Implicit Memory and Anesthesia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility and Inclusion
2.3. Data Extraction
2.4. End-Points
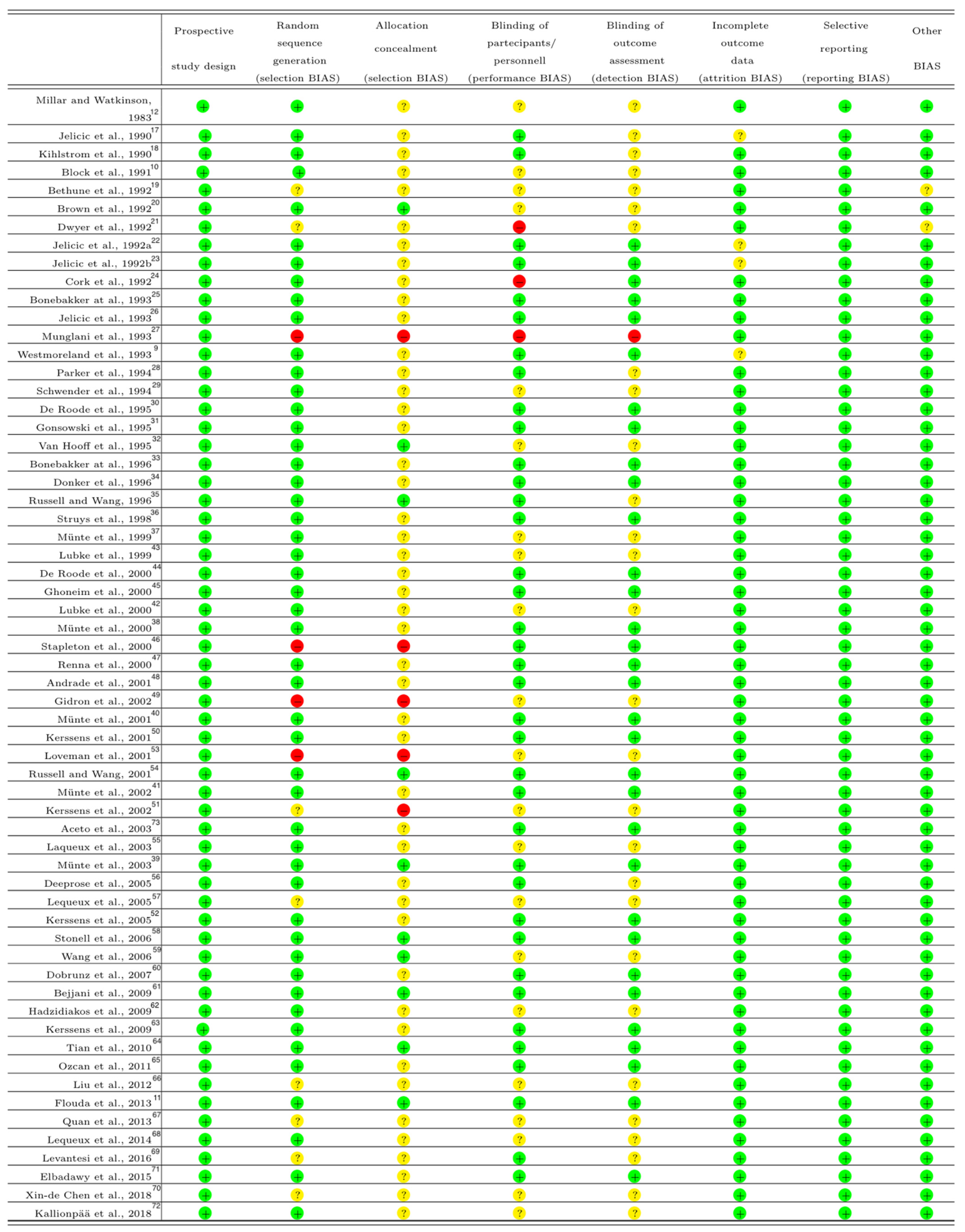
2.5. Assessment of Risk of Bias
2.6. Statistical Analysis
3. Results
3.1. Implicit Memory Rates after General Anesthesia and OAA/S 1 Sedation
3.2. Implicit Memory Rates after General Anesthesia
3.3. Implicit Memory Rates after General Anesthesia with Inhalational Maintenance
3.4. Implicit Memory Rates after General Anesthesia with Intravenous Maintenance
4. Discussion
4.1. Patient Characteristic Considerations
4.2. Anesthesiologic Considerations
4.3. Auditory Task Characteristic Considerations
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, R.D.; Tononi, G.; Laureys, S.; Sleigh, J.W. Unresponsiveness ≠ unconsciousness. Anesthesiology 2012, 116, 946–959. [Google Scholar] [CrossRef] [Green Version]
- Eger, E.I.; Sonner, J.M. Anaesthesia defined (gentlemen, this is no humbug). Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 23–29. [Google Scholar] [CrossRef]
- Chernik, A.D.; Gillings, D.; Laine, H.; Hendler, J.; Silver, J.M.; Davidson, A.B.; Schwam, E.M.; Siegel, J.L. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J. Clin. Psychopharmacol. 1990, 10, 244–251. [Google Scholar]
- Linassi, F.; Zanatta, P.; Tellaroli, P.; Ori, C.; Carron, M. Isolated forearm technique: A meta-analysis of connected consciousness during different general anaesthesia regimens. Br. J. Anaesth. 2018, 121, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Osterman, J.E.; Hopper, J.; Heran, W.J.; Keane, T.M.; van der Kolk, B.A. Awareness under anesthesia and the development of post-traumatic stress disorder. Gen. Hosp. Psychiatry 2001, 23, 198–204. [Google Scholar] [CrossRef]
- Avidan, M.S.; Mashour, G.A. Prevention of intraoperative awareness with explicit recall: Making sense of the evidence. Anesthesiology 2013, 118, 449–456. [Google Scholar] [CrossRef]
- Polster, M.R.; Gray, P.A.; O’Sullivan, G.; McCarthy, R.A.; Park, G.R. Comparison of the sedative and amnesic effects of midazolam and propofol. Br. J. Anaesth. 1993, 70, 612–616. [Google Scholar] [CrossRef]
- Kihlstrom, J.F.; Dorfman, J.; Park, L. Conscious and Unconscious Memory. Blackwell Companion Conscious 2017, 562–575. [Google Scholar] [CrossRef]
- Westmoreland, C.L.; Sebel, P.S.; Winograd, E.; Goldman, W.P. Indirect Memory during Anesthesia. Anesthesiology 1993, 78, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Block, R.; Ghoneim, M.M.M.; Ping, M.S.T.S.; Ali, M.M.A. Human Learning during General Anaesthesia and Surgery. Br. J. Anaesth. 1991, 66, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Flouda, L.; Pandazi, A.; Papageorgiou, C.; Perrea, D.; Krepi, E.; Kostopanagiotou, G. Comparative effects of sevoflurane and propofol based general anaesthesia for elective surgery on memory. Arch. Med. Sci. 2013, 1, 105–111. [Google Scholar] [CrossRef]
- Millar, K.; Watkinson, N. Recognition of words presented during general anaesthesia. Ergonomics 1983, 26, 585–594. [Google Scholar] [CrossRef]
