Omalizumab Restores Response to Corticosteroids in Patients with Eosinophilic Chronic Rhinosinusitis and Severe Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Cell Preparation
2.3. Corticosteroid Sensitivity
2.4. Quantitative RT-PCR
2.5. Cell Lysis, Immunoprecipitation, and Fluorometric Assay
2.6. Immunoassay for CCL4, IL-13, and CD69
2.7. RNA Interference
2.8. Immunofluorescence Staining
2.9. Cell Survival
2.10. Viscoelasticity
2.11. Statistical Analysis
3. Results
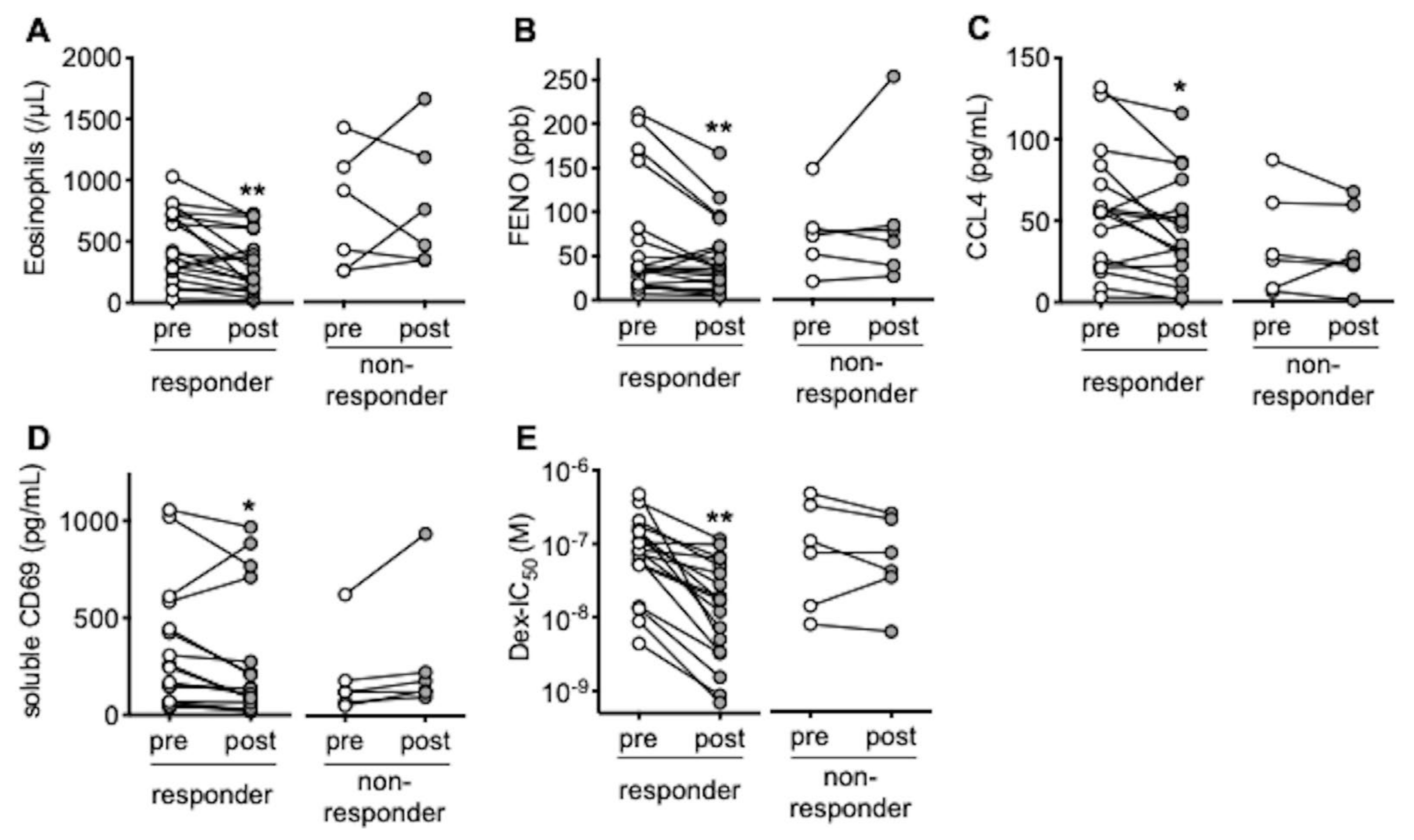
3.1. In Responders to Omalizumab, Blood Eosinophils and FENO Are Reduced with Restoration of Response to Corticosteroids
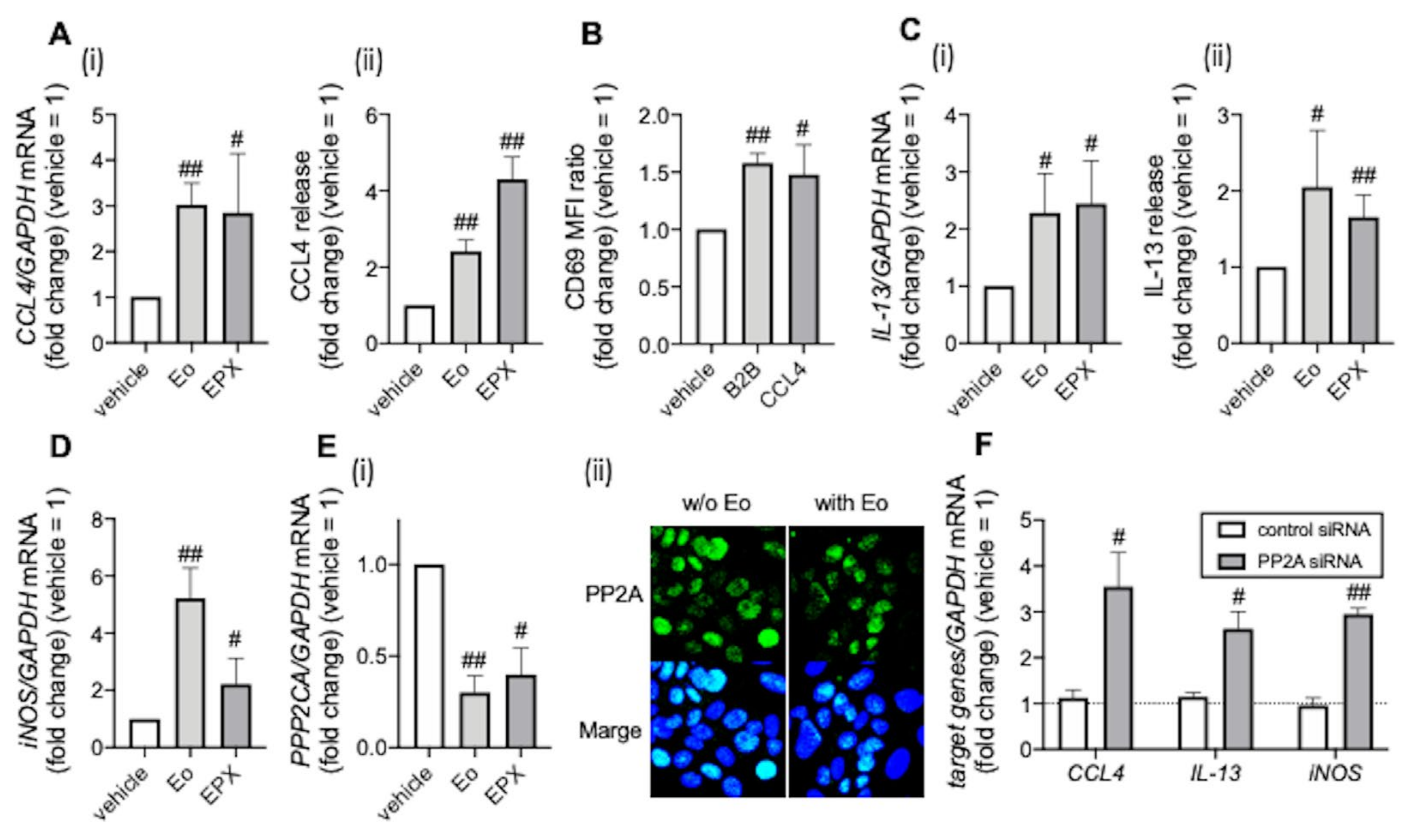
3.2. PP2A Is Associated with Eosinophilic Airway Inflammation
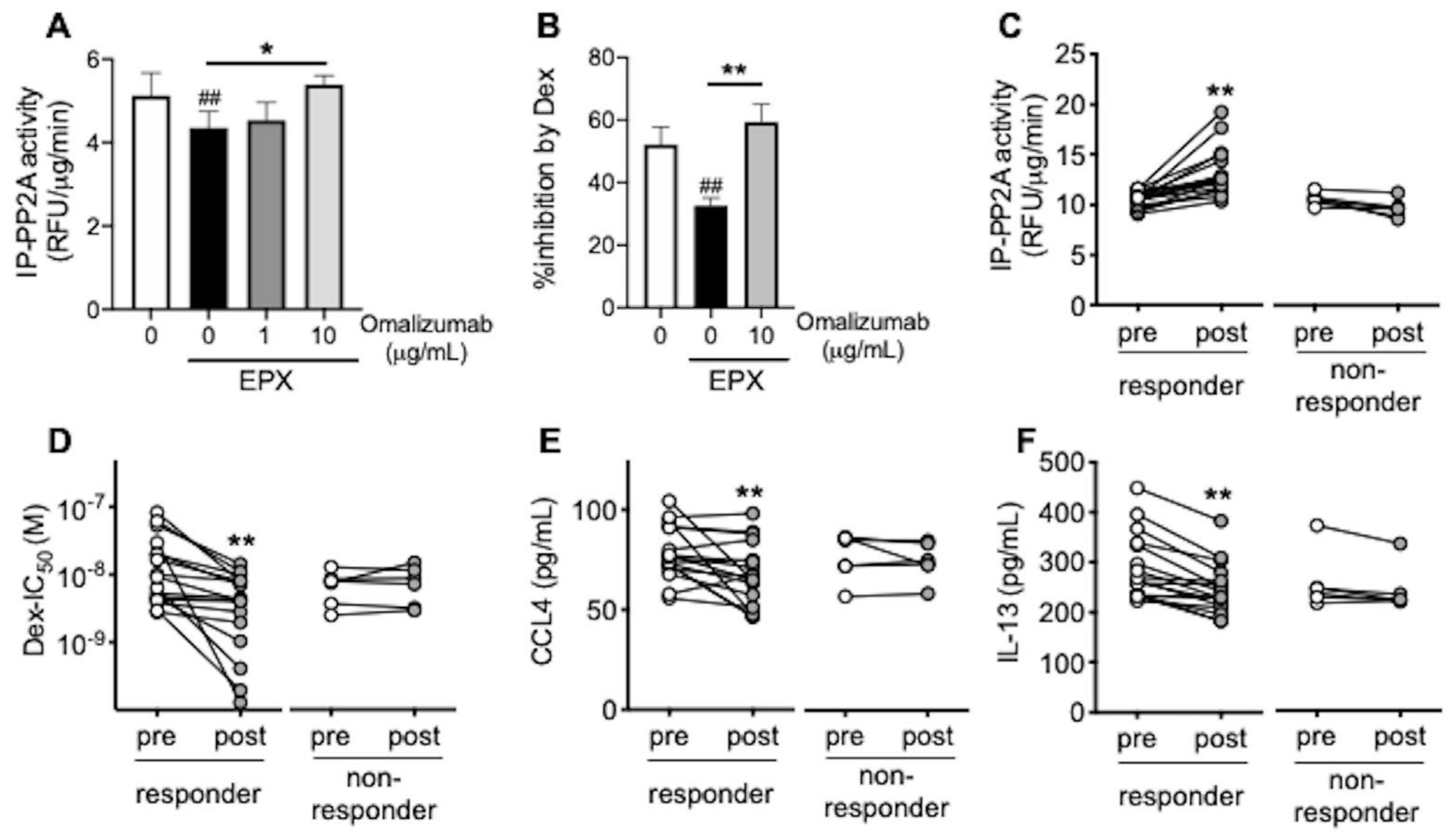
3.3. Omalizumab Restores PP2A Activity and Corticosteroid Sensitivity in Airway Epithelial Cells
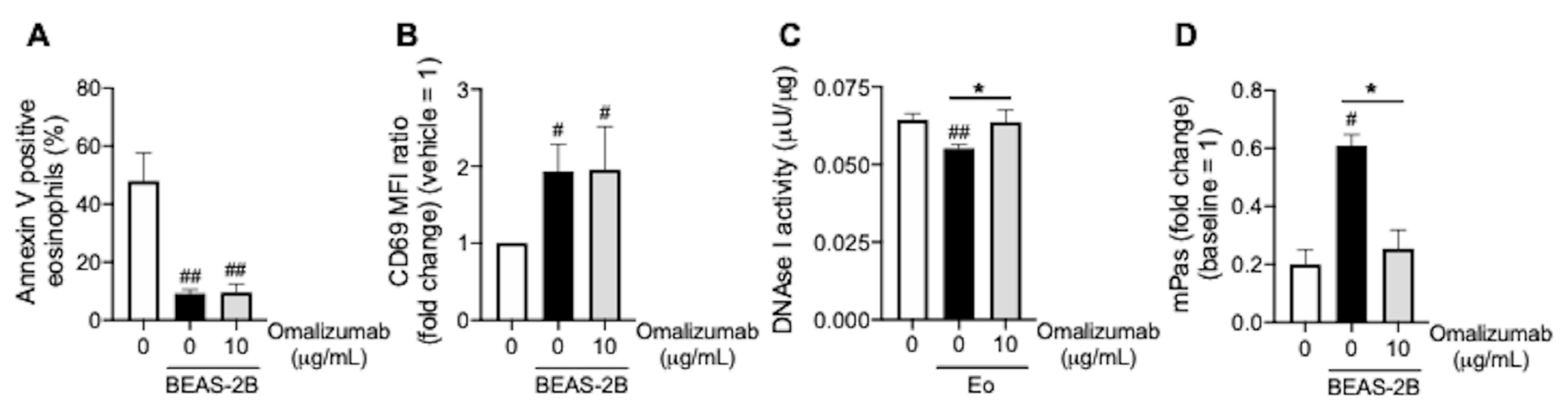
3.4. Omalizumab Promotes Mucin Decomposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ishidoya, J.; Sakuma, Y.; Tsukuda, M. Eosinophilic chronic rhinosinusitis in Japan. Allergol. Int. 2010, 59, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Fujieda, S.; Imoto, Y.; Kato, Y.; Ninomiya, T.; Tokunaga, T.; Tsutsumiuchi, T.; Yoshida, K.; Kidoguchi, M.; Takabayashi, T. Eosinophilic chronic rhinosinusitis. Allergol. Int. 2019, 68, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Asako, M.; Ooka, H.; Kanda, A.; Tomoda, K.; Yasuba, H. Residual exhaled nitric oxide elevation in asthmatics is associated with eosinophilic chronic rhinosinusitis. J. Asthma. 