The Role of Dupilumab in Severe Asthma
Abstract
:1. Introduction
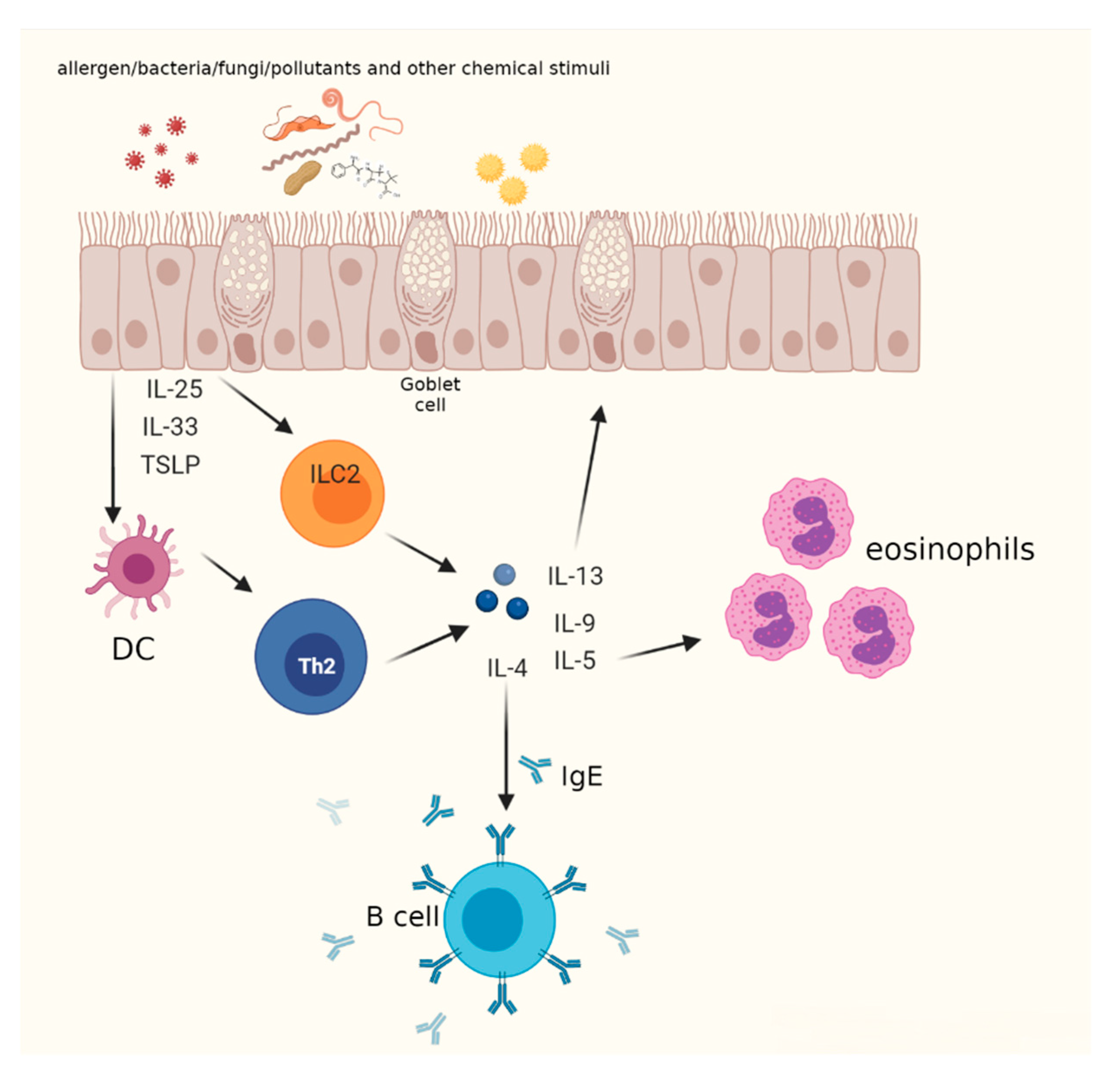
2. T2 High Mechanisms
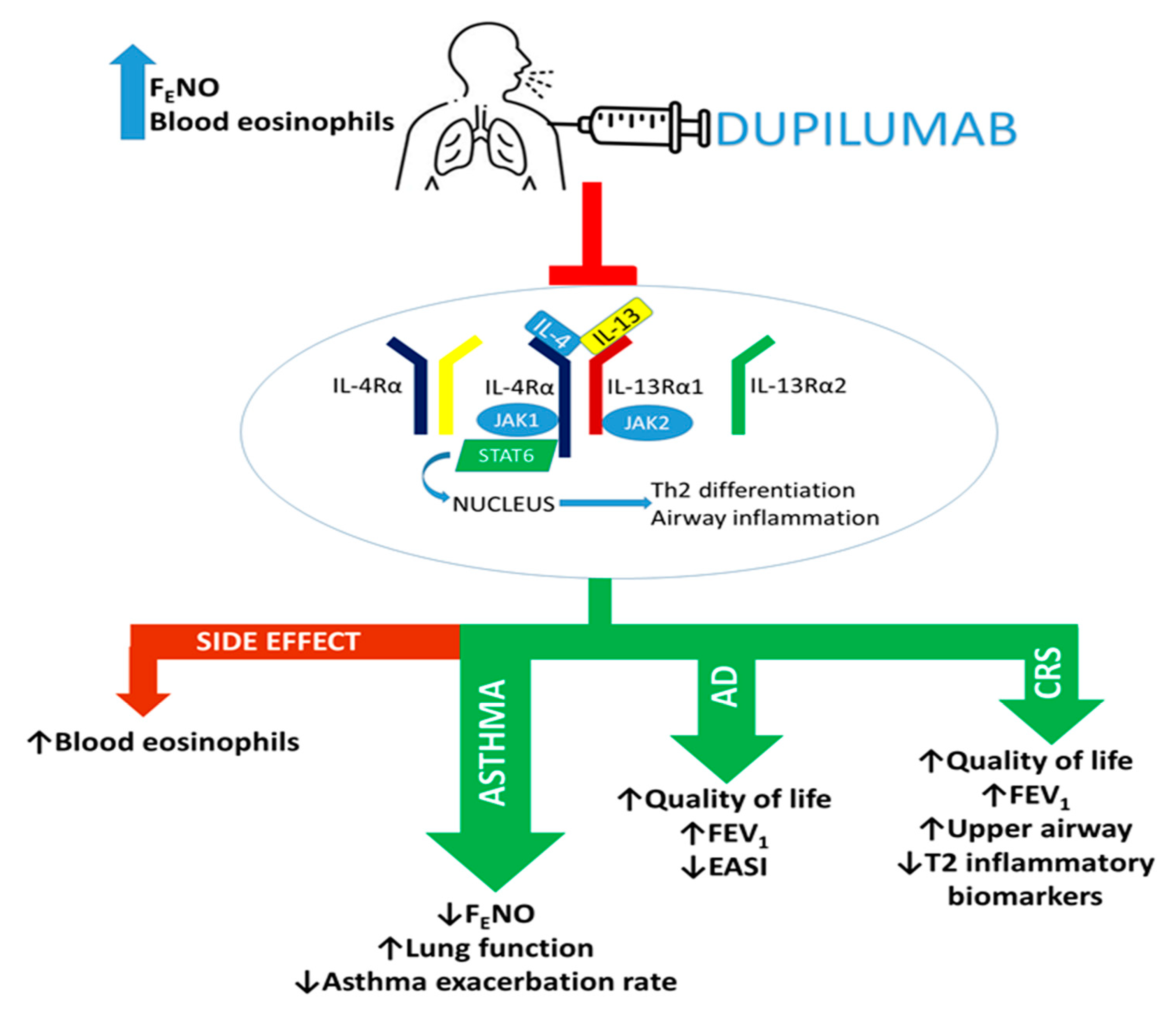
3. Mechanism of Action of Dupilumab
4. Dupilumab Efficacy in Severe Asthma
5. Dupilumab Efficacy in Asthma Comorbidity and Coexisting Conditions
6. Dupilumab Safety in T2 Diseases
7. Dupilumab Effects in Selected Populations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2017. Available online: www.ginasthma.org (accessed on 1 February 2021).