- Buchner, A.; Erdfelder, E.; Vaterrodt-Plunnecke, B. Toward unbiased measurement of conscious and unconscious memory pro-cesses within the process dissociation framework. J. Exp. Psychol. Gen. 1995, 124, 137–160. [Google Scholar] [CrossRef]
- Andrade, J. Learning during anaesthesia: A review. Br. J. Psychol. 1995, 86, 479–506. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, G.; Carpenter, J.; Rücker, G. Meta-Analysis with R.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Jelicic, M.; Bonke, B.; Appelboom, D. Indirect memory for words presented during anaesthesia. Lancet 1990, 336, 249. [Google Scholar] [CrossRef]
- Kihlstrom, J.F.; Schacter, D.L.; Cork, R.C.; Hurt, C.A.; Behr, S.E. Implicit and Explicit Memory following Surgical Anesthesia. Psychol. Sci. 1990, 1, 303–306. [Google Scholar] [CrossRef]
- Bethune, D.W.; Ghosh, S.; Gray, B.; Kerr, L.; Walker, I.A.; Doolan, L.A.; Harwood, R.J.; Sharples, L.D. Learning during General Anaesthesia: Implicit Recall after Methohexitone or Propofol Infusion. Br. J. Anaesth. 1992, 69, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Best, M.R.; Mitchell, D.B.; Haggard, L.C. Memory under anesthesia: Evidence for response suppression. Bull. Psychon. Soc. 1992, 30, 244–246. [Google Scholar] [CrossRef]
- Dwyer, R.; Bennett, H.L.; Eger, E.I.; Peterson, N. Isoflurane anesthesia prevents unconscious learning. Anesth. Analg. 1992, 75, 107–112. [Google Scholar] [CrossRef]
- Jelicic, M.; Bonke, B.; Wolters, G.; Phaf, R.H. Implicit memory for words presented during anaesthesia. Eur. J. Cogn. Psychol. 1992, 4, 71–80. [Google Scholar] [CrossRef]
- Jelicic, M.; De Roode, A.; Bovill, J.G.; Bonke, B. Unconscious learning during anaesthesia. Anaesthesia 1992, 47, 835–837. [Google Scholar] [CrossRef]
- Cork Randall, C.; Kihistrom John, F.; Schacter Daniel, L. Absence of Explicit or Implicit Memory in Patients Anesthetized with Sufentanil/Nitrous Oxide. Anesthesiology 1992, 76, 892–898. [Google Scholar] [CrossRef]
- Bonebakker, A.E.; Bonke, B.; Klein, J.; Wolters, G.; Hop, W.C.J. Implicit memory during balanced anaesthesia. Anaesthesia 1993, 48, 657–660. [Google Scholar] [CrossRef]
- Jelicic, M.; Asbury, A.J.; Millar, K.; Bonke, B. Implicit learning during enflurane anaesthesia in spontaneously breathing patients? Anaesthesia 1993, 48, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Munglani, R.; Andrade, J.; Sapsford, D.J.; Baddeley, A.; Jones, J.G. A measure of consciousness and memory during isoflurane ad-ministration: The coherent frequency. Br. J. Anaesth. 1993, 71, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.J.R.; Oates, J.D.L.; Boyd, A.H.; Thomas, J.D. Memory for auditory material presented during anaesthesia. Br. J. Anaesth. 1994, 73, 123–124. [Google Scholar] [CrossRef] [Green Version]
- Schwender, D.; Kaiser, A.; Klasing, S.; Peter, K.; Pöppel, E. Midlatency Auditory Evoked Potentials and Explicit and Implicit Memory in Patients Undergoing Cardiac Surgery. Anesthesiology 1994, 80, 493–501. [Google Scholar] [CrossRef] [PubMed]
- De Roode, A.; Jelicic, M.; Bonke, B.; Bovill, J.G. The effect of midazolam premedication on implicit memory activation during alfentanil-nitrous oxide anaesthesia. Anaesthesia 1995, 50, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Gonsowski, C.T.; Chortkoff, B.S.; Eger, E.I.; Bennett, H.L.; Weiskopf, R.B. Subanesthetic concentrations of desflurane and isoflu-rane suppress explicit and implicit learning. Anesth. Analg. 1995, 80, 568–572. [Google Scholar]