2015, 52, 1060–1064. [Google Scholar] [CrossRef]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Yasuba, H.; Asako, M.; Yamamoto, T.; Takano, H.; Tomoda, K.; Kanda, A.; Iwai, H. HFA-BDP Metered-Dose Inhaler Exhaled Through the Nose Improves Eosinophilic Chronic Rhinosinusitis with Bronchial Asthma: A Blinded, Placebo-Controlled Study. Front. Immunol. 2018, 9, 2192. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Asako, M.; Kanda, A.; Tomoda, K.; Yasuba, H. A novel therapeutic use of HFA-BDP metereddose inhaler for asthmatic patients with rhinosinusitis: Case series. Int. J. Clin. Pharmacol. Ther. 2014, 52, 914–919. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Asako, M.; Yamamoto, T.; Yasuba, H.; Tomoda, K.; Kanda, A. Replacement of SFC-DPI with SFC-MDI exhaled through the nose improves eosinophilic chronic rhinosinusitis in patients with bronchial asthma. Int. J. Clin. Pharmacol. Ther. 2017, 55, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Peiro, T.; Armengot, M.; Frias, S.; Morell, A.; Serrano, A.; Cortijo, J. Mucin 1 downregulation associates with corticosteroid resistance in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2015, 135, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kanda, A.; Yun, Y.; Dan Van, B.; Suzuki, K.; Sawada, S.; Asako, M.; Iwai, H. Reduced Local Response to Corticosteroids in Eosinophilic Chronic Rhinosinusitis with Asthma. Biomolecules 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; van Cauwenberge, P.; et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J. Allergy Clin. Immunol. 2013, 131, 110–116. [Google Scholar] [CrossRef]
- Tiotiu, A.; Oster, J.P.; Roux, P.R.; Nguyen Thi, P.L.; Peiffer, G.; Bonniaud, P.; Dalphin, J.C.; de Blay, F. Effectiveness of Omalizumab in Severe Allergic Asthma and Nasal Polyposis: A Real-Life Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bidder, T.; Sahota, J.; Rennie, C.; Lund, V.J.; Robinson, D.S.; Kariyawasam, H.H. Omalizumab