Assessing Forage Potential of the Global Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm Conserved at the ICRISAT Genebank
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germplasm Details
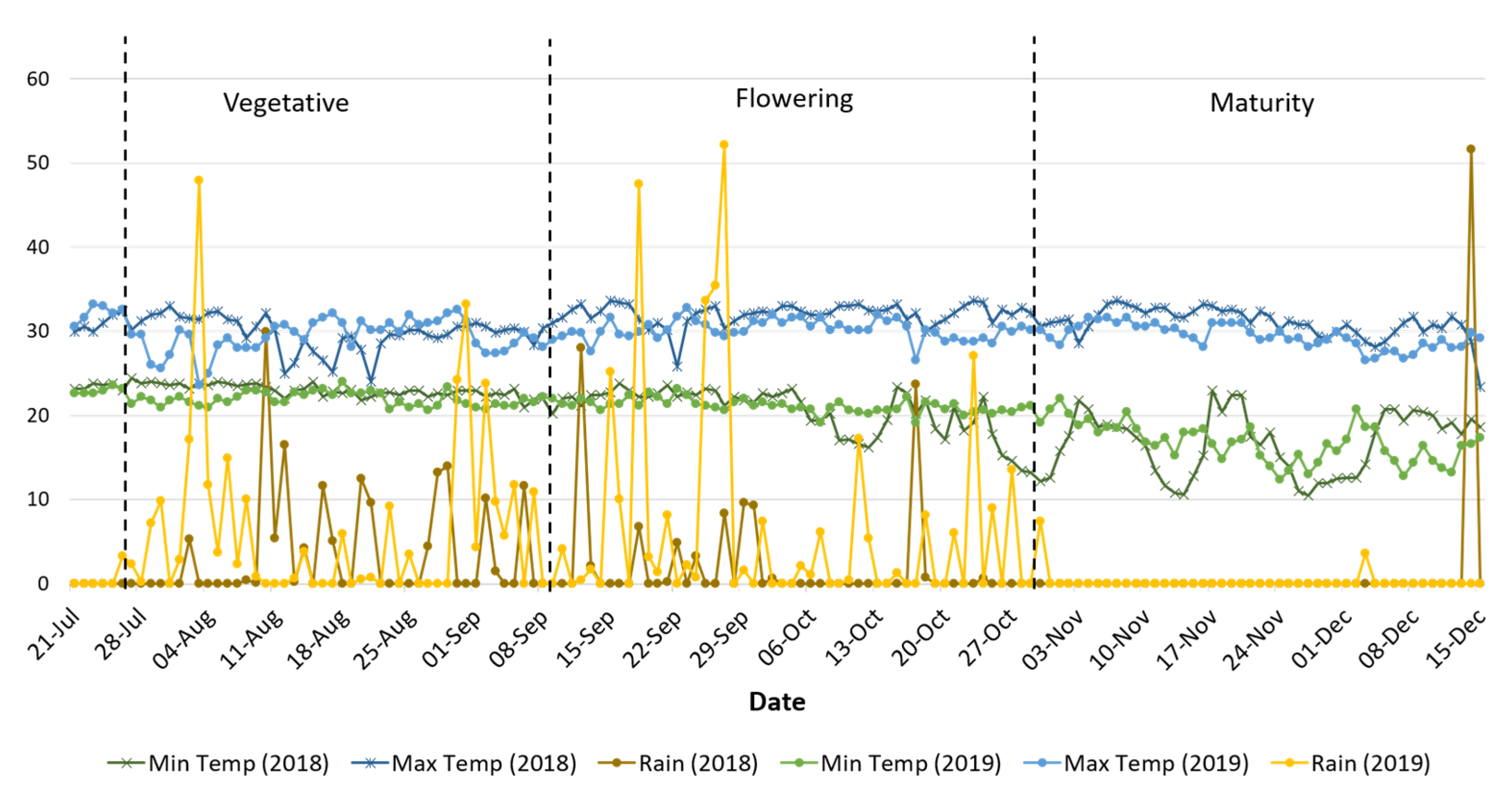
2.2. Experimental Details
2.3. Phenotyping for Agronomic Traits
2.4. Forage Quality Trait Assessment
2.5. Statistical Analysis
3. Results
3.1. ReML Variance Components Analysis
3.2. Genetic Variability and Heritability
3.3. Mean Comparison among Races, Regions, and Biological Status
3.4. Correlation among Forage Quality Traits and Agronomic Traits
3.5. Cluster Analysis
3.6. Promising Trait-Specific Accessions
4. Discussion
4.1. Variance Component and Heritability
4.2. Variability and Mean Performance of Finger Millet for Forage Quality Traits
4.3. Relationship among Agronomic and Forage Quality Traits
4.4. Promising Trait-Specific Sources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Wet, J.M.J. Eleusine coracana (L.) Gaertn. Record from Protabase. In PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale); Brink, M., Belay, G., Eds.; Animal Feed Resources Information System: Wageningen, The Netherlands, 2006. [Google Scholar]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Longvah, T.; Ananthan, R.; Bhaskarachary, K.; Venkaiah, K. Indian Food Composition Tables. In Indian Council of Medical Research; National Institute of Nutrition (NIN): Hyderabad, India, 2017; Volume 53. [Google Scholar]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger Millet (Ragi, Eleusine coracana L.). A Review of Its Nutritional Properties, Processing, and Plausible Health Benefits. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 69, pp. 1–39. [Google Scholar]
- Upadhyaya, H.D.; Ramesh, S.; Sharma, S.; Singh, S.K.; Varshney, S.K.; Sarma, N.D.R.K.; Ravishankar, C.R.; Narasimhudu, Y.; Reddy, V.G.; Sahrawat, K.L.; et al. Genetic Diversity for Grain Nutrients Contents in a Core Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm. Field Crop. Res. 2011, 121, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.M.; Arora, S.; Mirza, N.; Pande, A.; Lata, C.; Puranik, S.; Kumar, J.; Kumar, A. Finger Millet: A “Certain” Crop for an “Uncertain” Future and a Solution to Food Insecurity and Hidden Hunger under Stressful Environments. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetriventhan, M.; Azevedo, V.C.R.; Upadhyaya, H.D.; Nirmalakumari, A.; Kane-Potaka, J.; Anitha, S.; Ceasar, S.A.; Muthamilarasan, M.; Bhat, B.V.; Hariprasanna, K.; et al. Genetic and Genomic Resources, and Breeding for Accelerating Improvement of Small Millets: Current Status and Future Interventions. Nucleus (India); Springer: Berlin/Heidelberg, Germany, 2020; p. 13. Available online: https://doi.org/10.1007/s13237-020-00322-3 (accessed on 31 July 2020).
