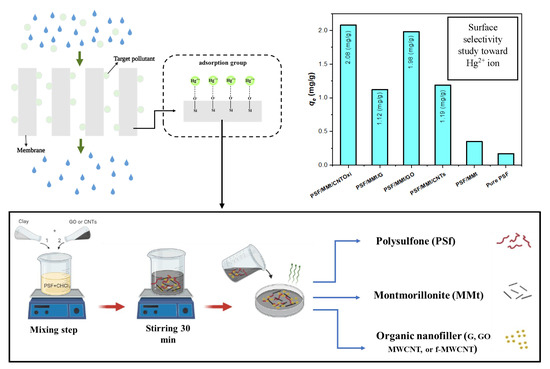
Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals and Reagents
2.2. Acid Treatment of CNTs
2.3. Preparation of Hybrid Nanocomposite Membranes
2.4. Adsorption Method Procedure
2.5. Instrumentation
3. Results and Discussion
3.1. Characterization of Hybrid Nanocomposite Membranes
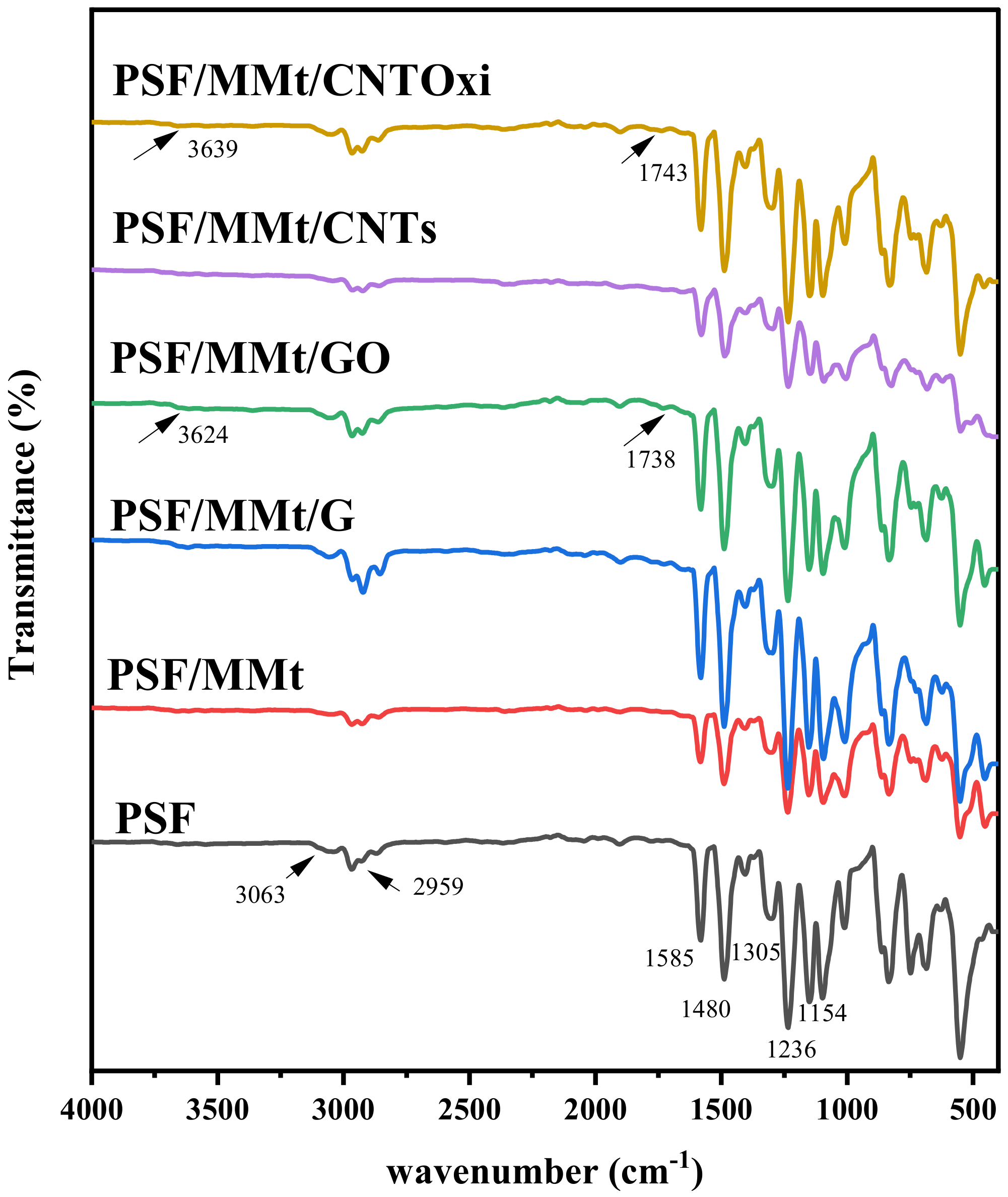
3.1.1. Fourier Transform Infrared (FTIR) Spectroscopy
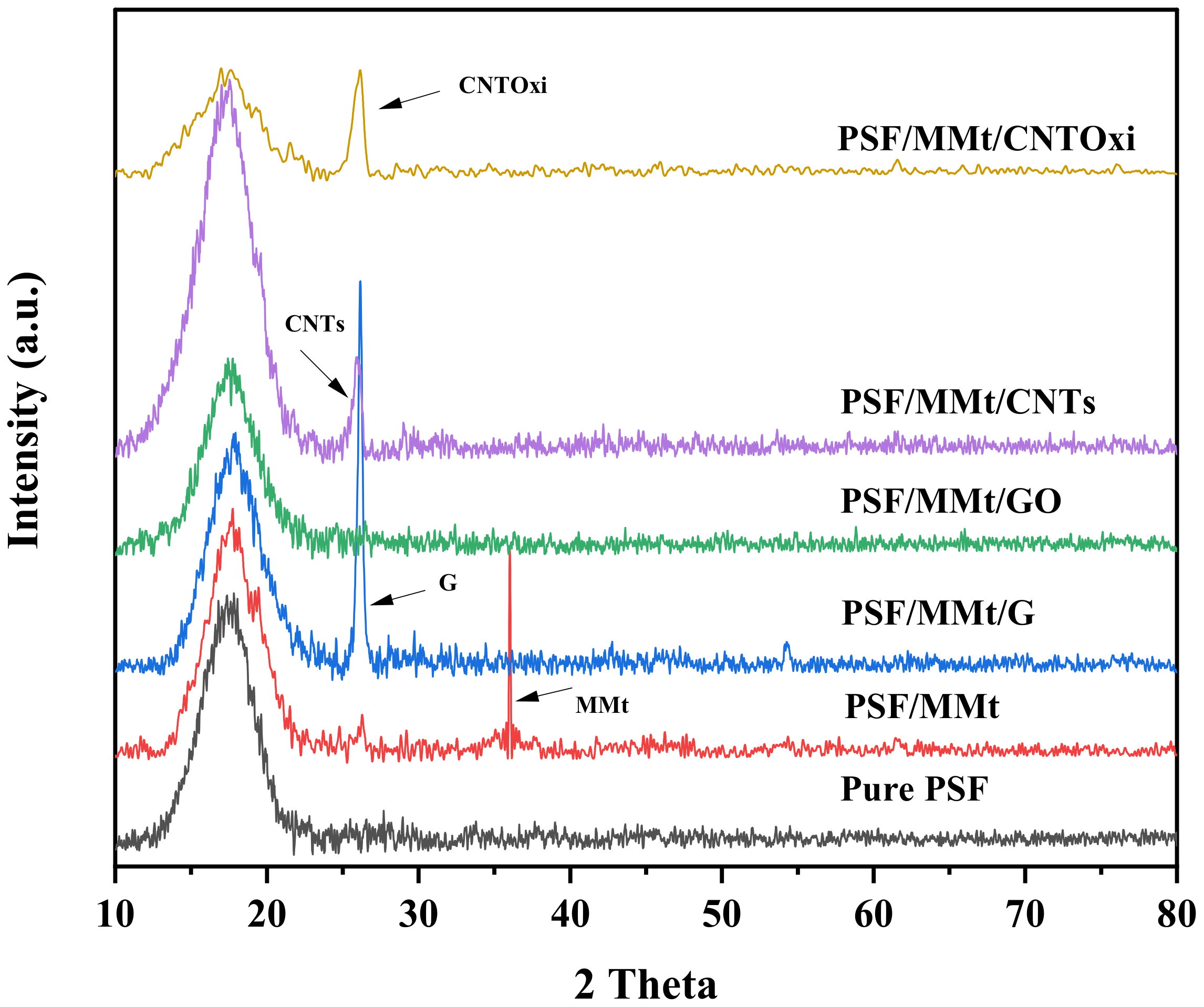
3.1.2. X-ray Diffraction (XRD)
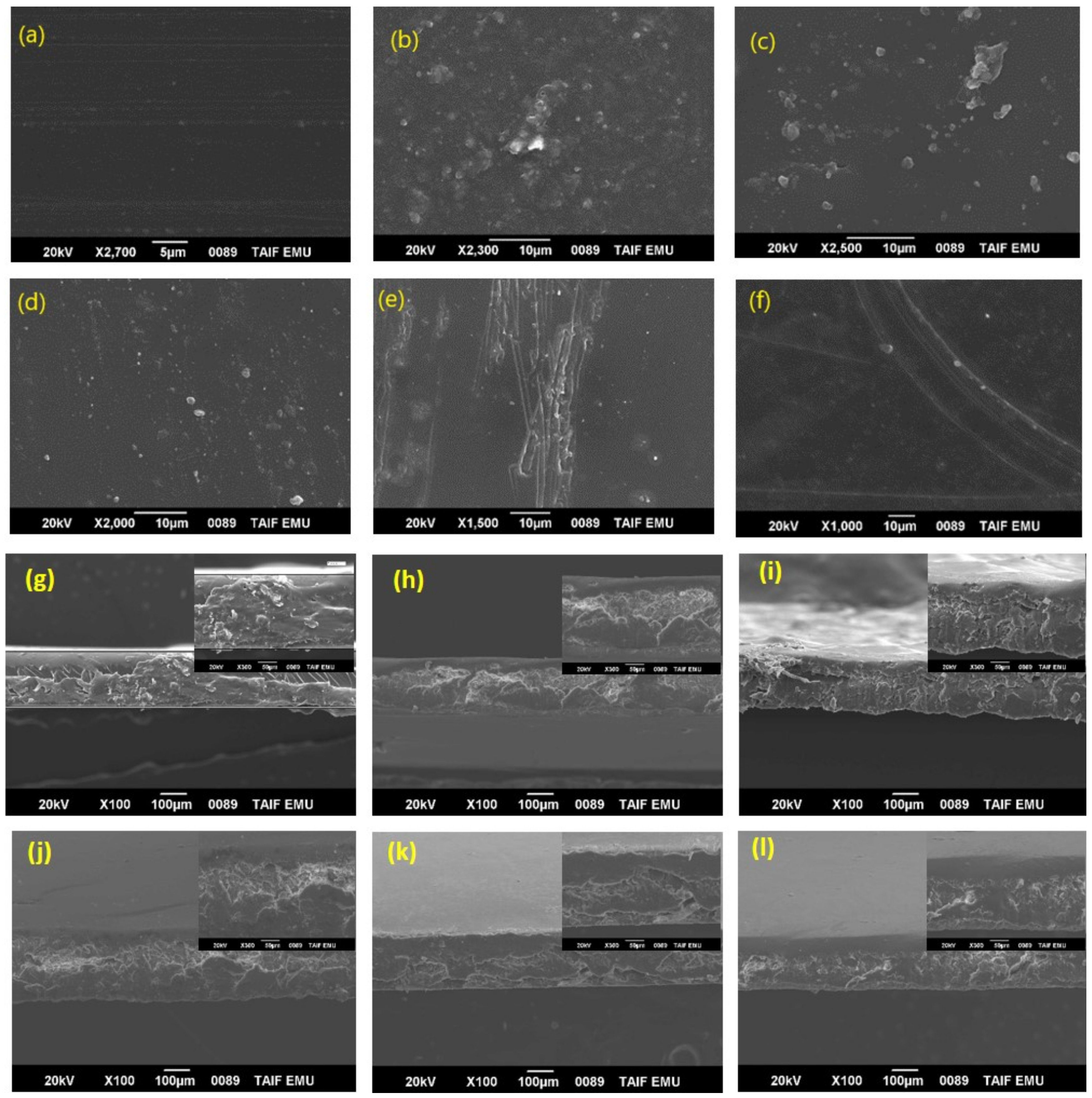
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Thermogravimetric Analysis (TGA)
3.1.5. Mechanical Properties
3.2. Adsorption Performance of Hybrid Nanocomposite Membranes
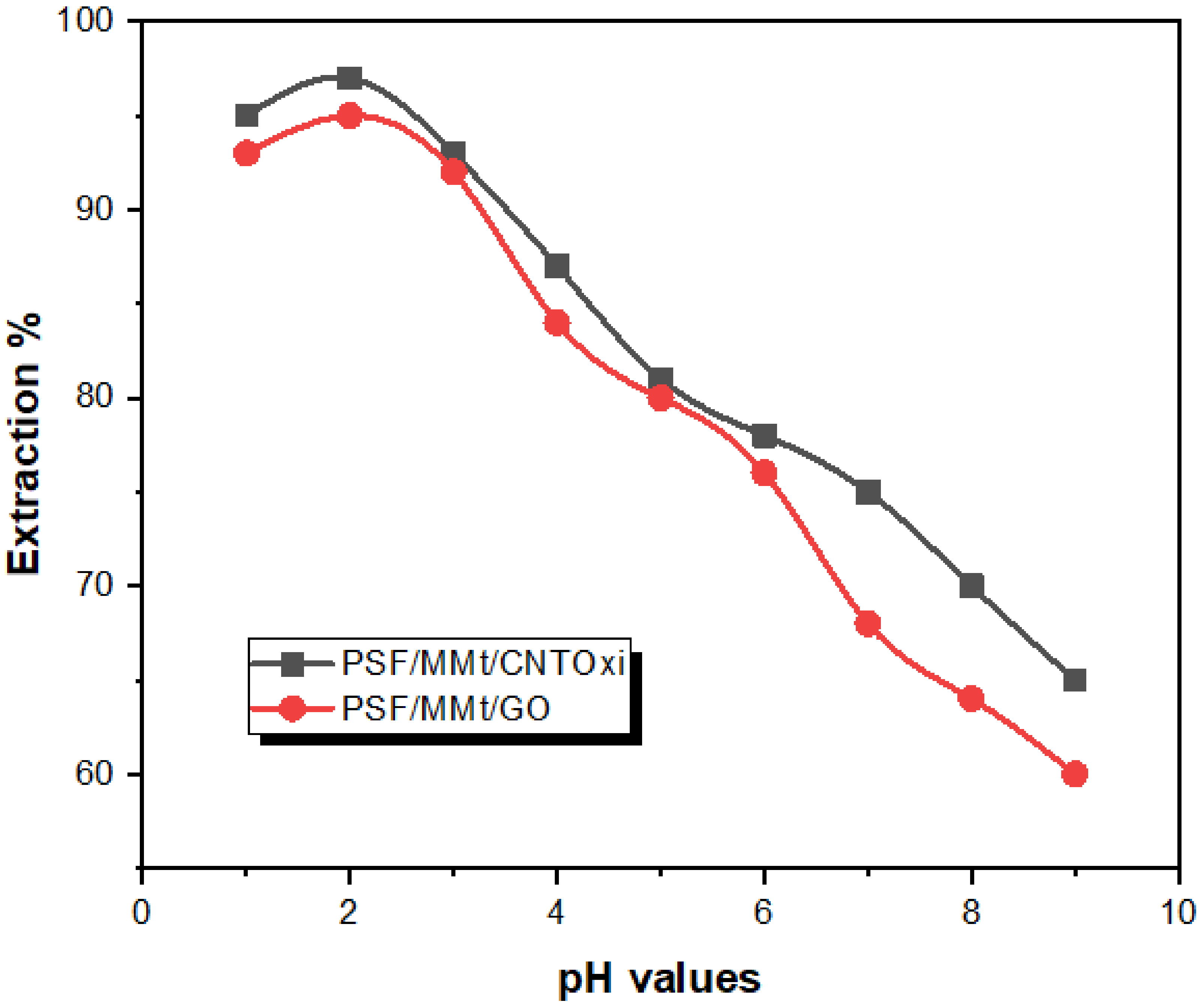
3.2.1. Effect of pH
3.2.2. Determination of Adsorption Capacity
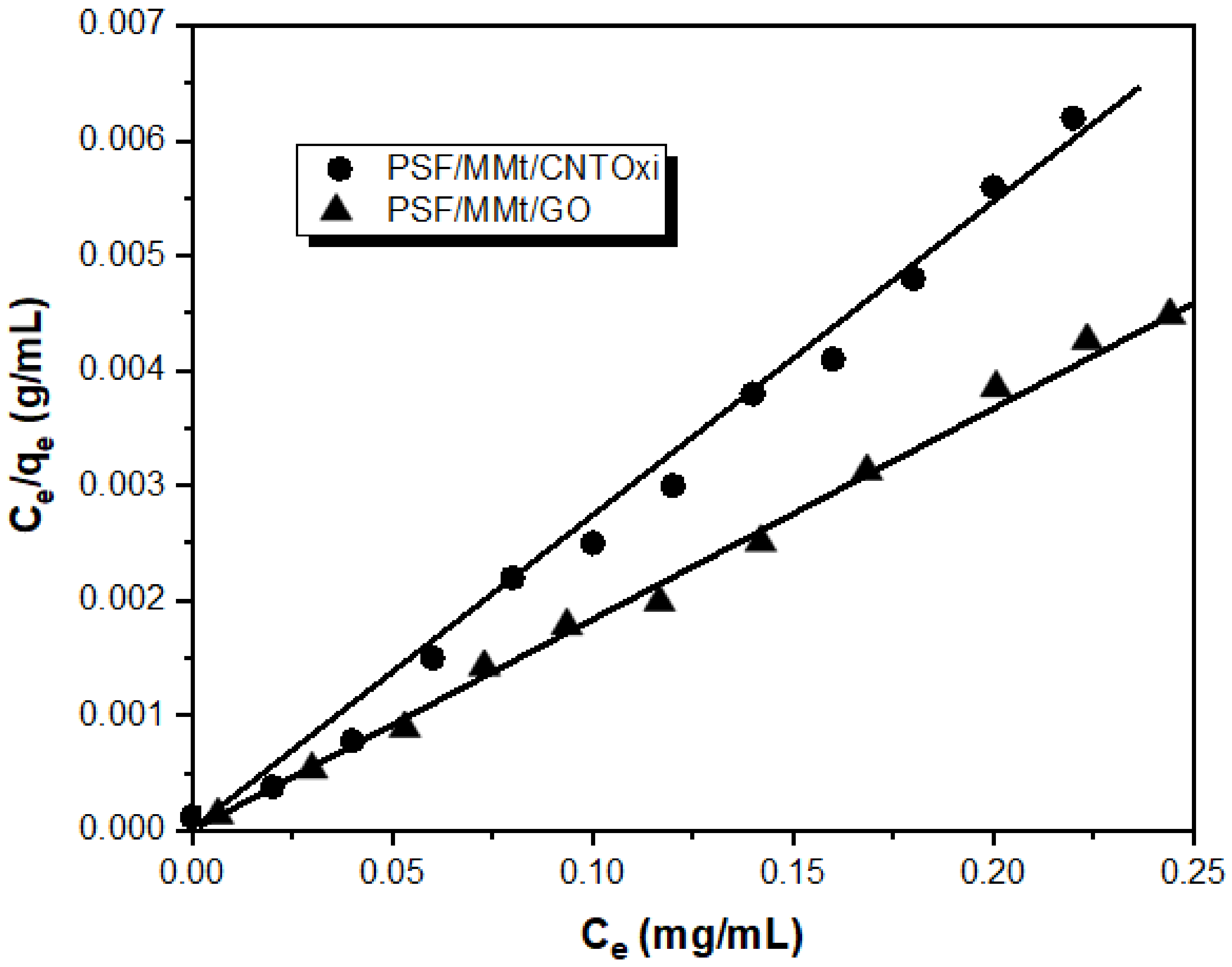
3.2.3. Adsorption Isotherm Models
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Lu, X.; Lu, X.; Wang, Z.; Ma, J.; Wang, P. Improved surface hydrophilicity and antifouling property of polysulfone ultrafiltration membrane with poly(ethylene glycol) methyl ether methacrylate grafted graphene oxide nanofillers. Appl. Surf. Sci. 2017, 425, 603–613. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.-H.; Kim, I.S. Novel sulfonated graphene oxide incorporated polysulfone nanocomposite membranes for enhanced-performance in ultrafiltration process. Chemosphere 2018, 207, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Sun, W.; Liu, H.-Y.; Huang, Y.; Gao, J.; Mai, Y.-W. Effects of carboxylated carbon nanotubes on the phase separation behaviour and fracture-mechanical properties of an epoxy/polysulfone blend. Compos. Sci. Technol. 2018, 159, 180–188. [Google Scholar] [CrossRef]