treats chronic rhinosinusitis with nasal polyps and asthma together-a real life study. Rhinology 2018, 56, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Mercado, N.; Barnes, P.J.; Ito, K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS One 2011, 6, e27627. [Google Scholar] [CrossRef]
- Cloutier, M.M.; Baptist, A.P.; Blake, K.V.; Brooks, E.G.; Bryant-Stephens, T.; DiMango, E.; Dixon, A.E.; Elward, K.S.; Hartert, T.; Krishnan, J.A.; et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J. Allergy Clin. Immunol. 2020, 146, 1217–1270. [Google Scholar] [CrossRef]
- Bousquet, J.; Brusselle, G.; Buhl, R.; Busse, W.W.; Cruz, A.A.; Djukanovic, R.; Domingo, C.; Hanania, N.A.; Humbert, M.; Menzies Gow, A.; et al. Care pathways for the selection of a biologic in severe asthma. Eur. Respir. J. 2017, 50, 1701782. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Adinoff, A.D.; Bachert, C.; Borish, L.; Chinchilli, V.M.; et al. Rhinosinusitis: Developing guidance for clinical trials. J. Allergy Clin. Immunol. 2006, 118, S17–S61. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Jia, C.E.; Zhang, H.P.; Lv, Y.; Liang, R.; Jiang, Y.Q.; Powell, H.; Fu, J.J.; Wang, L.; Gibson, P.G.; Wang, G. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013, 131, 695–703. [Google Scholar] [CrossRef]
- Lloyd, A.; Turk, F.; Leighton, T.; Walter Canonica, G. Psychometric evaluation of Global Evaluation of Treatment Effectiveness: A tool to assess patients with moderate-to-severe allergic asthma. J. Med. Econ. 2007, 10, 285–296. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Konno, Y.; Kanda, A.; Yamada, Y.; Yasuba, H.; Sakata, Y.; Fukuchi, M.; Tomoda, K.; Iwai, H.; Ueki, S. Critical role of CCL4 in eosinophil recruitment into the airway. Clin. Exp. Allergy 2019, 49, 853–860. [Google Scholar] [CrossRef]
