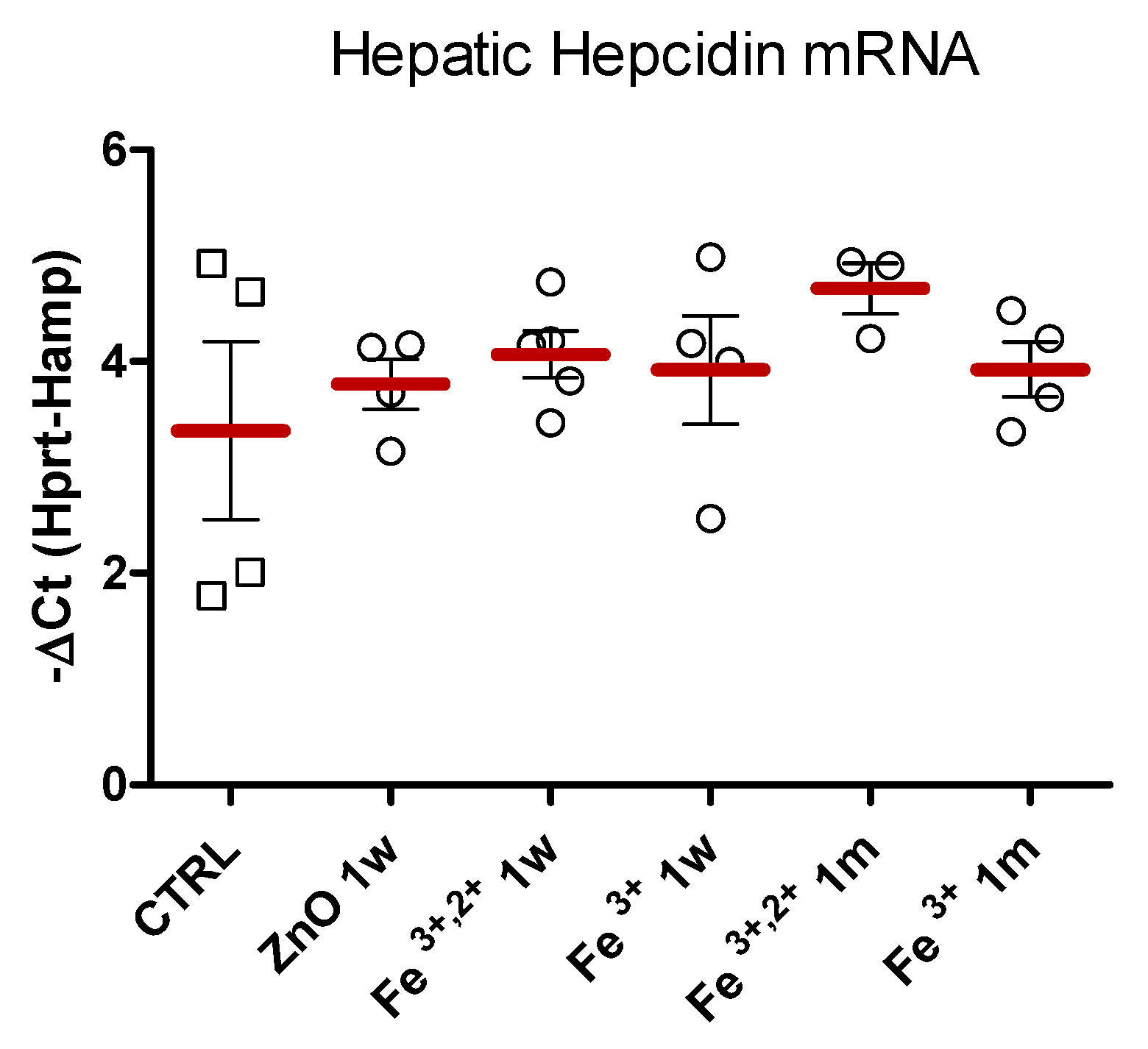
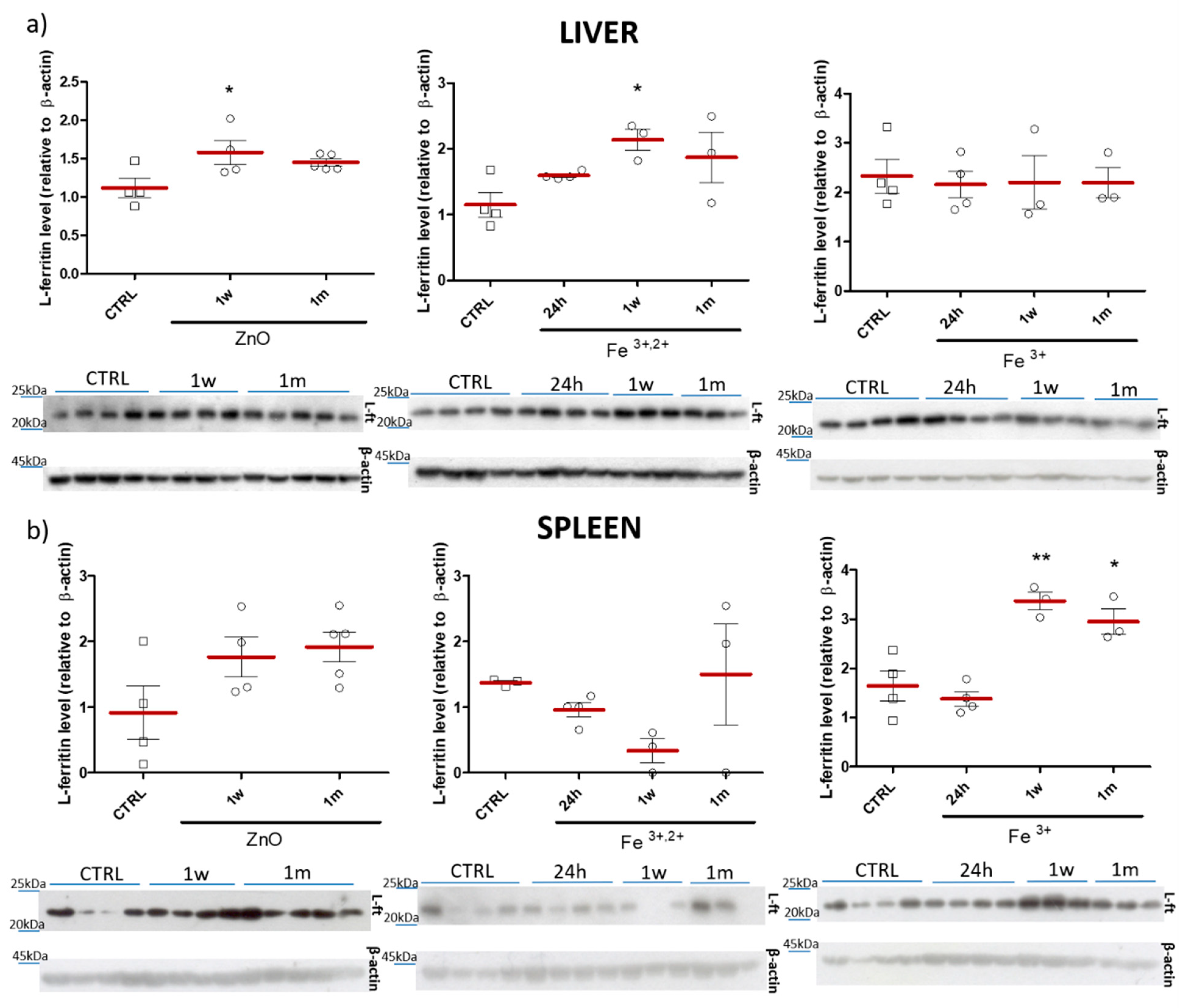
2.2. Selection of ZnO Nanoparticles Doping-Fe3+ or Fe3+,2+
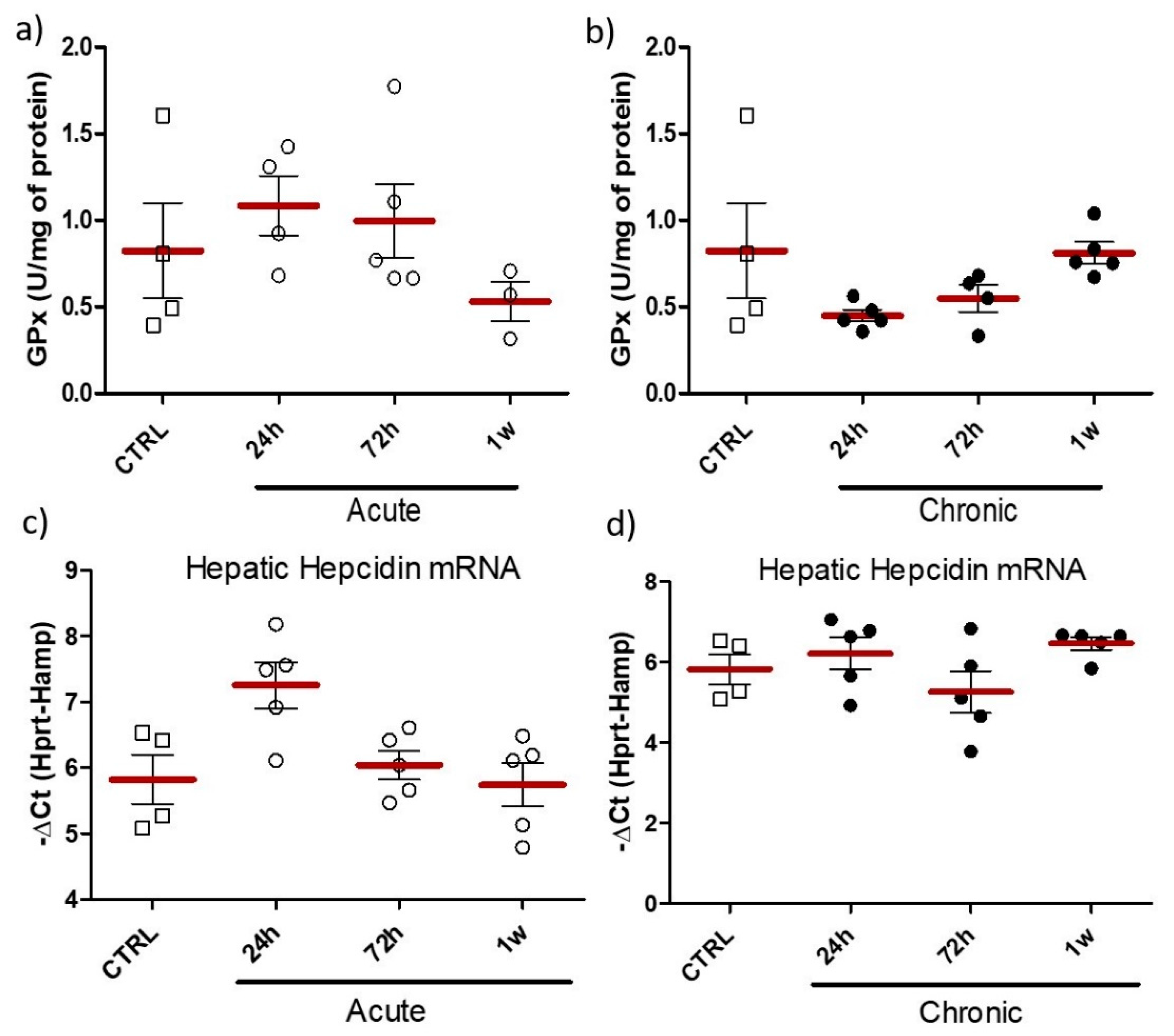
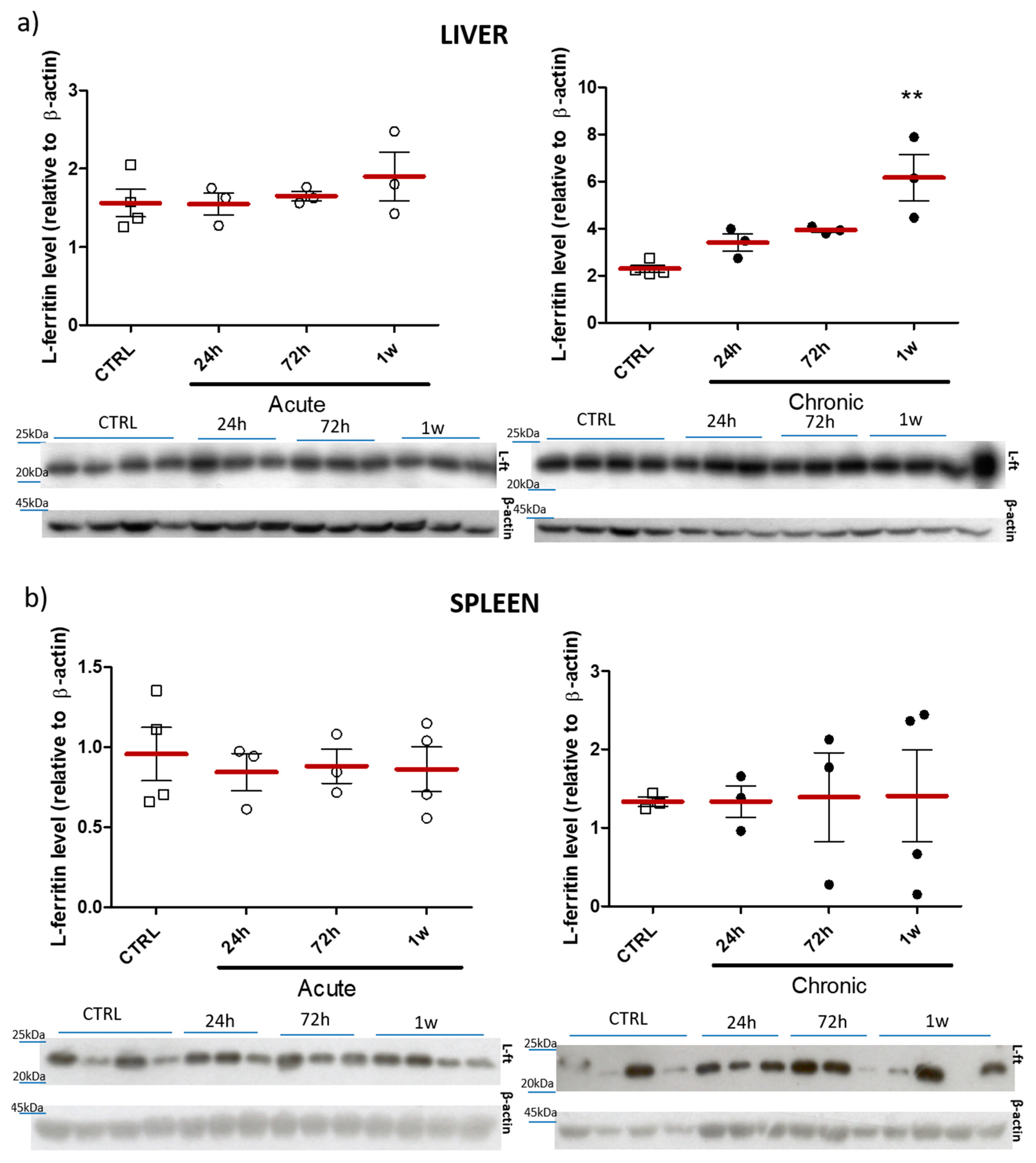
2.3. Effect of Acute/Chronic Exposure to ZnO Nanoparticles Doped with Fe3+,2+
2.4. Histopathology
3. Discussion
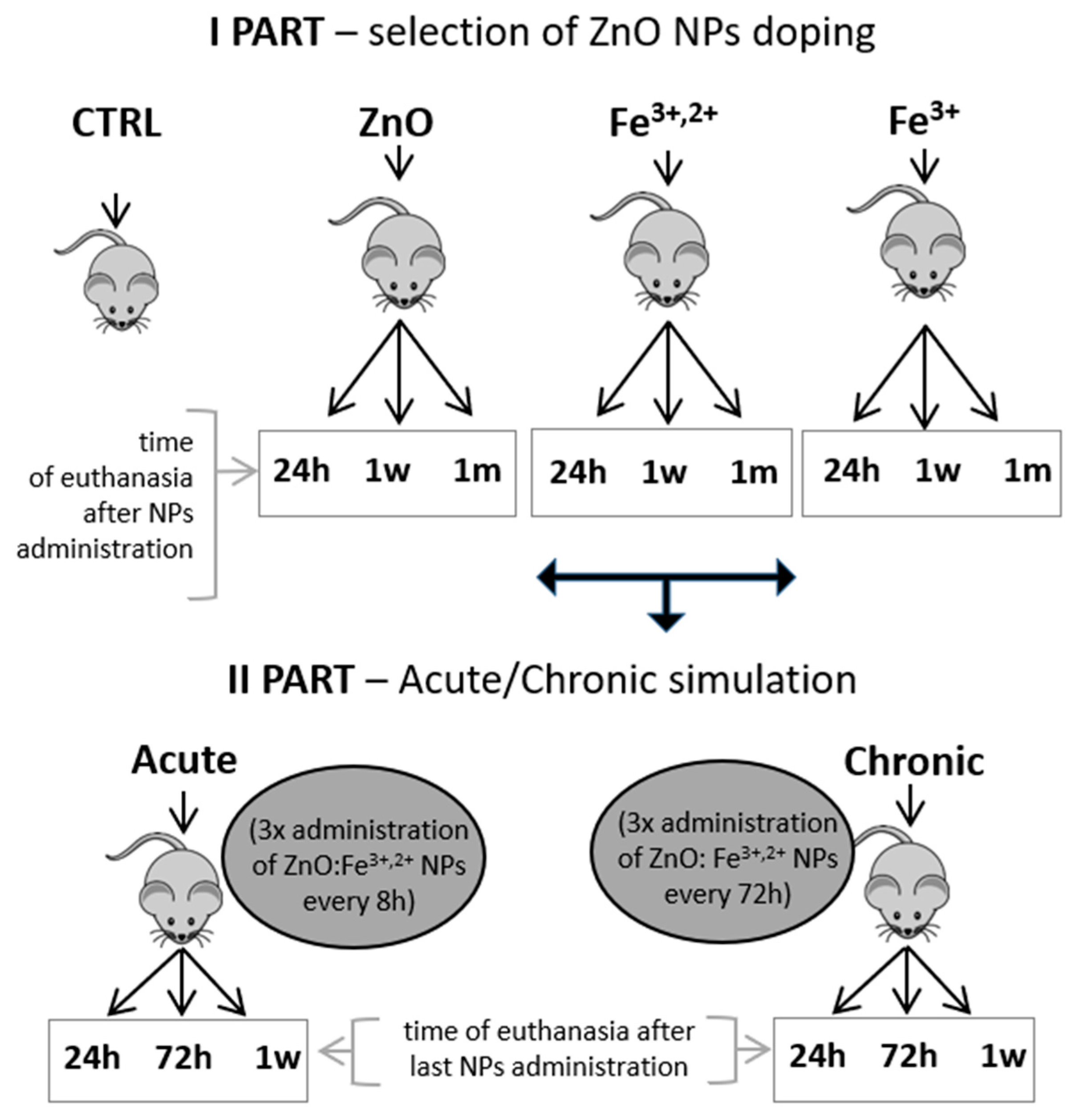
4. Materials and Methods
4.1. Preparation of Nanoparticles
4.2. Characterization of Nanoparticles
4.3. Experimental Design, Animal Model and Sample Collection
4.4. Hematological Parameters
4.5. Plasma Ferritin and Iron Measurement
4.6. Evaluation of Iron Level in Tissues
4.7. Hepatic Hepcidin mRNA Quantification
4.8. Immunoblot Analyses
4.9. Analysis of Glutathione Peroxidase (GPX) Activity
4.10. Histopathology
4.11. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2008, 23, 444–454. [Google Scholar] [CrossRef] [Green Version]
- de Benoist, B.; McLean, E.; Egli, I.; Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Lasocki, S.; Gaillard, T.; Rineau, E. Iron is essential for living! Crit. Care 2014, 18, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Allen, L.H. Anemia and iron deficiency: Effects on pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 1280S–1284S. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Corapci, F.; Calatroni, A.; Kaciroti, N.; Jimenez, E.; Lozoff, B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. J. Pediatr. Psychol. 2010, 35, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Mikulková, K. Oral iron administration in suckling piglets—A review. Acta Vet. Brno 2018, 87, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, P.; Starzynski, R.R.; Canonne-Hergaux, F.; Tudek, B.; Olinski, R.; Kowalczyk, P.; Dziaman, T.; Thibaudeau, O.; Gralak, M.A.; Smuda, E.; et al. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 2010, 177, 1233–1243. [Google Scholar] [CrossRef]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2012, 142, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Svoboda, M.; Drábek, J. Iron deficiency in suckling piglets: Ethiology, clinical aspects and diagnosis (a review). Folia Vet. 2005, 49, 104–111. [Google Scholar]
- Svoboda, M.; Drabek, J. Intramuscular versus subcutaneous administration of iron dextran in suckling piglets. Acta Vet. Brno 2007, 76, S11–S15. [Google Scholar] [CrossRef] [Green Version]
- Szudzik, M.; Starzyński, R.R.; Jończy, A.; Mazgaj, R.; Lenartowicz, M.; Lipiński, P. Iron Supplementation in Suckling Piglets: An Ostensibly Easy Therapy of Neonatal Iron Deficiency Anemia. Pharmaceuticals 2018, 11, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeli, A.K.; Framstad, T. An evaluation of iron-dextran supplementation in piglets administered by injection on the first, third or fourth day after birth. Res. Vet. Sci. 1999, 66, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I. More iron? Pig. Int. 1998, 28, 21–22. [Google Scholar]
- Strzalkowski, A.; Godlewski, M.; Hallay, N.; Kulasek, G.; Gajewski, Z.; Zabielski, R. The effect of suplementing sow with bioactive substances on neonatal small intestinal epithelium. J. Physiol. Pharmacol. 2007, 58 (Suppl. 3), 115–122. [Google Scholar] [PubMed]