- Hwang, T.; Oh, J.-S.; Yim, W.; Nam, J.-D.; Bae, C.; Kim, H.-I.; Kim, K. Ultrafiltration using graphene oxide surface-embedded polysulfone membranes. Sep. Purif. Technol. 2016, 166, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, M.R.; Tyler, J.; Stretz, H.; Wells, M.J. Effects of a dual nanofiller, nano-TiO2 and MWCNT, for polysulfone-based nanocomposite membranes for water purification. Desalination 2015, 372, 47–56. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Efficiently improved oil/water separation using high flux and superior antifouling polysulfone hollow fiber membranes modified with functionalized carbon nanotubes/graphene oxide nanohybrid. J. Environ. Chem. Eng. 2019, 7, 102944. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Alamry, K.A.; Hussein, M.A.; Marwani, H.M.; Asiri, A.M. Sulfone-modified chitosan as selective adsorbent for the extraction of toxic Hg(II) metal ions. Adsorpt. Sci. Technol. 2018, 37, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Bassyouni, M.; Abdel-Aziz, M.; Zoromba, M.S.; Abdel-Hamid, S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Buruga, K.; Song, H.; Shang, J.; Bolan, N.; Jagannathan, T.K.; Kim, K.-H. A review on functional polymer-clay based nanocomposite membranes for treatment of water. J. Hazard. Mater. 2019, 379, 120584. [Google Scholar] [CrossRef] [PubMed]
- Kochameshki, M.G.; Marjani, A.; Mahmoudian, M.; Farhadi, K. Grafting of diallyldimethylammonium chloride on graphene oxide by RAFT polymerization for modification of nanocomposite polysulfone membranes using in water treatment. Chem. Eng. J. 2017, 309, 206–221. [Google Scholar] [CrossRef]