- Baker, R. Millet Production. Guide A-414; New Mexico State University: Las Cruces, NM, USA, 2003. [Google Scholar]
- Mgonja, M.A.; Lenné, J.M.; Manyasa, E.; Sreenivasaprasad, S. Finger Millet Blast Management in East Africa. In Creating Opportunities for Improving Production and Utilization of Finger Millet, Proceedings of the First International Finger Millet Stakeholder Workshop, Projects R8030 & R8445 UK Department for International Development—Crop Protection Programme, Nairobi, Kenya, 13–14 September 2005; ICRISAT: Andhra Pradesh, India, 2007; p. 196. [Google Scholar]
- Baath, G.S.; Northup, B.K.; Gowda, P.H.; Rocateli, A.C.; Turner, K.E. Adaptability and Forage Characterization of Finger Millet Accessions in U.S. Southern Great Plains. Agronomy 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Sampath, S.R. Scope for Using Small Millets as Forage in India. In Small Millets in Global Agriculture, Proceedings of the 1st International Small Millets Workshop, Bangalore, India, 29 October–2 November 1986; Seetharam, A., Riley, K.W., Harinarayana, G., Eds.; Mohan Primlani for Oxford & IBH Publishing Co. Pvt. Ltd: New Delhi, India, 1986. [Google Scholar]
- Madibela, O.R.; Modiakgotla, E. Chemical Composition and In Vitro Dry Matter Digestibility of Indigenous Finger Millet (Eleusine coracana) in Botswana. Livest. Res. Rural Dev. 2004, 16, 60–65. [Google Scholar]
- Gowda, P.H.; Prasad, P.V.V.; Angadi, S.V.; Rangappa, U.M.; Wagle, P. Finger Millet: An Alternative Crop for the Southern High Plains. Am. J. Plant Sci. 2015, 6, 2686–2691. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Finger Millet in Lost Crops of Africa. Volume I: Grains; National Academy of Sciences: Washington, DC, USA, 1996. [Google Scholar]
- Blümmel, M.; Vrik, P.; Xianglin, I. Opportunities for Improving the Fodder Value of Rice Straw by Multidimensional Crop Improvement. In Proceedings of the International Symposium on Production and Utilization of Paddy as Feed, Tokyo, Japan, 9–12 October 2007; pp. 17–21. [Google Scholar]
- Virk, P.; Xianglin, L.; Blümmel, M. A Note on Variation in Grain and Straw Fodder Quality Traits in 437 Cultivars of Rice from the Varietal Groups of Aromatic, Hybrids, Indica, New Planting Types and Released Varieties in the Philippines. Field Crop. Res. 2019, 233, 96–100. [Google Scholar] [CrossRef]
- Duncan, A.J.; Samaddar, A.; Blümmel, M. Rice and Wheat Straw Fodder Trading in India: Possible Lessons for Rice and Wheat Improvement. Field Crop. Res. 2020, 246, 107680. [Google Scholar] [CrossRef]
- Bidinger, F.R.; Blümmel, M. Determinants of Ruminant Nutritional Quality of Pearl Millet [Pennisetum Glaucum (L.) R. Br.] Stover. I. Effects of Management Alternatives on Stover Quality and Productivity. Field Crop. Res. 2007, 103, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Subudhi, H.N.; Prasad, K.V.S.V.; Ramakrishna, C.; Rameswar, P.S.; Pathak, H.; Ravi, D.; Khan, A.A.; Padmakumar, V.; Blümmel, M. Genetic Variation for Grain Yield, Straw Yield and Straw Quality Traits in 132 Diverse Rice Varieties Released for Different Ecologies Such as Upland, Lowland, Irrigated and Salinity Prone Areas in India. Field Crop. Res. 2020, 245, 96–100. [Google Scholar] [CrossRef]
- Blümmel, M.; Updahyay, S.R.; Gautam, N.; Barma, N.C.D.; Abdul Hakim, M.; Hussain, M.; Yaqub Mujahid, M.; Chatrath, R.; Sohu, V.S.; Mavi, G.; et al. Comparative Assessment of Food-Fodder Traits in a Wide Range of Wheat Germplasm for Diverse Biophysical Target Domains in South Asia. Field Crop. Res. 2019, 236, 68–74. [Google Scholar] [CrossRef]