- Vaziri, N. Understanding iron: Promoting its safe use in patient with chronic kidney failure treated by hemodialysis. Am. J. Kidney Dis. 2013, 61, 992–1000. [Google Scholar] [CrossRef]
- Macdougall, I.C. Strategies for iron supplementation: Oral versus intravenous. Kidney Int. Suppl. 1999, 69, S61–S66. [Google Scholar] [CrossRef] [Green Version]
- Malyszko, J. Hepcidin assays: Ironing out some details. Clin. J. Am. Soc. Nephrol. 2009, 4, 1015–1016. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Rishi, G.; Wallace, F.F.; Subramaniam, V.N. Hepcidin: Regulation of the master iron regulator. Biosci. Rep. 2015, 35, e00192. [Google Scholar] [CrossRef] [Green Version]
- Staroń, R.; Van Swelm, R.P.; Lipiński, P.; Gajowiak, A.; Lenartowicz, M.; Bednarz, A.; Gajewska, M.; Pieszka, M.; Laarakkers, C.M.; Swinkels, D.W.; et al. Urinary hepcidin levels in iron-deficient and iron-supplemented piglets correlate with hepcidin hepatic mRNA and serum levels and with body iron status. PLoS ONE 2015, 10, e0136695. [Google Scholar] [CrossRef] [Green Version]
- Starzyński, R.R.; Laarakkers, C.M.; Tjalsma, H.; Swinkels, D.W.; Pieszka, M.; Styś, A.; Mickiewicz, M.; Lipiński, P. Iron supplementation in suckling piglets: How to correct iron deficiency anemia without affecting plasma hepcidin levels. PLoS ONE 2013, 8, e64022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godlewski, M.; Kaszewski, J.; Kielbik, P.; Olszewski, J.; Lipinski, W.; Słońska, A.; Rosowska, J.; Witkowski, B.; Gralak, M.; Gajewski, Z.; et al. New generation of oxide-based nanoparticles for the applications in early cancer detection and diagnostics. Nanotechnol. Rev. 2020, 9, 274–302. [Google Scholar] [CrossRef]