- Zambianchi, M.; Durso, M.; Liscio, A.; Treossi, E.; Bettini, C.; Capobianco, M.; Aluigi, A.; Kovtun, A.; Ruani, G.; Corticelli, F.; et al. Graphene oxide doped polysulfone membrane adsorbers for the removal of organic contaminants from water. Chem. Eng. J. 2017, 326, 130–140. [Google Scholar] [CrossRef]
- Serbanescu, O.; Voicu, S.; Thakur, V. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Mocanu, A.; Rusen, E.; Diacon, A.; Isopencu, G.; Mustățea, G.; Şomoghi, R.; Dinescu, A. Antimicrobial properties of polysulfone membranes modified with carbon nanofibers and silver nanoparticles. Mater. Chem. Phys. 2018, 223, 39–45. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Hocine, N.A. Clays and carbon nanotubes as hybrid nanofillers in thermoplastic-based nanocomposites—A review. Appl. Clay Sci. 2019, 185, 105408. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Development of novel SiO2–GO nanohybrid/polysulfone membrane with enhanced performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Chai, P.; Mahmoudi, E.; Teow, Y.; Mohammad, A. Preparation of novel polysulfone-Fe3O4/GO mixed-matrix membrane for humic acid rejection. J. Water Process. Eng. 2017, 15, 83–88. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, J.; Wang, S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Kumar, M.; Algamdi, M.S.; Khan, M.A.; Nolan, K.; Lawler, J. Antifouling hybrid ultrafiltration membranes with high selectivity fabricated from polysulfone and sulfonic acid functionalized TiO2 nanotubes. Chem. Eng. J. 2017, 316, 573–583. [Google Scholar] [CrossRef]