- Van Hooff, J.C.; De Beer, N.A.; Brunia, C.H.; Cluitmans, P.J.; Korsten, H.H.; Tavilla, G.; Grouls, R. Information processing during cardiac surgery: An event related potential study. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 433–452. [Google Scholar] [CrossRef]
- Bonebakker, A.; Bonke, B.; Klein, J.; Wolters, G.; Stijnen, T.; Passchier, J.; Merikle, P.M. Information processing during general anesthesia: Evidence for unconscious memory. Mem. Cogn. 1996, 24, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Donker, A.G.; Phaf, R.H.; Porcelijn, T.; Bonke, B. Processing familiar and unfamiliar auditory stimuli during general anesthesia. Anesth. Analg. 1996, 82, 452–455. [Google Scholar]
- Russell, I.F.; Wang, M. Absence of memory for intraoperative information during surgery under adequate general anaesthesia. Br. J. Anaesth. 1997, 78, 3–9. [Google Scholar] [CrossRef]
- Struys, M.; Versichelen, L.; Byttebier, G.; Mortier, E.; Moerman, A.; Rolly, G. Clinical usefulness of the bispectral index for titrating propofol target effect-site concentration. Anaesthesia 1998, 53, 4–12. [Google Scholar] [CrossRef]
- Munte, S.; Kobbe, I.; Demertzis, A.; Lullwitz, E.; Munte, T.F.; Piepenbrock, S.; Leuwer, M. Increased Reading Speed for Stories Presented during General Anesthesia. Anesthesiology 1999, 90, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Münte, S.; Lüllwitz, E.; Leuwer, M.; Mitzlaff, B.; Münte, T.F.; Hussein, S.; Piepenbroc, S.A. No implicit memory for stories played during isoflurane/alfentanil/nitrous oxide anes-thesia: A reading speed measurement. Anesth. Analg. 2000, 90, 733–738. [Google Scholar] [CrossRef]
- Münte, S.; Münte, T.F.; Grotkamp, J.; Haeseler, G.; Raymondos, K.; Piepenbrock, S.; Kraus, G. Implicit memory varies as a function of hypnotic electroencephalogram stage in surgi-cal patients. Anesth. Analg. 2003, 97, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Münte, S.; Münte, T.F.; Mitzlaff, B.; Walz, R.; Leuwer, M.; Piepenbrock, S. Postoperative reading speed does not indicate implicit memory in elderly cardiac patients after propofol and remifentanyl anaesthesia. Acta Anaesthesiol. Scand. 2001, 45, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Münte, S.; Schmidt, M.; Meyer, M.; Nager, W.; Lüllwitz, E.; Münte, T.F.; Piepenbrock, S. Implicit memory for words played during isoflurane-or propofol-based anesthesia: The lexical decision task. Anesthesiology 2002, 96, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Lubke, G.H.; Kerssens, C.; Gershon, R.Y.; Sebel, P.S. Memory Formation during General Anesthesia for Emergency Cesarean Sections. Anesthesiology 2000, 92, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Lubke, G.H.; Kerssens, C.; Phaf, H.; Sebel, P.S. Dependence of Explicit and Implicit Memory on Hypnotic State in Trauma Patients. Anesthesiology 1999, 90, 670–680. [Google Scholar] [CrossRef]