- Joshi, A.K.; Barma, N.C.D.; Hakim, M.A.; Kalappanavar, I.K.; Naik, V.R.; Biradar, S.S.; Prasad, S.V.S.; Singh, R.P.; Blümmel, M. Opportunities for Wheat Cultivars with Superior Straw Quality Traits Targeting the Semi-Arid Tropics. Field Crop. Res. 2019, 231, 51–56. [Google Scholar] [CrossRef]
- Kim, K.S.; Anderson, J.D.; Newell, M.A.; Grogan, S.M.; Byrne, P.F.; Stephen Baenziger, P.; Butler, T.J. Genetic Diversity of Great Plains Hard Winter Wheat Germplasm for Forage. Crop Sci. 2016, 56, 2297–2305. [Google Scholar] [CrossRef] [Green Version]
- Vaswani, S.; Kumar, R.; Kumar, V.; Roy, D.; Kumar, M. In Vitro Evaluation of Wheat Straw Varieties for Chemical Composition, Gas Production and Digestibility. Indian J. Anim. Res. 2013, 47, 555–557. [Google Scholar]
- Zaidi, P.H.; Vinayan, M.T.; Blümmel, M. Genetic Variability of Tropical Maize Stover Quality and the Potential for Genetic Improvement of Food-Feed Value in India. Field Crop. Res. 2013, 153, 94–101. [Google Scholar] [CrossRef]
- Hash, C.; Blümmel, M.; Bidinger, F. Genotype × Environment Interactions in Food-Feed Traits in Pearl Millet Cultivars. Int. Sorghum Millets Newsl. 2006, 47, 153–157. [Google Scholar]
- Nigam, S.N.; Blümmel, M. Cultivar-Dependent Variation in Food-Feed-Traits in Groundnut (Arachis Hypogaea L.). Anim. Nutr. Feed Technol. 2010, 10, 39–48. [Google Scholar]
- Somegowda, V.K.; Vemula, A.; Naravula, J.; Prasad, G.; Rayaprolu, L.; Rathore, A.; Blümmel, M.; Deshpande, S.P. Evaluation of Fodder Yield and Fodder Quality in Sorghum and Its Interaction with Grain Yield under Different Water Availability Regimes. Curr. Plant Biol. 2021, 25, 100191. [Google Scholar] [CrossRef]
- Wafula, W.; Siambi, M.; Ojulong, H.; Korir, N.; Gweyi-Onyango, J. Finger Millet (Eleusine coracana) Fodder Yield Potential and Nutritive Value under Different Levels of Phosphorus in Rainfed Conditions. J. Agric. Ecol. Res. Int. 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Blümmel, M.; Bidinger, F.R.; Hash, C.T. Management and Cultivar Effects on Ruminant Nutritional Quality of Pearl Millet (Pennisetum Glaucum (L.) R. Br.) Stover. II. Effects of Cultivar Choice on Stover Quality and Productivity. Field Crop. Res. 2007, 103, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Wald, A. Tests of Statistical Hypotheses Concerning Several Parameters When the Number of Observations Is Large. Trans. Am. Math. Soc. 1943, 54, 426–482. [Google Scholar] [CrossRef]
- Keuls, M. The Use of the “Studentized Range” in Connection with an Analysis of Variance. Euphytica 1952, 1, 112–122. [Google Scholar] [CrossRef]
- Newman, D. The Distribution of Range in Samples from a Normal Population, Expressed in Terms of an Independent Estimate of Standard Deviation. Biometrika 1939, 31, 20. [Google Scholar] [CrossRef]
- de Mendiburu, F. Package ‘Agricolae’, Statistical Procedures for Agricultural Research. R Package. Version 1.3-3. 2020. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 31 July 2020).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. R Package “Corrplot”: Visualization of a Correlation Matrix. Statistician 2017, 56, 316–324. [Google Scholar]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’, Community Ecology Package Version-2.5-7. 2020. Available online: https://github.com/vegandevs/vegan (accessed on 31 July 2020).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Studer, M.; Roudier, P.; Gonzalez, J.; Kozlowski, K.; Schubert, E.; et al. Package “Cluster” for R: ‘Finding Groups in Data’: Cluster Analysis Extended. 2019. Available online: https://github.com/jkrijthe/Rtsne (accessed on 31 July 2020).