- Ammar, A.; Elzatahry, A.; Al-Maadeed, S.; Alenizi, A.M.; Huq, A.F.; Karim, A. Nanoclay compatibilization of phase separated polysulfone/polyimide films for oxygen barrier. Appl. Clay Sci. 2017, 137, 123–134. [Google Scholar] [CrossRef]
- Daraei, P.; Ghaemi, N. Synergistic effect of Cloisite 15A and 30B nanofillers on the characteristics of nanocomposite polyethersulfone membrane. Appl. Clay Sci. 2019, 172, 96–105. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Biswas, J.K.; Vithanage, M.; Bhatnagar, A.; et al. Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2019, 383, 121125. [Google Scholar] [CrossRef]
- Rodrigues, R.; Mierzwa, J.C.; Vecitis, C.D. Mixed matrix polysulfone/clay nanoparticles ultrafiltration membranes for water treatment. J. Water Process. Eng. 2019, 31, 100788. [Google Scholar] [CrossRef]
- Singla, P.; Mehta, R.; Upadhyay, S.N. Clay Modification by the Use of Organic Cations. Green Sustain. Chem. 2012, 2, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Miao, L.; Zhao, Y.; Lü, C. Azide-assisted crosslinked quaternized polysulfone with reduced graphene oxide for highly stable anion exchange membranes. J. Membr. Sci. 2017, 530, 84–94. [Google Scholar] [CrossRef]
- Lei, T.; Xue, Q.; Chu, L.; Han, Z.; Sun, J.; Xia, F.; Zhang, Z.; Guo, Q. Excellent dielectric properties of polymer composites based on core-shell structured carbon/silica nanohybrid. Appl. Phys. Lett. 2013, 103, 12902. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H.; Ding, L.; Zhu, L.; Dai, X.; Fei, X.; Li, F.; Gong, X. Utilization of silane functionalized carbon nanotubes-silica hybrids as novel reinforcing fillers for solution styrene butadiene rubber. Polym. Compos. 2013, 34, 690–696. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. Part B Eng. 2014, 59, 133–139. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Biswas, P.; Fortner, J.D. Graphene oxides as nanofillers in polysulfone ultrafiltration membranes: Shape matters. J. Membr. Sci. 2019, 581, 453–461. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Miguel, V.S.; Baselga, J.; Fernández-Blázquez, J.P.; Gedler, G.; Ozisik, R.; Cabanelas, J.C. Effect of polysulfone brush functionalization on thermo-mechanical properties of melt extruded graphene/polysulfone nanocomposites. Carbon 2019, 151, 84–93. [Google Scholar] [CrossRef]
- Jiang, Y.; Biswas, P.; Fortner, J.D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 915–922. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, M.; Xu, Z.; Jiang, C.; Shen, C.; Meng, Q. Guanidyl-functionalized graphene/polysulfone mixed matrix ultrafiltration membrane with superior permselective, antifouling and antibacterial properties for water treatment. J. Colloid Interface Sci. 2019, 540, 295–305. [Google Scholar] [CrossRef]
- Zunita, M. Graphene Oxide-Based Nanofiltration for Hg Removal from Wastewater: A Mini Review. Membranes 2021, 11, 269. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- Saleh, T.A.; Parthasarathy, P.; Irfan, M. Advanced functional polymer nanocomposites and their use in water ultra-purification. Trends Environ. Anal. Chem. 2019, 24. [Google Scholar] [CrossRef]
- Xu, L.; He, J.; Yu, Y.; Chen, J.P. Effect of CNT content on physicochemical properties and performance of CNT composite polysulfone membranes. Chem. Eng. Res. Des. 2017, 121, 92–98. [Google Scholar] [CrossRef]