- De Roode, A.; van Gerven, J.M.; Schoemaker, R.C.; Engbers, F.H.; Olieman, W.; Kroon, J.R.; Cohen, A.F.; Bovill, J.G. A comparison of the effects of propofol and midazolam on memory dur-ing two levels of sedation by using target-controlled infusion. Anesth. Analg. 2000, 91, 1056–1061. [Google Scholar] [PubMed]
- Ghoneim, M.M.; Block, R.; Dhanaraj, V.J.; Todd, M.M.; Choi, W.W.; Brown, C.K. Auditory evoked responses and learning and awareness during general anesthesia. Acta Anaesthesiol. Scand. 2000, 44, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, C.L.; Andrade, J. An Investigation of Learning during Propofol Sedation and Anesthesia Using the Process Dissociation Procedure. Anesthesiology 2000, 93, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Lang, E.M.; Lockwood, G.G. The effect of sevoflurane on implicit memory: A double-blind, randomised study. Anaesthesia 2000, 55, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Englert, L.; Harper, C.; Edwards, N. Comparing the effects of stimulation and propofol infusion rate on implicit and explicit memory formation. Br. J. Anaesth. 2001, 86, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidron, Y.; Barak, T.; Henik, A.; Gurman, G.; Stiener, O. Implicit learning of emotional information under anesthesia. NeuroReport 2002, 13, 139–142. [Google Scholar] [CrossRef]
- Kerssens, C.; Klein, J.; van der Woerd, A.; Bonke, B. Auditory information processing during adequate propofol anesthesia moni-tored by electroencephalogram bispectral index. Anesth. Analg. 2001, 92, 1210–1214. [Google Scholar] [CrossRef] [Green Version]
- Kerssens, C.; Lubke, G.H.; Klein, J.; van der Woerd, A.; Bonke, B. Memory function during propofol and alfentanil anesthesia: Pre-dictive value of individual differences. Anesthesiology 2002, 97, 382–389. [Google Scholar] [CrossRef]
- Kerssens, C.; Ouchi, T.; Sebel, P.S. No evidence of memory function during anesthesia with propofol or isoflurane with close con-trol of hypnotic state. Anesthesiology 2005, 102, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Loveman, E.; Van Hooff, J.; Smith, D. The auditory evoked response as an awareness monitor during anaesthesia. Br. J. Anaesth. 2001, 86, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, I.F.; Wang, M. Absence of memory for intra-operative information during surgery with total intravenous anaesthesia. Br. J. Anaesth. 2001, 86, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lequeux, P.-Y.; Cantraine, F.; Levarlet, M.; Barvais, L. Absence of explicit and implicit memory in unconscious patients using a TCI of propofol. Acta Anaesthesiol. Scand. 2003, 47, 833–837. [Google Scholar] [CrossRef]
- Deeprose, C.; Andrade, J.; Harrison, D.; Edwards, N. Unconscious auditory priming during surgery with propofol and nitrous oxide anaesthesia: A replication. Br. J. Anaesth. 2005, 94, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lequeux, P.Y.; Velghe-Lenelle, C.E.; Cantraine, F.; Sosnowski, M.; Barvais, L. Absence of implicit and explicit memory during propofol/remifentanil anaesthesia. Eur. J. Anaesthesiol. 2005, 22, 333–336. [Google Scholar] [CrossRef]
- Stonell, C.; Leslie, K.; He, C.; Lee, L. No Sex Differences in Memory Formation during General Anesthesia. Anesthesiology 2006, 105, 920–926. [Google Scholar] [CrossRef]