- Nishikawa, K.; Morii, T.; Ako, H.; Hamada, K.; Saito, S.; Narita, N. In vivo expression of CD69 on lung eosinophils in eosinophilic pneumonia: CD69 as a possible activation marker for eosinophils. J. Allergy Clin. Immunol. 1992, 90, 169–174. [Google Scholar] [CrossRef]
- Suresh, V.; Mih, J.D.; George, S.C. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2007, 37, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Noga, O.; Hanf, G.; Brachmann, I.; Klucken, A.C.; Kleine-Tebbe, J.; Rosseau, S.; Kunkel, G.; Suttorp, N.; Seybold, J. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J. Allergy Clin. Immunol. 2006, 117, 1493–1499. [Google Scholar] [CrossRef]
- Holgate, S.; Casale, T.; Wenzel, S.; Bousquet, J.; Deniz, Y.; Reisner, C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 2005, 115, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Martin, R.J.; Szefler, S.J.; Sher, E.R.; Ying, S.; Kay, A.B.; Hamid, Q. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J. Exp. Med. 1995, 181, 33–40. [Google Scholar] [CrossRef]
- van Rensen, E.L.; Evertse, C.E.; van Schadewijk, W.A.; van Wijngaarden, S.; Ayre, G.; Mauad, T.; Hiemstra, P.S.; Sterk, P.J.; Rabe, K.F. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: Effect of anti-IgE treatment. Allergy 2009, 64, 72–80. [Google Scholar] [CrossRef]
- Maurer, M.; von Stebut, E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell. Biol. 2004, 36, 1882–1886. [Google Scholar] [CrossRef]