- Carr, T.F.; Zeki, A.A.; Kraft, M. Eosinophilic and Noneosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sabatini, F.; Sorbello, V.; Benedetto, S.; Defilippi, I.; Petecchia, L.; Usai, C.; Gnemmi, I.; Balbi, B.; De Rose, V.; et al. Expression of vascular remodelling markers in relation to bradykinin receptors in asthma and COPD. Thorax 2013, 68, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Rackemann, F.M. A working classification of asthma. Am. J. Med. 1947, 3, 601–606. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster Analysis and Clinical Asthma Phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; Jr, R.D.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.; et al. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [Green Version]
- Heffler, E.; Blasi, F.; Latorre, M.; Menzella, F.; Paggiaro, P.; Pelaia, G.; Senna, G.; Canonica, G.W.; Barbuto, S.; Bradicich, M.; et al. The Severe Asthma Network in Italy: Findings and Perspectives. J. Allergy Clin. Immunol. Pr. 2019, 7, 1462–1468. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Vatrella, A.; Tinello, C.; Terracciano, R.; Pelaia, G. Molecular Targets for Biological Therapies of Severe Asthma. Front. Immunol. 2020, 11, 11–603312. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nat. Cell Biol. 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Bakakos, A.; Loukides, S. Severe Eosinophilic Asthma. J. Clin. Med. 2019, 8, 1375. [Google Scholar] [CrossRef] [Green Version]
- Van Hulst, G.; Batugedara, H.M.; Jorssen, J.; Louis, R.; Bureau, F.; Desmet, C.J. Eosinophil diversity in asthma. Biochem. Pharmacol. 2020, 179, 113963. [Google Scholar] [CrossRef]
- Busse, W.W. Biological treatments for severe asthma: A major advance in asthma care. Allergol. Int. 2019, 68, 158–166. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Le Floch-Ramondou, A.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Murphy, A.; Sleeman, M.; Orengo, J. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. J. Allergy Clin. Immunol. 2020, 145, AB158. [Google Scholar] [CrossRef]
- Boyce, J.A.; Mellor, E.A.; Perkins, B.; Lim, Y.-C.; Luscinskas, F.W. Human mast cell progenitors use α4-integrin, VCAM-1, and PSGL-1 E-selectin for adhesive interactions with human vascular endothelium under flow conditions. Blood 2002, 99, 2890–2896. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.; Humbert, M.; Buhl, R.; Cruz, A.A.; Inoue, H.; Korom, S.; Hanania, N.A.; Nair, P. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 2017, 47, 161–175. [Google Scholar] [CrossRef]
- Medrek, S.; Parulekar, A.D.; Hanania, N.A. Predictive Biomarkers for Asthma Therapy. Curr. Allergy Asthma Rep. 2017, 17, 69. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Silkoff, P.E. Perspectives on exhaled nitric oxide. J. Breath Res. 2017, 11, 047104. [Google Scholar] [CrossRef] [Green Version]
- Ul-Haq, Z.; Naz, S.; Mesaik, M.A. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor Rev. 2016, 32, 3–15. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Gallelli, L.; Terracciano, R.; Navalesi, P.; Maselli, R.; Pelaia, G. Dupilumab for the treatment of asthma. Expert Opin. Biol. Ther. 2017, 17, 1565–1572. [Google Scholar] [CrossRef]
- Matsunaga, K.; Katoh, N.; Fujieda, S.; Izuhara, K.; Oishi, K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol. Int. 2020, 69, 187–196. [Google Scholar] [CrossRef]
- LaPorte, S.L.; Juo, Z.S.; Vaclavikova, J.; Colf, L.A.; Qi, X.; Heller, N.M.; Keegan, A.D.; Garcia, K.C. Molecular and Structural Basis of Cytokine Receptor Pleiotropy in the Interleukin-4/13 System. Cell 2008, 132, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, T.R.; Pesce, J.T.; Sheikh, F.; Cheever, A.W.; Mentink-Kane, M.M.; Wilson, M.S.; Stevens, S.; Valenzuela, D.M.; Murphy, A.; Yancopoulos, G.D.; et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat. Immunol. 2007, 9, 25–33. [Google Scholar] [CrossRef]
- Moran, A.; Pavord, I.D. Anti-IL-4/IL-13 for the treatment of asthma: The story so far. Expert Opin. Biol. Ther. 2020, 20, 283–294. [Google Scholar] [CrossRef]