- Govindaraj, M.; Rai, K.N.; Kanatti, A.; Upadhyaya, H.D.; Shivade, H.; Rao, A.S. Exploring the Genetic Variability and Diversity of Pearl Millet Core Collection Germplasm for Grain Nutritional Traits Improvement. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blümmel, M.; Vishala, A.; Ravi, D.; Prasad, K.V.S.V.; Ramakrishna Reddy, C.; Seetharama, N. Multi-Environmental Investigations of Food-Feed Trait Relationships in Kharif and Rabi Sorghum (Sorghum bicolor (L) Moench) over Several Years of Cultivars Testing in India. In Animal Nutrition and Feed Technology; International Livestock Research Institute: Nairobi, Kenya, 2010. [Google Scholar]
- Sood, S.; Joshi, D.C.; Chandra, A.K.; Kumar, A. Phenomics and Genomics of Finger Millet: Current Status and Future Prospects. In Planta; Springer: Berlin/Heidelberg, Germany, 2019; pp. 731–751. [Google Scholar]
- Van Saun, R.J. Determining Forage Quality: Understanding Feed Analysis. Lamalink 2013, 3, 18–19. [Google Scholar]
- Mahyuddin, P. Chemical Composition of Leaf and Stem of Tropical Grassess at Different Stages of Growth. Anim. Prod. 2007, 9, 153–159. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Mahyuddin, P. Relationship between Chemical Component and In Vitro Digestibility of Tropical Grasses. HAYATI J. Biosci. 2008, 15, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.J.; Moser, L.E. Plant Factors Affecting Forage Quality. In Forage Quality, Evaluation, and Utilization; American Society of Agronomy, Inc.: Madison, WI, USA; Crop Science Society of America, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1994; pp. 115–154. [Google Scholar]
- Ravi, D.; Khan, A.A.; Saibutcharao, M.; Blümmel, M. A Note on Suitable Laboratory Stover Quality Traits for Multidimensional Maize Improvement. Field Crop. Res. 2013, 153, 58–62. [Google Scholar] [CrossRef]
- Liu, W.; Liu, G.H.; Liao, R.B.; Chang, Y.L.; Huang, X.Y.; Wu, Y.B.; Yang, H.M.; Yan, H.J.; Cai, H.Y. Apparent Metabolizable and Net Energy Values of Corn and Soybean Meal for Broiler Breeding Cocks. Poult. Sci. 2017, 96, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mlay, P.S.; Pereka, A.; Phiri, E.C.; Balthazary, S.; Igusti, J.; Hvelplund, T.; Weisbjerg, M.R.; Madsen, J. Feed Value of Selected Tropical Grasses, Legumes and Concentrates. Vet. Arh. 2006, 76, 53–63. [Google Scholar]
- Habte, E.; Muktar, M.S.; Abdena, A.; Hanson, J.; Sartie, A.M.; Negawo, A.T.; Machado, J.C.; da Silva Ledo, F.J.; Jones, C.S. Forage Performance and Detection of Marker Trait Associations with Potential for Napier Grass (Cenchrus purpureus) Improvement. Agronomy 2020, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Bezabih, M.; Adie, A.; Ravi, D.; Prasad, K.V.S.V.; Jones, C.; Abeyo, B.; Tadesse, Z.; Zegeye, H.; Solomon, T.; Blümmel, M. Variations in Food-Fodder Traits of Bread Wheat Cultivars Released for the Ethiopian Highlands. Field Crop. Res. 2018, 229, 1–7. [Google Scholar] [CrossRef]

| Trait | 2018 | 2019 | Pooled | |||||
|---|---|---|---|---|---|---|---|---|
| σ2g # | σ2error | σ2g | σ2error | σ2g | σ2ge | Wald Statistic for Years | σ2error | |
| Days to 50% flowering | 89.01 ** | 2.076 | 54.095 ** | 2.545 | 65.996 ** | 5.52 ** | 8.85 * | 2.411 |
| Days to maturity | 106.442 ** | 4.599 | 57.588 ** | 4.366 | 73.099 ** | 9.023 ** | 16.52 ** | 4.526 |
| Grain yield (kg ha−1) | 483,310 ** | 182,705 | 213,483 ** | 98,715 | 185,838 ** | 164,167 ** | 465.66 ** | 150,149 |
| Stover yield (kg ha−1) | 4,536,125 ** | 2,344,396 | 1,140,943 ** | 695,538 | 1,791,173 ** | 1,015,857 ** | 839.20 ** | 1,562,927 |
| Harvest index | 0.002493 ** | 0.00159 | 0.002178 ** | 0.00112 | 0.001738 ** | 0.000553 ** | 10.46 ** | 0.001 |