- Yun, Y.; Kanda, A.; Kobayashi, Y.; Van Bui, D.; Suzuki, K.; Sawada, S.; Baba, K.; Yagi, M.; Asako, M.; Okazaki, H.; et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol. Int. 2020, 69, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Nonaka, M.; Tagaya, E.; Tamaoki, J.; Yoshihara, T. Eosinophilic otitis media is associated with asthma severity and smoking history. ORL J. Otorhinolaryngol. Relat. Spec. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Ueki, S.; Ohta, N.; Takeda, M.; Konno, Y.; Hirokawa, M. Eosinophilic Otitis Media: The Aftermath of Eosinophil Extracellular Trap Cell Death. Curr. Allergy Asthma Rep. 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Takahashi, E.; Ida, S.; Kikuchi, S. Clinical efficacy of anti-IL-5 monoclonal antibody mepolizumab in the treatment of eosinophilic otitis media. Auris Nasus Larynx 2019, 46, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Sekine, Y.; Yoshida, S.; Kikuchi, S. Dupilumab therapy for patients with refractory eosinophilic otitis media associated with bronchial asthma. Auris Nasus Larynx 2021, 48, 353–360. [Google Scholar] [CrossRef] [PubMed]
| Responder (n = 19) | Non-Responder (n = 6) | |
|---|---|---|
| Age | 52.1 ± 13.1 | 54.0 ± 15.1 |
| Gender (M/F) | 8/11 | 2/4 |
| Body mass index | 23.6 ± 3.8 | 21.3 ± 3.6 |
| NSAIDs intolerance | 8 | 2 |
| EOM * | 4 | 5 |
| Smoking history (never/ex) | 13/6 | 4/2 |
| ESS history (Y/N) | 17/2 | 6/0 |
| Total IgE (IU/mL) | 429 ± 403 | 401 ± 275 |
| Positive RAST (single/multi) | 2/17 | 1/5 |
| Eosinophils (/μL) [peak value] | 466 ± 287 [699 ± 328] | 741 ± 495 [1022 ± 457] |
| FENO (ppb) | 65.2 ± 67.6 | 75.8 ± 42.4 |
| Lund-Mackay scale | 16.0 ± 4.5 | 15.3 ± 4.8 |
| Polyp score | 4.6 ± 1.6 | 4.7 ± 2.0 |
| JESREC score | 15.1 ± 1.7 | 16.7 ± 0.8 |
| Impaired sense of smell | 13 | 5 |
| FEV1%pred. | 82.6 ± 18.2 | 77.6 ± 22.7 |
| FEF25–75%pred. | 55.5 ± 30.4 | 48.3 ± 25.9 |
| FVC %pred. | 93.9 ± 15.8 | 96.0 ± 11.8 |