- Hart, T.K.; Blackburn, M.N.; Brigham-Burke, M.; DeDe, K.; Al-Mahdi, N.; Zia-Amirhosseini, P.; Cook, R.M. Preclinical efficacy and safety of pascolizumab (SB 240683): A humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin. Exp. Immunol. 2002, 130, 93–100. [Google Scholar] [CrossRef]
- Borish, L.C.; Nelson, H.S.; Lanz, M.J.; Claussen, L.; Whitmore, J.B.; Agosti, J.M.; Garrison, L. Interleukin-4 Receptor in Moderate Atopic Asthma. Am. J. Respir. Crit. Care Med. 1999, 160, 1816–1823. [Google Scholar] [CrossRef]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab Treatment in Adults with Asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef] [Green Version]
- Hanania, N.A.; Noonan, M.; Corren, J.; Korenblat, P.; Zheng, Y.; Fischer, S.K.; Cheu, M.; Putnam, W.S.; Murray, E.; Scheerens, H.; et al. Lebrikizumab in moderate-to-severe asthma: Pooled data from two randomised placebo-controlled studies. Thorax 2015, 70, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.J.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Piper, E.; Brightling, C.; Niven, R.; Oh, C.; Faggioni, R.; Poon, K.; She, D.; Kell, C.; May, R.; Geba, G.P.; et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur. Respir. J. 2012, 41, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.; Chanez, P.; Leigh, R.; O’Byrne, P.; Korn, S.; She, D.; May, R.; Streicher, K.; Ranade, K.; Piper, E. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 692–701. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Sjöbring, U.; Péterffy, A.; Wessman, P.; Bowen, K.; Piper, E.; Colice, G.; Brightling, C. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): Two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir. Med. 2018, 6, 511–525. [Google Scholar] [CrossRef] [Green Version]
- Bourdin, A.; Papi, A.A.; Corren, J.; Virchow, J.C.; Rice, M.S.; Deniz, Y.; Djandji, M.; Rowe, P.; Pavord, I.D. Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline. Allergy 2021, 76, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Maspero, J.F.; Rabe, K.F.; Papi, A.; Wenzel, S.; Ford, L.B.; Pavord, I.D.; Zhang, B.; Staudinger, H.; Pirozzi, G.; et al. Liberty Asthma QUEST: Phase 3 Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate Dupilumab Efficacy/Safety in Patients with Uncontrolled, Moderate-to-Severe Asthma. Adv. Ther. 2018, 35, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy Clin. Immunol. Pr. 2020, 8, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Dupin, C.; Belhadi, D.; Guilleminault, L.; Gamez, A.; Berger, P.; De Blay, F.; Bonniaud, P.; Leroyer, C.; Mahay, G.; Girodet, P.; et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin. Exp. Allergy 2020, 50, 789–798. [Google Scholar] [CrossRef]
- Mümmler, C.; Munker, D.; Barnikel, M.; Veit, T.; Kayser, M.Z.; Welte, T.; Behr, J.; Kneidinger, N.; Suhling, H.; Milger, K. Dupilumab Improves Asthma Control and Lung Function in Patients with Insufficient Outcome During Previous Antibody Therapy. J. Allergy Clin. Immunol. Pr. 2021, 9, 1177–1185.e4. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Levra, S.; Sprio, A.E.; Bertolini, F.; Carriero, V.; Gallo, F.; Ciprandi, G. A real-world assessment of asthma with chronic rhinosinusitis. Ann. Allergy Asthma Immunol. 2020, 125, 65–71. [Google Scholar] [CrossRef]
- Ravnborg, N.; Ambikaibalan, D.; Agnihotri, G.; Price, S.; Rastogi, S.; Patel, K.R.; Singam, V.; Andersen, Y.; Halling, A.-S.; Silverberg, J.I.; et al. Prevalence of asthma in patients with atopic dermatitis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 471–478. [Google Scholar] [CrossRef]
- Ledford, D.K.; Lockey, R.F. Asthma and comorbidities. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Maspero, J.F.; Lu, Y.; Corren, J.; Hanania, N.A.; Chipps, B.E.; Katelaris, C.H.; FitzGerald, J.M.; Quirce, S.; Ford, L.B.; et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann. Allergy, Asthma Immunol. 2020, 125, 565–576.e1. [Google Scholar] [CrossRef] [PubMed]
- Samitas, K.; Carter, A.; Kariyawasam, H.H.; Xanthou, G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy 2017, 73, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef]