- Wang, Y.; Yue, Y.; Sun, Y.-H.; Wu, A.-S. Can bispectral index or auditory evoked potential index predict implicit memory during propofol-induced sedation? Chin. Med. J. 2006, 119, 894–898. [Google Scholar] [CrossRef]
- Dobrunz, U.E.G.; Jaeger, K.; Vetter, G. Memory priming during light anaesthesia with desflurane and remifentanil anaesthesia. Br. J. Anaesth. 2007, 98, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Bejjani, G.; Lequeux, P.-Y.; Schmartz, D.; Engelman, E.; Barvais, L. No Evidence of Memory Processing During Propofol-Remifentanil Target-Controlled Infusion Anesthesia With Bispectral Index Monitoring in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2009, 23, 175–181. [Google Scholar] [CrossRef]
- Hadzidiakos, D.A.; Horn, N.; Degener, R.; Buchner, A.; Rehberg, B. Analysis of Memory Formation during General Anesthesia (Propofol/Remifentanil) for Elective Surgery Using the Process-dissociation Procedure. Anesthesiology 2009, 111, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerssens, C.; Gaither, J.R.; Sebel, P.S. Preserved memory function during bispectral index-guided anesthesia with sevoflurane for major orthopedic surgery. Anesthesiology 2009, 111, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.Y.; Zou, L.; Quan, X.; Zhang, Y.; Xue, F.S.; Ye, T.H. Effect of midazolam on memory: A study of process dissociation procedure and functional magnetic resonance imaging. Anaesthesia 2010, 65, 586–594. [Google Scholar] [CrossRef]
- Ozcan, M.; Gronlund, S.; Trojan, R.; Khan, Q.; Cure, J.; Wong, C. Does a BIS-Guided Maintenance of Anesthetic Depth Prevent Implicit Memory? Psychology 2011, 2, 143. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lauer, K.K.; Ward, B.D.; Rao, S.; Li, S.-J.; Hudetz, A.G. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum. Brain Mapp. 2012, 33, 2487–2498. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.; Yi, J.; Ye, T.H.; Tian, S.Y.; Zou, L.; Yu, X.R.; Huang, Y.G. Propofol and memory: A study using a process dissociation procedure and functional magnetic resonance imaging. Anaesthesia 2013, 68, 391–399. [Google Scholar] [CrossRef]
- Lequeux, P.-Y.; Hecquet, F.; Bredas, P. Does Anesthetic Regimen Influence Implicit Memory During General Anesthesia? Anesth. Analg. 2014, 119, 1174–1179. [Google Scholar] [CrossRef]
- Levantesi, L.; Oggiano, M.; Sicuranza, R.; Canistro, G.; Sessa, F.; de Waure, C.; Congedo, E.; De Cosmo, G. Relationships between Bispectral Index, Implicit Memory, Dream Recall and Minimum Alveolar Concentration in Blended Anaesthesia. J. Anesth. Crit. Care Open Access 2016, 5, 00194. [Google Scholar]
- Chen, X.D.; Xie, W.; Zhou, Q.H. Effect of propofol and sevoflurane on cognitive function among elderly patients undergoing elec-tive surgery under anesthesia. Pak. J. Pharm. Sci. 2018, 31, 2909–2913. [Google Scholar]