| Asthma Control Test | 21.3 ± 3.3 | 20.7 ± 5.1 |
| Asthma exacerbation (per year) ** | 1.4 ± 1.5 | 1.2 ± 0.4 |
| Treatment | ||
| Inhaled corticosteroids (μg) *** | 1200 ± 330 | 1133 ± 350 |
| LABA | 19 | 6 |
| LAMA | 5 | 1 |
| LTRA | 16 | 4 |
| Theophylline | 3 | 1 |
| Anti-histamine | 7 | 3 |
| Inhaled nasal corticosteroids | 12 | 3 |
| Oral corticosteroids | 2 | 0 |
| Omalizumab (mg) (per month) | 426 ± 251 | 425 ± 148 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Kanda, A.; Bui, D.V.; Yun, Y.; Nguyen, L.M.; Chu, H.H.; Mitani, A.; Suzuki, K.; Asako, M.; Iwai, H. Omalizumab Restores Response to Corticosteroids in Patients with Eosinophilic Chronic Rhinosinusitis and Severe Asthma. Biomedicines 2021, 9, 787. https://doi.org/10.3390/biomedicines9070787
Kobayashi Y, Kanda A, Bui DV, Yun Y, Nguyen LM, Chu HH, Mitani A, Suzuki K, Asako M, Iwai H. Omalizumab Restores Response to Corticosteroids in Patients with Eosinophilic Chronic Rhinosinusitis and Severe Asthma. Biomedicines. 2021; 9(7):787. https://doi.org/10.3390/biomedicines9070787
Chicago/Turabian StyleKobayashi, Yoshiki, Akira Kanda, Dan Van Bui, Yasutaka Yun, Linh Manh Nguyen, Hanh Hong Chu, Akitoshi Mitani, Kensuke Suzuki, Mikiya Asako, and Hiroshi Iwai. 2021. "Omalizumab Restores Response to Corticosteroids in Patients with Eosinophilic Chronic Rhinosinusitis and Severe Asthma" Biomedicines 9, no. 7: 787. https://doi.org/10.3390/biomedicines9070787
APA StyleKobayashi, Y., Kanda, A., Bui, D. V., Yun, Y., Nguyen, L. M., Chu, H. H., Mitani, A., Suzuki, K., Asako, M., & Iwai, H. (2021). Omalizumab Restores Response to Corticosteroids in Patients with Eosinophilic Chronic Rhinosinusitis and Severe Asthma. Biomedicines, 9(7), 787. https://doi.org/10.3390/biomedicines9070787