- Amjad, M.S.; Sadiq, N.; Qureshi, H.; Fareed, G.; Sabir, S. Nano particles: An emerging tool in biomedicine. Asian Pac. J. Trop. Dis. 2015, 5, 767–771. [Google Scholar] [CrossRef]
- Hasirci, N. Micro and Nano Systems in Biomedicine and Drug Delivery. In Nanomaterials and Nanosystems for Biomedical Applications; Mozafari, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Hammond, P.T. A Growing Place for Nano in Medicine. ACS Nano 2014, 8, 7551–7552. [Google Scholar] [CrossRef]
- Kielbik, P.; Kaszewski, J.; Dominiak, B.; Damentko, M.; Serafińska, I.; Rosowska, J.; Gralak, M.A.; Krajewski, M.; Witkowski, B.W.; Gajewski, Z.; et al. Preliminary Studies on Biodegradable Zinc Oxide Nanoparticles Doped with Fe as a Potential Form of Iron Delivery to the Living Organism. Nanoscale Res. Lett. 2019, 14, 373. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Chen, B.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar]
- Elbialy, N.S.; Aboushoushah, S.F.; Alshammari, W.W. Long-term biodistribution and toxicity of curcumin capped iron oxide nanoparticles after single-dose administration in mice. Life Sci. 2019, 230, 76–83. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Kaszewski, J.; Kielbik, P.; Grzyb, J.; Godlewski, M.M.; Godlewski, M. Rare earth activated ZnO nanoparticles as biomarkers. Opt. Mater. 2014, 36, 1655–1659. [Google Scholar] [CrossRef]
- Kielbik, P.; Kaszewski, J.; Godlewski, M.; Godlewski, M.M. Cytometric analysis of Zn-based nanoparticles for biomedical applications. In Microscopy and Imaging Science: Practical Approaches to Applied Research and Education; Mendez-Vilas, A., Ed.; Microscopy Book Series #7; Formatex: Badajoz, Spain, 2017; pp. 89–96. [Google Scholar]