| Basal tillers number | 0.9857 ** | 0.484 | 0.5001 ** | 0.424 | 0.4667 ** | 0.2728 ** | 1285.83 ** | 0.476 |
| Plant height (cm) | 125.41 ** | 43.22 | 123.52 ** | 36.04 | 105.35 ** | 17.98 ** | 153.17 ** | 43.340 |
| Dry matter (Dm) (%) | 0.0938 ** | 0.222 | 0.0404 ** | 0.254 | 0.0242 ** | 0.0445 ** | 898.19 ** | 0.517 |
| Ash content (%) | 0.3439 ** | 0.733 | 0.752 ** | 4.767 | 0.248 ** | 0.297 ** | 2276.71 ** | 2.794 |
| Crude protein (CP) (%) | 0.2856 ** | 0.511 | 0.1049 ** | 0.597 | 0.1597 ** | 0.0051 ns | 289.64 ** | 0.713 |
| Neutral detergent fiber (NDF) (%) | 2.15 ** | 3.654 | 1.616 ** | 5.376 | 0.963 ** | 0.902 ** | 895.79 ** | 4.575 |
| Acid detergent fiber (ADF) (%) | 2.472 ** | 2.945 | 1.283 ** | 1.682 | 1.195 ** | 0.413 ** | 1315.96 ** | 3.272 |
| Acid detergent lignin (ADL) (%) | 0.04788 ** | 0.0699 | 0.03828 ** | 0.0964 | 0.03183 ** | 0.00832 * | 2184.52 ** | 0.092 |
| Metabolizable energy (ME) (MJ/kg Dm) | 0.03261 ** | 0.0587 | 0.016 ** | 0.112 | 0.01766 ** | 0.00582 * | 16.48 ** | 0.090 |
| In vitro organic matter digestibility (IVOMD) (%) | 1.118 ** | 2.022 | 0.557 ** | 3.639 | 0.555 ** | 0.249 * | 73.63 ** | 2.992 |
| Trait | Season | Mean | Range | Heritability (H2b) | # CV% | SED | LSD (p ≤ 0.05) |
|---|---|---|---|---|---|---|---|
| Days to 50% flowering | Pooled | 74 ± 0.47 | 51–97 | 0.95 | 2.08 | 1.27 | 2.49 |
| 2018 | 74 ± 0.53 a † | 50–99 | 0.98 | 1.94 | 1.18 | 2.31 | |
| 2019 | 75 ± 0.41 a | 53–97 | 0.96 | 2.13 | 1.30 | 2.56 | |
| Days to maturity | Pooled | 107 ± 0.5 | 85–130 | 0.93 | 1.98 | 1.74 | 3.41 |
| 2018 | 108 ± 0.58 a | 84–134 | 0.96 | 1.99 | 1.75 | 3.44 | |
| 2019 | 107 ± 0.42 a | 86–128 | 0.93 | 1.96 | 1.71 | 3.35 | |
| Grain yield (kg ha−1) | Pooled | 2267 ± 28 | 810–3698 | 0.63 | 17.09 | 316 | 620 |
| 2018 | 2666 ± 37 a | 615–4416 | 0.73 | 16.03 | 349 | 685 | |
| 2019 | 1865 ± 24 b | 731–3331 | 0.68 | 16.85 | 256 | 503 | |
| Stover yield (kg ha−1) | Pooled | 7078 ± 81 | 2890–10,779 | 0.70 | 17.66 | 1020 | 2001 |
| 2018 | 8559 ± 111 a | 2998–13,423 | 0.66 | 17.89 | 1250 | 2454 | |
| 2019 | 5598 ± 55 b | 2952–8944 | 0.62 | 14.90 | 680 | 1336 | |
| Harvest index | Pooled | 0.25 ± 0.002 | 0.12–0.37 | 0.77 | 15.68 | 0.03 | 0.06 |
| 2018 | 0.24 ± 0.003 b | 0.12–0.39 | 0.61 | 16.50 | 0.03 | 0.06 | |
| 2019 | 0.25 ± 0.002 a | 0.11–0.36 | 0.66 | 13.36 | 0.03 | 0.05 | |
| Basal tillers number | Pooled | 4 ± 0.04 | 3–7 | 0.68 | 15.69 | 0.56 | 1.10 |
| 2018 | 5 ± 0.05 a | 4–9 | 0.67 | 13.03 | 0.57 | 1.12 | |
| 2019 | 3 ± 0.04 b | 2–6 | 0.54 | 18.87 | 0.53 | 1.04 | |
| Plant height (cm) | Pooled | 120 ± 0.59 | 84–143 | 0.87 | 5.50 | 5.38 | 10.54 |
| 2018 | 123 ± 0.6 a | 85–150 | 0.74 | 5.36 | 5.37 | 10.54 | |
| 2019 | 117 ± 0.6 b | 78–138 | 0.77 | 5.13 | 4.90 | 9.62 | |
| Dry matter (Dm) (%) | Pooled | 90.42 ± 0 | 90.39–90.44 | 0.18 | 0.80 | 0.59 | 1.15 |
| 2018 | 90.84 ± 0.01 a | 90.15–91.78 | 0.14 | 0.52 | 0.38 | 0.76 | |
| 2019 | 89.99 ± 0.01 b | 89.64–90.36 | 0.14 | 0.56 | 0.41 | 0.81 | |
| Ash content (%) | Pooled | 12.42 ± 0.02 | 11.47–14.17 | 0.29 | 13.46 | 1.36 | 2.68 |
| 2018 | 10.31 ± 0.03 b | 9.12–11.69 | 0.32 | 8.30 | 0.70 | 1.37 | |
| 2019 | 14.53 ± 0.03 a | 13.44–16.75 | 0.28 | 15.02 | 1.78 | 3.50 | |
| Crude protein (CP) (%) | Pooled | 7.23 ± 0.02 | 6.47–8.15 | 0.57 | 11.68 | 0.69 | 1.35 |
| 2018 | 7.56 ± 0.02 a | 6.50–8.82 | 0.36 | 9.45 | 0.58 | 1.15 | |
| 2019 | 6.9 ± 0.01 b | 6.42–7.60 | 0.30 | 11.19 | 0.63 | 1.24 | |
| Neutral detergent fiber (NDF) (%) | Pooled | 64.94 ± 0.05 | 62.36–67.73 | 0.44 | 3.29 | 1.75 | 3.42 |
| 2018 | 66.8 ± 0.07 a | 62.96–69.55 | 0.37 | 2.86 | 1.56 | 3.06 | |
| 2019 | 63.08 ± 0.05 b | 60.03–65.39 | 0.23 | 3.68 | 1.89 | 3.72 | |