- Maspero, J.F.; Katelaris, C.H.; Busse, W.W.; Castro, M.; Corren, J.; Chipps, B.E.; Peters, A.T.; Pavord, I.D.; Ford, L.B.; Sher, L.; et al. Dupilumab Efficacy in Uncontrolled, Moderate-to-Severe Asthma with Self-Reported Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pr. 2020, 8, 527–539.e9. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Hellings, P.W.; Mullol, J.; Naclerio, R.M.; Chao, J.; Amin, N.; Grabher, A.; Swanson, B.N.; Hamilton, J.D.; Guillonneau, S.; et al. Dupilumab improves patient-reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. J. Allergy Clin. Immunol. Pr. 2019, 7, 2447–2449.e2. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Bachert, C.; Amin, N.; Desrosiers, M.; Hellings, P.W.; Mullol, J.; Maspero, J.F.; Gevaert, P.; Zhang, M.; Mao, X.; et al. Dupilumab improves upper and lower airway disease control in chronic rhinosinusitis with nasal polyps and asthma. Ann. Allergy Asthma Immunol. 2021, 126, 584–592.e1. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Brehler, A.-C.; Novak, N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzecry, V.; Pravettoni, V.; Segatto, G.; Marzano, A.; Ferrucci, S. Type 2 Inflammation: Atopic Dermatitis, Asthma, and Hypereosinophilia Successfully Treated With Dupilumab. J. Investig. Allergol. Clin. Immunol. 2021, 31, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Tolino, E.; Proietti, I.; Sarni, A.; Bernardini, N.; Mambrin, A.; Balduzzi, V.; Maddalena, P.; Marchesiello, A.; Michelini, S.; Volpe, S.; et al. Success of dupilumab as a monotherapy in an adult patient affected by severe uncontrolled asthma and atopic dermatitis. Dermatol. Ther. 2021, 34, e14596. [Google Scholar] [CrossRef]
- Paller, A.S.; Siegfried, E.C.; Thaçi, D.; Wollenberg, A.; Cork, M.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef]
- Ramratnam, S.K.; Bacharier, L.B.; Guilbert, T.W. Severe Asthma in Children. J. Allergy Clin. Immunol. Pr. 2017, 5, 889–898. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M. Severe Asthma in Children: Lessons Learned and Future Directions. J. Allergy Clin. Immunol. Pr. 2016, 4, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Licari, A.; Castagnoli, R.; Marseglia, A.; Olivero, F.; Votto, M.; Ciprandi, G.; Marseglia, G.L. Dupilumab to Treat Type 2 Inflammatory Diseases in Children and Adolescents. Pediatr. Drugs 2020, 22, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Ruggiero, A.; Fontanella, G.; Fabbrocini, G.; Patruno, C. New emergent therapies for atopic dermatitis: A review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol. Ther. 2021, 34, e14475. [Google Scholar] [CrossRef] [PubMed]
- Kage, P.; Simon, J.; Treudler, R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Dupixent (Dupilumab) Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf (accessed on 23 July 2021).
| Authors | Population | Summary of Outcomes | |
|---|---|---|---|
| DUPILUMAB | Wenzel et al. [41] | 104 adults with persistent, moderate-to-severe asthma who used ICS and LABAs, characterized by elevated blood (≥300 cells/µL) and sputum eosinophil levels (≥3%). | ↓ asthma exacerbations rate and β-agonist use, improved FEV1, ACQ5 score, and asthma symptoms. ↓ levels of FENO, serum IgE, plasma eotaxin-3, and TARC. |
| Wenzel et al. [42] | 769 patients with uncontrolled persistent asthma on medium-to-high-dose ICS plus a LABA | Improvement of FEV1 in patients with blood eosinophils (≥ 300 cells/µL) and higher ICS dose therapy. | |
| Castro et al. [43] | 1900 patients with uncontrolled asthma | Highest efficacy in patients with elevated blood eosinophils (≥150 cells/µL) and FENO (≥25 ppb): preventing asthma exacerbations and improving FEV1. | |
| Busse et al. [44] | 1902 patients characterized by uncontrolled, moderate-to-severe asthma who were receiving continuous treatment with ICS plus one or two other asthma controller medications | Improvement in lung function, quality of life, asthma control, and severe exacerbation rate. | |