- de Lannoy, C.-F.; Soyer, E.; Wiesner, M.R. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J. Membr. Sci. 2013, 447, 395–402. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Vatanpour, V.; Haghighat, N. Improvement of polyvinyl chloride nanofiltration membranes by incorporation of multiwalled carbon nanotubes modified with triethylenetetramine to use in treatment of dye wastewater. J. Environ. Manag. 2019, 242, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, S.; Rubiolo, G.; Salazar, A.; Jimeno, A.; Corcuera, M.A.; Mondragon, I. Carboxylation treatment of multiwalled carbon nanotubes monitored by infrared and ultraviolet spectroscopies and scanning probe microscopy. Diam. Relat. Mater. 2007, 16, 412–417. [Google Scholar] [CrossRef]
- Jyothi, M.; Nayak, V.; Padaki, M.; Balakrishna, R.G.; Soontarapa, K. Aminated polysulfone/TiO2 composite membranes for an effective removal of Cr(VI). Chem. Eng. J. 2016, 283, 1494–1505. [Google Scholar] [CrossRef]
- Alosaimi, A.; Hussein, M.; Abdelaal, M.; Elfaky, M.; Sobahi, T.; Abdel-Daiem, A. Polysulfone-based modified organoclay nanocomposites as a promising breast anticancer agent. Cogent Chem. 2017, 3, 1417672. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Li, J.; Mamba, B.B. Critical review of montmorillonite/polymer mixed-matrix filtration membranes: Possibilities and challenges. Appl. Clay Sci. 2018, 168, 21–30. [Google Scholar] [CrossRef]
- Benally, C.; Li, M.; El-Din, M.G. The effect of carboxyl multiwalled carbon nanotubes content on the structure and performance of polysulfone membranes for oil sands process-affected water treatment. Sep. Purif. Technol. 2018, 199, 170–181. [Google Scholar] [CrossRef]