- Kielbik, P.; Kaszewski, J.; Rosowska, J.; Wolska, E.; Witkowski, B.S.; Gralak, M.A.; Gajewski, Z.; Godlewski, M.; Godlewski, M.M. Biodegradation of the ZnO:Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomedicine 2017, 13, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, P.; Kaszewski, J.; Dabrowski, S.; Faundez, R.; Witkowski, B.S.; Wachnicki, L.; Zhydachevskyy, Y.; Sapierzynski, R.; Gajewski, Z.; Godlewski, M.; et al. Transfer of orally administered ZnO:Eu nanoparticles through the blood-testis barrier: The effect on kinetic sperm parameters and apoptosis in mice testes. Nanotechnology 2019, 30, 455101. [Google Scholar] [CrossRef]
- Crist, B.V. Handbooks of Monochromatic XPS Spectra; XPS International LLC: Mountain View, CA, USA, 2005; Volume 2, p. 820. [Google Scholar]
- Morkoc, H.; Ozgur, U. Zinc Oxide: Fundamentals, Materials and Device Technology, 1st ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Hilty, F.M.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.N.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Wolfgang Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380. [Google Scholar] [CrossRef]
- Pham, B.; Colvin, E.K.; Pham, N.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S.; et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Liu, Y.; Zhang, N.; Yao, Y.; Ma, P.; Wen, H.; Huang, S.; Luo, Y.; Fan, H. Effects of core size and PEG coating layer of iron oxide nanoparticles on the distribution and metabolism in mice. Int. J. Nanomed. 2018, 13, 5719–5731. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Rajak, S.; Singh, S.P.; Murty, U.S.; Mahboob, M.; Grover, P.; Rahman, M.F. Biochemical alterations induced by acute oral doses of iron oxide nanoparticles in Wistar rats. Drug Chem. Toxicol. 2013, 36, 296–305. [Google Scholar] [CrossRef]