| Rabe et al. [45] | 210 severe asthmatic patients who used OCS to maintain control of asthma | ↓ OCS use without loss of asthma control, ↓ asthma exacerbation rate, and lung function improvement. Asthmatics with higher blood eosinophils (≥300 cells/µL) had ↓ of exacerbations (71%) | |
| Corren et al. [46] | 1902 uncontrolled moderate-to-severe asthmatics: 1083, allergic asthma and 819 non-allergic asthma. | ↓ annualized rate of severe asthma exacerbations, serum IgE concentrations, and FENO levels and improved ACQ5 score and FEV1 in allergic asthmatic group with IgE levels IgE ≥700 UI/mL. In allergic moderate-to-severe asthmatics, dupilumab determined better clinical outcomes and decreased levels of specific T2 inflammatory biomarkers. | |
| Bourdin et al. [40] | 465 asthmatics who used high (>1000 μg/day)- or medium (500–1000 μg/day)-dose ICS plus LABA; and patients of QUEST study [43] | ↓ severe asthma exacerbation rate and improved asthma control and FEV1. Patients with a high baseline concentration of at least one T2 biomarker, such as FENO (≥25ppb) and blood eosinophil counts (≥150 or ≥300 cells/µL), had a better outcome in lung function. | |
| Dupin et al. [47] | 64 uncontrolled severe asthma patients | ↑ asthma control, FEV1, and a reduction of the OCS dose intake. | |
| Mümmler et al. [48] | 38 severe asthmatics treated previously with other biologic therapies without achieving a better clinical response | Asthmatics had improvement when switched from omalizubam, benralizumab, or mepolizumab to dupilumab. After 3 to 6 months of treatment: ↑ asthma control, lung function, and ↓ exacerbation rate and FENO and IgE levels. |
| Authors | Population | Summary of Outcomes |
|---|---|---|
| Busse et al. [52] | 814 uncontrolled, moderate-to-severe asthmatics with perennial allergic rhinitis determined by a history of allergic rhinitis and sensitization to one or more perennial aeroallergen-specific IgEs (≥0.35 kU/L) at baseline | Rapid improvement in the key standard asthma outcomes analyzed, except for the severe exacerbation rate; better score concerning the HRQoL questionnaire; reduction in total serum IgE, FENO, and TARC. |
| Maspero et al. [55] | 1897 asthmatic patients with or without self-reported CRS (both CRSwNP and CRSsNP) | Patients with CRS showed ↓ in severe asthma exacerbations rate and ↑FEV1. |
| Bachert et al. [56] | 60 patients with CRSwNP and comorbid asthma | ↑ nasal polyp burden, asthma control, FEV1, HRQoL, and the patient perception of general health and physical functioning. |
| Laidlaw et al. [57] | 724 patients with CRSwNP with or without comorbid asthma | ↑ FEV1, ACQ-6 score, HRQoL; ↓ upper airway obstruction, |
| Benzecry et al. [59] | 1 adult patient affected by severe uncontrolled asthma and atopic dermatitis | ↑ in Eczema Area and Severity Index, a better quality of life, and raised in FEV1 |
| Tolino et al. [60] | 1 adult patient affected by severe uncontrolled asthma and atopic dermatitis | ↑ in Eczema Area and Severity Index, a better quality of life, and raised in FEV1 |
| Dupilumab Dosage | Adverse Events |
|---|---|
| 300 mg once weekly [41] | Injection-site reaction |
| 200 or 300 mg every 2 or 4 weeks [42,55] | Headache |
| 200 or 300 mg every 2 weeks [43] | Upper respiratory tract infections |
| 300 mg every 2 weeks [45,47] | Nasopharyngitis |
| 200 or 300 mg every 2 weeks [43] | Increase in eosinophils counts |
| 300 mg every 2 weeks [45,47] | |
| 300 mg every 2 weeks [45,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardolo, F.L.M.; Bertolini, F.; Carriero, V. The Role of Dupilumab in Severe Asthma. Biomedicines 2021, 9, 1096. https://doi.org/10.3390/biomedicines9091096
Ricciardolo FLM, Bertolini F, Carriero V. The Role of Dupilumab in Severe Asthma. Biomedicines. 2021; 9(9):1096. https://doi.org/10.3390/biomedicines9091096
Chicago/Turabian StyleRicciardolo, Fabio Luigi Massimo, Francesca Bertolini, and Vitina Carriero. 2021. "The Role of Dupilumab in Severe Asthma" Biomedicines 9, no. 9: 1096. https://doi.org/10.3390/biomedicines9091096
APA StyleRicciardolo, F. L. M., Bertolini, F., & Carriero, V. (2021). The Role of Dupilumab in Severe Asthma. Biomedicines, 9(9), 1096. https://doi.org/10.3390/biomedicines9091096