- Shah, P.; Murthy, C. Studies on the porosity control of MWCNT/polysulfone composite membrane and its effect on metal removal. J. Membr. Sci. 2013, 437, 90–98. [Google Scholar] [CrossRef]


| Code | Clay % (g) | Carbon-Filler Loading (%) | Carbon-Filler Loading (g) |
|---|---|---|---|
| Pure PSF | - | - | - |
| PSF/MMt | 5 (0.2) | - | - |
| PSF/MMt/G | 2.5 (0.1) | 2.5 | 0.1 |
| PSF/MMt/GO | 2.5 (0.1) | 2.5 | 0.1 |
| PSF/MMt/CNTs | 2.5 (0.1) | 2.5 | 0.1 |
| PSF/MMt/CNTOxi | 2.5 (0.1) | 2.5 | 0.1 |
| T10 (°C) | T50 (°C) | Residue at 750 (°C) % | |
|---|---|---|---|
| Pure PSF | 288 | 411 | 4.7 |
| PSF/MMt | 90 | 460 | 6.6 |
| PSF/MMt/G | 316 | 515 | 10.8 |
| PSF/MMt/GO | 246 | 505 | 4.7 |
| PSF/MMt/CNTs | 350 | 566 | 10.8 |
| PSF/MMt/CNTOxi | 316 | 452 | 4.7 |
| Code | Flexural Properties | Modulus of Elasticity in Tension (MPa) | Tensile Strain at Break (%) | ||
|---|---|---|---|---|---|
| Strength (MPa) | Modulus (GPa) | Toughness (kJ/m2) | |||
| PSF | 3.86 (±0.61) | 96.68 (±0.28) | 3.54 (±0.65) | 73.15 (±1.6) | 30.22 (±0.54) |
| PSF/MMt | 4.47 (±0.98) | 132.61 (±1.22) | 4.03 (±0.23) | 70.08 (±1.2) | 29.43 (±0.27) |
| PSF/MMt/G | 5.83 (±0.22) | 134.92 (±1.07) | 5.54 (±0.12) | 54.21 (±1.5) | 18.65 (±0.71) |
| PSF/MMt/GO | 6.03 (±0.28) | 139.32 (±1.02) | 5.88 (±0.82) | 58.27 (±1.7) | 14.03(±0.56) |
| PSF/MMt/CNTs | 5.46 (±0.12) | 140.67 (±1.43) | 4.98 (±0.75) | 52.34 (±1.3) | 16.05 (±0.32) |
| PSF/MMt/CNTOxi | 6.84 (±0.16) | 143.42 (±0.99) | 5.15 (±0.41) | 60.22 (±1.4) | 12.94 (±0.19) |
| Code | Tensile Properties | Impact Strength (KJ/m2) | ||
|---|---|---|---|---|
| Strength (MPa) | Modulus (GPa) | Toughness (kJ/m2) | ||
| PSF | 3.96 (±0.13) | 99.26 (±0.87) | 6.43 (±1.94) | 1.25 (±0.86) |
| PSF/MMt | 4.32 (±0.52) | 114.57 (±1.08) | 7.28 (±0.76) | 1.45 (±1.18) |