- Kumari, M.; Rajak, S.; Singh, S.P.; Kumari, S.I.; Kumar, P.U.; Murty, U.S.; Mahboob, M.; Grover, P.; Rahman, M.F. Repeated oral dose toxicity of iron oxide nanoparticles: Biochemical and histopathological alterations in different tissues of rats. J. Nanosci. Nanotechnol. 2012, 12, 2149–2159. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Turuntas, V.; De Luka, S.R.; Jankovic, S.; Stefanovic, S.; Puskas, N.; Zaletel, I.; Milutinović-Smiljanic, S.; Trbovich, A.M. Effects of Il-33/St2 pathway on alteration of iron and hematological parameters in acute inflammation. Exp. Mol. Pathol. 2015, 99, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.F.; Frazer, D.M.; Faria, N.; Bruggraber, S.F.; Wilkins, S.J.; Mirciov, C.; Powell, J.J.; Anderson, G.J.; Pereira, D.I. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. FASEB J. 2014, 28, 3671–3678. [Google Scholar] [CrossRef]
- Rohner, F.; Ernst, F.O.; Arnold, M.; Hilbe, M.; Biebinger, R.; Ehrensperger, F.; Pratsinis, S.E.; Langhans, W.; Hurrell, R.F.; Zimmermann, M.B. Synthesis, characterization, and bioavailability in rats of ferric phosphate nanoparticles. J. Nutr. 2007, 137, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Vyoral, D.; Petrak, J. Therapeutic potential of hepcidin—The master regulator of iron metabolism. Pharmacol. Res. 2017, 115, 242–254. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Garcés, V.; Rodríguez-Nogales, A.; González, A.; Gálvez, N.; Rodríguez-Cabezas, M.E.; García-Martin, M.L.; Gutiérrez, L.; Rondón, D.; Olivares, M.; Gálvez, J.; et al. Bacteria-Carried Iron Oxide Nanoparticles for Treatment of Anemia. Bioconjug. Chem. 2018, 29, 1785–1791. [Google Scholar] [CrossRef] [Green Version]
- Líšková, S.; Bališ, P.; Mičurová, A.; Kluknavský, M.; Okuliarová, M.; Puzserová, A.; Škrátek, M.; Sekaj, I.; Maňka, J.; Valovič, P.; et al. Effect of iron oxide nanoparticles on vascular function and nitric oxide production in acute stress-exposed rats. Physiol. Res. 2020, 69, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Luciani, N.; Alloyeau, D.; Elgrabli, D.; Deveaux, V.; Pechoux, C.; Chat, S.; Wang, G.; Vats, N.; Gendron, F.; et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 2011, 32, 3988–3999. [Google Scholar] [CrossRef]
- Ruiza, A.; Gutiérreza, L.; Cáceres-Vélezc, P.R.; Santosc, D.; Chavesc, S.B.; Fascinelic, M.L.; Garciac, M.P.; Azevedoc, R.B.; Moralesa, M.P. Biotransformation of magnetic nanoparticles as a function of coating in a rat model. Nanoscale 2015, 7, 16321–16329. [Google Scholar] [CrossRef]
 —NaNO3 (PDF no. 36-1474).
—NaNO3 (PDF no. 36-1474).
 —NaNO3 (PDF no. 36-1474).
—NaNO3 (PDF no. 36-1474).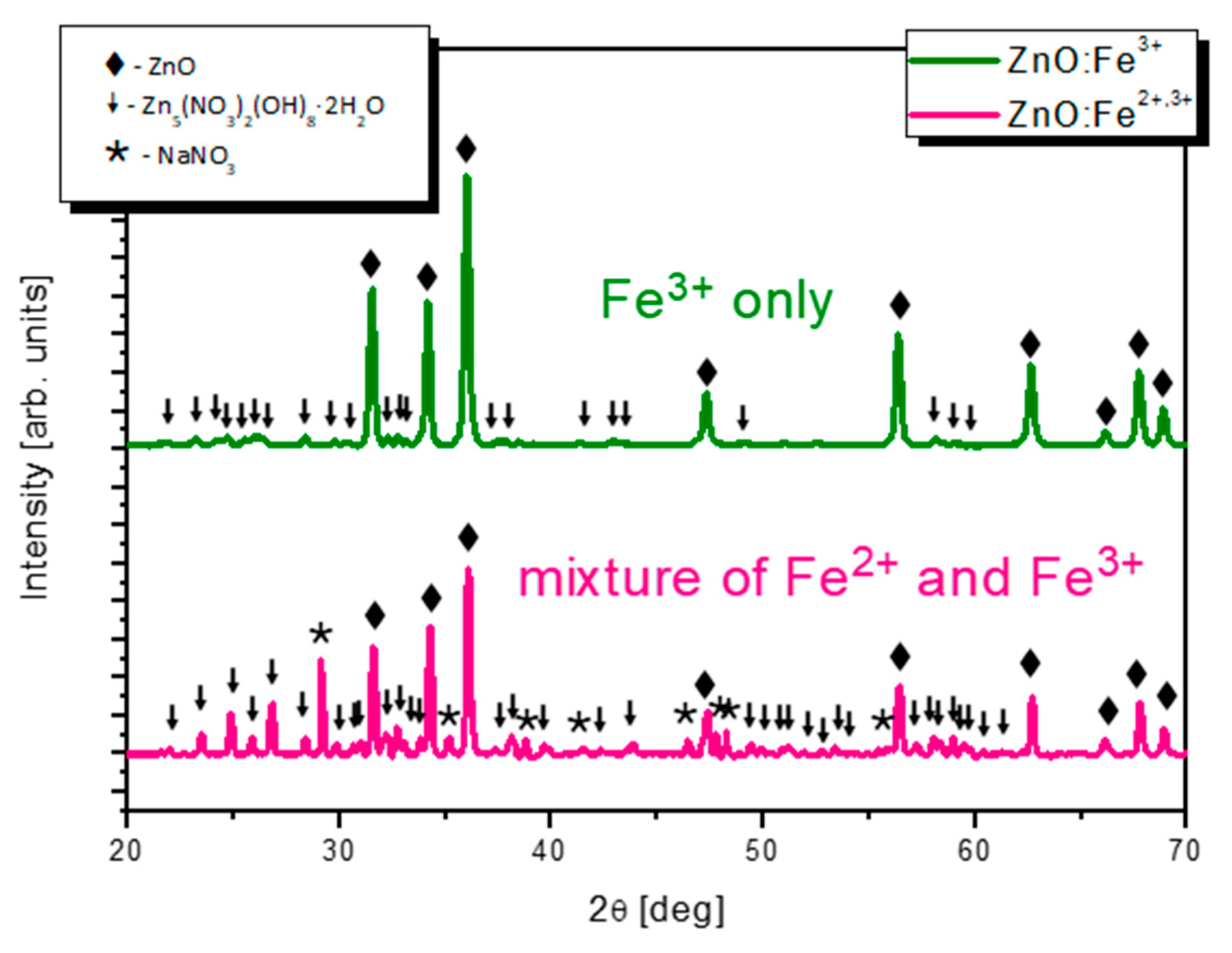




| Zn | O | Fe | N | Cl | |
|---|---|---|---|---|---|
| ZnO:Fe3+ | 38.9 ± 0.5 | 54.7 ± 0.4 | 1.4 ± 0.1 | 5.1 ± 0.4 | - |
| ZnO:Fe3+,2+ | 22.0 ± 0.4 | 65.8 ± 0.5 | 0.4 ± 0.0 | 11.6 ± 0.8 | 0.1 ± 0.0 |
| Zn | O | Fe | N | Cl | |
|---|---|---|---|---|---|
| ZnO:Fe3+ | 37.9 | 59.0 | 0.8 | 2.3 | - |
| ZnO:Fe3+,2+ | 42.0 | 52.3 | 0.3 | 1.8 | 3.6 |
| Gene | Primer Sequence 5′-3′ | |