| Acid detergent fiber (ADF) (%) | Pooled | 35.87 ± 0.06 | 33.07–38.37 | 0.61 | 5.04 | 1.48 | 2.90 |
| 2018 | 37.64 ± 0.08 a | 34.33–40.58 | 0.46 | 4.56 | 1.40 | 2.75 | |
| 2019 | 34.1 ± 0.05 b | 31.43–36.80 | 0.43 | 3.80 | 1.06 | 2.08 | |
| Acid detergent lignin (ADL) (%) | Pooled | 4.39 ± 0.01 | 3.95–4.80 | 0.62 | 6.90 | 0.25 | 0.48 |
| 2018 | 4.76 ± 0.01 a | 4.31–5.19 | 0.41 | 5.56 | 0.22 | 0.42 | |
| 2019 | 4.02 ± 0.01 b | 3.65–4.36 | 0.28 | 7.71 | 0.25 | 0.50 | |
| Metabolizable energy (ME) (MJ/kg Dm) | Pooled | 6.57 ± 0.01 | 6.18–6.89 | 0.50 | 4.56 | 0.24 | 0.48 |
| 2018 | 6.54 ± 0.01 b | 6.08–7.09 | 0.36 | 3.70 | 0.20 | 0.39 | |
| 2019 | 6.6 ± 0 a | 6.27–6.77 | 0.33 | 5.07 | 0.27 | 0.54 | |
| In vitro organic matter digestibility (IVOMD) (%) | Pooled | 47.28 ± 0.04 | 45.21–49.09 | 0.47 | 3.66 | 1.41 | 2.77 |
| 2018 | 46.9 ± 0.05 b | 43.87–49.84 | 0.36 | 3.03 | 1.16 | 2.28 | |
| 2019 | 47.66 ± 0.02 a | 45.83–48.75 | 0.33 | 4.00 | 1.56 | 3.06 |
| Trait # | Race | Region | Biological Status | |||||
|---|---|---|---|---|---|---|---|---|
| Compacta | Elongata | Plana | Vulgaris | Africa | Asia | Breeding Lines | Landraces | |
| DF | 75 ± 1.82 ab † | 78 ± 1.43 a | 79 ± 0.95 a | 73 ± 0.56 b | 78 ± 0.52 a | 71 ± 0.72 b | 70 ± 1.14 b | 75 ± 0.5 a |
| DM | 108 ± 1.94 ab | 111 ± 1.55 a | 112 ± 1.04 a | 105 ± 0.59 b | 111 ± 0.56 a | 103 ± 0.75 b | 103 ± 1.19 b | 108 ± 0.53 a |
| GY | 2261 ± 86 a | 2149 ± 120 a | 2295 ± 63 a | 2267 ± 331 a | 2252 ± 34 a | 2307 ± 48 a | 2583 ± 57 a | 2206 ± 29 b |
| SY | 7374 ± 300 ab | 7227 ± 241 ab | 7721 ± 169 a | 6833 ± 100 b | 7440 ± 83 a | 6620 ± 142 b | 7184 ± 253 a | 7058 ± 83 a |
| HI | 0.24 ± 0.01 a | 0.23 ± 0.01 a | 0.23 ± 0.01 a | 0.25 ± 0.02 a | 0.23 ± 0.01 b | 0.26 ± 0.01 a | 0.27 ± 0.01 a | 0.24 ± 0.01 b |
| BTN | 4 ± 0.10 a | 4 ± 0.14 a | 4 ± 0.06 a | 5 ± 0.05 b | 4 ± 0.05 b | 5 ± 0.06 a | 5 ± 0.09 a | 4 ± 0.04 b |
| PH | 118 ± 1.96 a | 121 ± 2.15 a | 124 ± 1.45 a | 119 ± 0.71 a | 123 ± 0.65 a | 115 ± 0.88 b | 116 ± 1.48 b | 121 ± 0.63 a |
| Dm | 90.42 ± 0.001 a | 90.42 ± 0.001 a | 90.42 ± 0.001 a | 90.42 ± 0.001 a | 90.42 ± 0.001 a | 90.42 ± 0.001 a | 90.42 ± 0.001 a | 90.42 ± 0.001 a |
| Ash | 12.46 ± 0.08 a | 12.26 ± 0.08 a | 12.41 ± 0.06 a | 12.45 ± 0.03 a | 12.28 ± 0.03 b | 12.60 ± 0.04 a | 12.55 ± 0.05 a | 12.40 ± 0.03 b |
| CP | 7.25 ± 0.05 a | 7.16 ± 0.06 a | 7.12 ± 0.04 a | 7.27 ± 0.02 a | 7.13 ± 0.02 b | 7.36 ± 0.02 a | 7.31 ± 0.03 a | 7.22 ± 0.02 b |
| NDF | 64.58 ± 0.19 b | 65.27 ± 0.21 a | 64.78 ± 0.14 b | 65.00 ± 0.06 ab | 65.07 ± 0.08 a | 64.78 ± 0.08 b | 64.67 ± 0.13 b | 64.99 ± 0.06 a |
| ADF | 35.55 ± 0.21 a | 36.08 ± 0.21 a | 35.64 ± 0.14 a | 35.96 ± 0.07 a | 35.89 ± 0.08 a | 35.86 ± 0.09 a | 35.58 ± 0.15 b | 35.93 ± 0.06 a |
| ADL | 4.33 ± 0.03 b | 4.42 ± 0.03 a | 4.35 ± 0.02 ab | 4.41 ± 0.01 ab | 4.40 ± 0.01 a | 4.39 ± 0.01 a | 4.37 ± 0.02 a | 4.40 ± 0.01 a |
| ME | 6.59 ± 0.02 ab | 6.58 ± 0.02 ab | 6.63 ± 0.02 a | 6.55 ± 0.01 b | 6.60 ± 0.01 a | 6.54 ± 0.01 b | 6.54 ± 0.01 a | 6.58 ± 0.01 a |
| IVOMD | 47.42 ± 0.11 ab | 47.28 ± 0.10 ab | 47.58 ± 0.09 a | 47.19 ± 0.04 b | 47.41 ± 0.05 a | 47.12 ± 0.05 b | 47.13 ± 0.08 a | 47.31 ± 0.04 a |
| Trait # | DM | GY | SY | HI | BTN | PH | Dm | Ash | CP | NDF | ADF | ADL | ME | IVOMD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | 0.996 ** | 0.024 | 0.647 ** | −0.621 ** | −0.408 ** | 0.405 ** | −0.379 ** | −0.395 ** | −0.325 ** | 0.034 | −0.211 ** | −0.251 ** | 0.405 ** | 0.358 ** |
| DM | 0.021 | 0.643 ** | −0.617 ** | −0.410 ** | 0.392 ** | −0.383 ** | −0.398 ** | −0.319 ** | 0.034 | −0.209 ** | −0.248 ** | 0.403 ** | 0.356 ** | |