| PSF/MMt/G | 5.68 (±0.45) | 128.28 (±1.25) | 7.77 (±0.58) | 1.60 (±1.04) |
| PSF/MMt/GO | 6.07 (±0.86) | 134.44 (±1.13) | 9.34 (±1.11) | 1.69 (±0.82) |
| PSF/MMt/CNTs | 6.82 (±0.82) | 139.92 (±1.05) | 7.08 (±1.26) | 1.62 (±1.07) |
| PSF/MMt/CNTOxi | 7.54 (±0.91) | 133.51 (±1.55) | 8.40 (±1.31) | 1.76 (±0.99) |
| Material | Metal Ions | qe (mg/g) | Kd (mL/g) |
|---|---|---|---|
| PSF/MMt/CNTOxi | Hg+2 | 2.08 | 61,357.25 |
| Ni+2 | 0.11 | 49.57 | |
| Fe+3 | 0.34 | 167.82 | |
| Cr+3 | 0.23 | 32.41 | |
| Y+3 | 0.37 | 45.67 | |
| Al+3 | 0.46 | 147.69 | |
| Pb+2 | 0.59 | 186.43 | |
| Zn+2 | 0.14 | 55.67 | |
| Co+2 | 0.29 | 42.83 | |
| Sr+2 | 0.04 | 12.87 | |
| PSF/MMt/G | Hg+2 | 1.12 | 1829.46 |
| PSF/MMt/GO | Hg+2 | 1.98 | 56,947.72 |
| PSF/MMt/CNTs | Hg+2 | 1.19 | 1958.31 |
| P/MMt | Hg+2 | 0.35 | 77.82 |
| Pure PSF | Hg+2 | 0.17 | 62.17 |
| Material | Qo (mg/g) | b (L/mg) | R2 | RL |
|---|---|---|---|---|
| PSF/MMt/CNTOxi | 149.36 | 0.36 | 0.992 | 0.04 |
| PSF/MMt/GO | 142.48 | 0.28 | 0.985 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alosaimi, A.M. Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions. Polymers 2021, 13, 2792. https://doi.org/10.3390/polym13162792
Alosaimi AM. Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions. Polymers. 2021; 13(16):2792. https://doi.org/10.3390/polym13162792
Chicago/Turabian StyleAlosaimi, Abeer M. 2021. "Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions" Polymers 13, no. 16: 2792. https://doi.org/10.3390/polym13162792
APA StyleAlosaimi, A. M. (2021). Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions. Polymers, 13(16), 2792. https://doi.org/10.3390/polym13162792