|---|---|---|
| Forward | Reverse | |
| Hamp | TCTCCTGCTTCTCCTCCTTG | CAATGTCTGCCCTGCTTTCT |
| Hprt | AGGGAGAGCGTTGGGCTTAC | TCGCTAATCACGACGCTGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbik, P.; Jończy, A.; Kaszewski, J.; Gralak, M.; Rosowska, J.; Sapierzyński, R.; Witkowski, B.; Wachnicki, Ł.; Lawniczak-Jablonska, K.; Kuzmiuk, P.; et al. Biodegradable Zinc Oxide Nanoparticles Doped with Iron as Carriers of Exogenous Iron in the Living Organism. Pharmaceuticals 2021, 14, 859. https://doi.org/10.3390/ph14090859
Kiełbik P, Jończy A, Kaszewski J, Gralak M, Rosowska J, Sapierzyński R, Witkowski B, Wachnicki Ł, Lawniczak-Jablonska K, Kuzmiuk P, et al. Biodegradable Zinc Oxide Nanoparticles Doped with Iron as Carriers of Exogenous Iron in the Living Organism. Pharmaceuticals. 2021; 14(9):859. https://doi.org/10.3390/ph14090859
Chicago/Turabian StyleKiełbik, Paula, Aneta Jończy, Jarosław Kaszewski, Mikołaj Gralak, Julita Rosowska, Rafał Sapierzyński, Bartłomiej Witkowski, Łukasz Wachnicki, Krystyna Lawniczak-Jablonska, Piotr Kuzmiuk, and et al. 2021. "Biodegradable Zinc Oxide Nanoparticles Doped with Iron as Carriers of Exogenous Iron in the Living Organism" Pharmaceuticals 14, no. 9: 859. https://doi.org/10.3390/ph14090859
APA StyleKiełbik, P., Jończy, A., Kaszewski, J., Gralak, M., Rosowska, J., Sapierzyński, R., Witkowski, B., Wachnicki, Ł., Lawniczak-Jablonska, K., Kuzmiuk, P., Lipiński, P., Godlewski, M., & Godlewski, M. M. (2021). Biodegradable Zinc Oxide Nanoparticles Doped with Iron as Carriers of Exogenous Iron in the Living Organism. Pharmaceuticals, 14(9), 859. https://doi.org/10.3390/ph14090859