| GY | 0.453 ** | 0.531 ** | 0.140 * | 0.019 | −0.071 | −0.044 | −0.232 ** | −0.043 | −0.137 * | −0.046 | 0.056 | 0.005 | ||
| SY | −0.469 ** | −0.231 ** | 0.540 ** | −0.216 ** | −0.396 ** | −0.500 ** | −0.185 ** | −0.442 ** | −0.378 ** | 0.479 ** | 0.384 ** | |||
| HI | 0.400 ** | −0.516 ** | 0.143 * | 0.343 ** | 0.225 ** | 0.100 | 0.249 ** | 0.308 ** | −0.379 ** | −0.344 ** | ||||
| BTN | −0.290 ** | 0.039 | 0.123 * | 0.070 | 0.175 ** | 0.301 ** | 0.283 ** | −0.357 ** | −0.364 ** | |||||
| PH | 0.000 | −0.423 ** | −0.521 ** | 0.163 ** | 0.000 | 0.011 | 0.283 ** | 0.183 ** | ||||||
| Dm | 0.195 ** | 0.082 | −0.043 | 0.113 * | 0.139 * | −0.150 ** | −0.151 ** | |||||||
| Ash | 0.658 ** | −0.374 ** | −0.054 | −0.095 | −0.482 ** | −0.338 ** | ||||||||
| CP | −0.287 ** | −0.108 | −0.119 * | −0.480 ** | −0.313 ** | |||||||||
| NDF | 0.777 ** | 0.843 ** | −0.395 ** | −0.493 ** | ||||||||||
| ADF | 0.845 ** | −0.539 ** | −0.566 ** | |||||||||||
| ADL | −0.646 ** | −0.730 ** | ||||||||||||
| ME | 0.976 ** |
| Trait # | Cluster 1 (64) | Cluster 2 (66) | Cluster 3 (41) | Cluster 4 (72) | Cluster 5 (71) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| DF | 77 b † | 67–91 | 83 a | 65–97 | 62 d | 51–75 | 76 b | 67–88 | 70 c | 60–76 |
| DM | 110 b | 99–125 | 116 a | 96–130 | 94 d | 85–107 | 109 b | 99–122 | 103 c | 92–109 |
| GY | 2368 a | 1554–3214 | 2006 b | 810–2909 | 2059 b | 1233–2924 | 2377 a | 1235–3538 | 2427 a | 1324–3698 |
| SY | 8290 a | 5957–10,779 | 7557 b | 3760–10,123 | 5012 d | 2891–6617 | 7244 b | 5805–10,186 | 6567 c | 4726–9478 |
| HI | 0.22 d | 0.17–0.27 | 0.21 e | 0.12–0.29 | 0.29 a | 0.21–0.37 | 0.25 c | 0.16–0.32 | 0.27 b | 0.2–0.34 |
| BTN | 4 d | 3–6 | 4 cd | 3–7 | 5 a | 4–7 | 5 b | 3–7 | 4 bc | 3–6 |
| PH | 124 a | 102–138 | 121 ab | 95–143 | 109 c | 84–122 | 124 a | 110–140 | 117 b | 93–132 |
| Dm | 90.42 b | 90.40–90.44 | 90.41 c | 90.40–90.43 | 90.42 a | 90.41–90.44 | 90.42 b | 90.40–90.43 | 90.42 b | 90.41–90.44 |
| Ash | 12.44 b | 11.64–13.78 | 12.18 c | 11.49–12.85 | 12.74 a | 11.82–14.17 | 12.28 c | 11.52–13.18 | 12.60 a | 11.47–13.57 |
| CP | 7.18 c | 6.58–8.05 | 7.17 c | 6.47–7.88 | 7.50 a | 7.09–8.15 | 7.07 c | 6.57–7.53 | 7.34 b | 6.77–7.91 |
| NDF | 63.88 d | 62.37–65.41 | 65.04 b | 63.8–66.42 | 65.31 b | 62.94–67.09 | 65.91 a | 64.07–67.74 | 64.61 c | 63.25–65.74 |
| ADF | 34.67 c | 33.08–36.16 | 35.73 b | 34.51–37.17 | 36.65 a | 34.56–37.54 | 36.72 a | 35.28–38.37 | 35.78 b | 34.26–37.04 |
| ADL | 4.20 c | 3.96–4.49 | 4.35 b | 4.18–4.59 | 4.54 a | 4.16–4.79 | 4.54 a | 4.4–4.81 | 4.37 b | 4.16–4.62 |
| ME | 6.68 a | 6.46–6.9 | 6.62 b | 6.47–6.76 | 6.44 d | 6.19–6.57 | 6.53 c | 6.37–6.69 | 6.55 c | 6.38–6.79 |
| IVOMD | 47.90 a | 46.77–49.09 | 47.56 b | 46.69–48.11 | 46.56 e | 45.21–47.53 | 46.94 d | 45.82–47.93 | 47.23 c | 46.21–48.57 |
| Cluster | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 0.163† | 0.203 | 0.303 | 0.219 | 0.284 |
| 2 | 0.144 | 0.193 | 0.173 | 0.191 | |
| 3 | 0.153 | 0.217 | 0.184 | ||
| 4 | 0.137 | 0.185 | |||
| 5 | 0.153 |
| Trait | Class | Top Ten Accessions with High Trait Values | Days to 50% Flowering (Days) | Grain Yield (kg ha−1) |
|---|---|---|---|---|
| Stover yield (SY) (kg ha−1) | High | IE 2039, IE 2045, IE 2939, IE 3473, IE 4699, IE 4700, IE 4701, IE 4707, IE 5435, MR 6 | 76–88 | 2088–3530 |
| Dm Dry matter (Dm) (%) | High | IE 501, IE 588, IE 615, IE 872, IE 1055, IE 2217, IE 3769, IE 5165, IE 5407, IE 5435 | 51–79 | 1616–3698 |
| Ash (%) | Low * | IE 2066, IE 2586, IE 2789, IE 2911, IE 4646, IE 3025, IE 3443, IE 4497, IE 6326, IE 6537 | 60–95 | 1233–3342 |
| Crude protein (CP) (%) | High | IE 588, IE 2030, IE 2437, IE 2957, IE 4660, IE 4700, IE 4816, IE 5736, IE 5817, IE 5956 | 51–85 | 1029–3140 |
| Neutral detergent fiber (NDF) (%) | Low * | IE 2437, IE 3129, IE 3130, IE 3723, IE 3821, IE 3947, IE 4654, IE 4737, IE 5435, IE 8602 | 55–81 | 1596–3313 |
| Acid detergent fiber (ADF) (%) | Low * | IE 2045, IE 3128, IE 3129, IE 3723, IE 3821, IE 3947, IE 4654, IE 4699, IE 5249, IE 5435 | 66–84 | 2088–3121 |
| Acid detergent lignin (ADL) (%) | Low * | IE 953, IE 2437, IE 2760, IE 3128, IE 3129, IE 3130, IE 3723, IE 3821, IE 4654, IE 5364 | 66–90 | 1596–3002 |
| Metabolizable energy (ME) (MJ/kg Dm) | High | IE 2066, IE 2341, IE 2760, IE 3614, IE 3723, IE 3821, IE 3952, IE 4646, IE 5435, IE 8790 | 60–90 | 1909–2870 |
| In vitro organic matter digestibility (IVOMD) (%) | High | IE 2217, IE2341, IE 2760, IE 3129, IE 3614, IE 3723, IE 3821, IE 4646, IE 5435, IE 8790 | 60–90 | 1909–2870 |
| Accession | Cluster No. | SY kg ha−1 | Dm (%) | Ash (%) | CP (%) | NDF (%) | ADF (%) | ADL (%) | ME (MJ/kg Dm) | IVOMD (%) | DF # (days) | GY kg ha−1 | Region | Biological Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IE 2066 | 5 | 7368 | 90.41 | 11.47 | 6.76 | 63.28 | 35.07 | 4.19 | 6.78 | 48.39 | 61 | 2496 | Asia | Improved cultivar |
| IE 2437 | 1 | 6623 | 90.42 | 13.06 | 8.05 | 62.97 | 34.77 | 4.01 | 6.60 | 47.90 | 81 | 1596 | Africa | Landrace |
| IE 3129 | 1 | 8924 | 90.41 | 12.14 | 7.46 | 63.09 | 33.09 | 4.03 | 6.77 | 48.51 | 80 | 2223 | Asia | Improved cultivar |
| IE 3614 | 1 | 8808 | 90.42 | 12.03 | 6.84 | 63.61 | 34.61 | 4.11 | 6.89 | 49.03 | 72 | 2118 | Unidentified | Landrace |
| IE 3723 | 1 | 9658 * | 90.43 | 12.29 | 7.18 | 62.58 | 33.61 | 4.04 | 6.89 | 49.09 | 79 | 2278 | Africa | Landrace |
| IE 3821 | 1 | 9791 | 90.42 | 12.29 | 7.13 | 62.36 | 33.07 | 3.98 | 6.85 | 48.94 | 79 | 2138 | Africa | Landrace |
| IE 3952 | 1 | 9820 | 90.42 | 12.02 | 6.58 | 64.01 | 34.33 | 4.16 | 6.79 | 48.36 | 77 | 2647 | Africa | Landrace |
| IE 4700 | 1 | 10,158 | 90.42 | 13.38 | 7.86 | 63.38 | 34.14 | 4.17 | 6.53 | 47.30 | 86 | 2787 | Asia | Improved cultivar |
| IE 5435 | 1 | 10,640 | 90.43 | 12.03 | 6.87 | 62.96 | 33.57 | 4.12 | 6.88 | 48.97 | 80 | 2088 | Africa | Landrace |
| IE 8790 | 1 | 8139 | 90.41 | 12.20 | 7.11 | 63.29 | 34.18 | 4.10 | 6.79 | 48.52 | 91 | 2022 | Africa | Landrace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Backiyalakshmi, C.; Babu, C.; Naresh Reddy, D.; Padmakumar, V.; Prasad, K.V.S.V.; Azevedo, V.C.R.; Vetriventhan, M. Assessing Forage Potential of the Global Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm Conserved at the ICRISAT Genebank. Agronomy 2021, 11, 1706. https://doi.org/10.3390/agronomy11091706
Backiyalakshmi C, Babu C, Naresh Reddy D, Padmakumar V, Prasad KVSV, Azevedo VCR, Vetriventhan M. Assessing Forage Potential of the Global Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm Conserved at the ICRISAT Genebank. Agronomy. 2021; 11(9):1706. https://doi.org/10.3390/agronomy11091706
Chicago/Turabian StyleBackiyalakshmi, Chinnadurai, Chakrapani Babu, Dagunapur Naresh Reddy, Varijakshapanicker Padmakumar, Kodukula V. S. V. Prasad, Vania Cristina Renno Azevedo, and Mani Vetriventhan. 2021. "Assessing Forage Potential of the Global Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm Conserved at the ICRISAT Genebank" Agronomy 11, no. 9: 1706. https://doi.org/10.3390/agronomy11091706
APA StyleBackiyalakshmi, C., Babu, C., Naresh Reddy, D., Padmakumar, V., Prasad, K. V. S. V., Azevedo, V. C. R., & Vetriventhan, M. (2021). Assessing Forage Potential of the Global Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm Conserved at the ICRISAT Genebank. Agronomy, 11(9), 1706. https://doi.org/10.3390/agronomy11091706