- Elbadawy, A.M.; Khidr, A.M.; Saleh, A.A. Comparative study of implicit memory during bispectral index guided total intrave-nous anesthesia versus sevoflurane inhalation anesthesia. Egypt. J. Anaesth. 2015, 31, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Kallionpaa, R.E.; Scheinin, A.; Kallionpaa, R.A.; Sandman, N.; Kallioinen, M.; Laitio, R.; Laitio, T.; Kaskinoro, K.; Kuusela, T.; Revonsuo, A. Spoken words are processed during dexmedetomidine-induced unresponsiveness. Br. J. Anaesth. 2018, 121, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Aceto, P.; Valente, A.; Gorgoglione, M.; Adducci, E.; De Cosmo, G. Relationship between awareness and middle latency auditory evoked responses during surgical anaesthesia. Br. J. Anaesth. 2003, 90, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.B.; Brown, A.S.; Murphy, D.R. Dissociations between procedural and episodic memory: Effects of time and aging. Psychol. Aging 1990, 5, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.M.; Weiskopf, R.B. Awareness during Anesthesia. Anesthesiology 2000, 92, 597. [Google Scholar] [CrossRef] [PubMed]
- Schnider, T.W.; Minto, C.F.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Shafer, S.L.; Youngs, E.J. The influence of method of administration and covariates on the pharmacokinet-ics of Propofol in adult volunteers. Anesthesiology 1998, 88, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Bertoncello, F.; Ieppariello, G. Profile of sugammadex for reversal of neuromuscular blockade in the elderly: Current perspectives. Clin. Interv. Aging 2017, 13, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Linassi, F.; Kreuzer, M.; Maran, E.; Farnia, A.; Zanatta, P.; Navalesi, P.; Carron, M. Age infuences on Propofol estimated brain con-centration and entropy during maintenance and at return of consciousenss during total intravenous anesthesia with target-controlled infusion in unparalyzed patients: An observational prospective trial. PLoS ONE 2020, 15, e0244145. [Google Scholar] [CrossRef]
- Ward, E.V.; Berry, C.J.; Shanks, D.R. Age effects on explicit and implicit memory. Front. Psychol. 2013, 4, 639. [Google Scholar] [CrossRef] [Green Version]
- Samuel, N.; Taub, A.; Paz, R.; Raz, A. Implicit aversive memory under anaesthesia in animal models: A narrative review. Br. J. Anaesth. 2018, 121, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Nashiro, K.; Sakaki, M.; Mather, M. Age differences in brain activity during emotion processing: Reflections of age-related de-cline or increased emotion regulation? Gerontology 2012, 58, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.E. Pharmacodynamic Considerations for Moderate and Deep Sedation. Anesth. Prog. 2012, 59, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Antognini, J.F.; Schwartz, K. Exaggerated Anesthetic Requirements in the Preferentially Anesthetized Brain. Anesthesiology 1993, 79, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.M.; Block, R.I.; Haffarnan, M.; Mathews, M.J. Awareness during anesthesia: Risk factors, causes and sequelae: A re-view of reported cases in the literature. Anesth. Analg. 2009, 108, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, T.M.; Andrade, J.; Bogod, D.G.; Hitchman, J.M.; Jonker, W.R.; Lucas, N.; Mackay, J.H.; Nimmo, A.F.; O’Connor, K.; O’Sullivan, E.P.; et al. The 5th National Audit Project (NAP5) on accidental awareness during general an-aesthesia: Patient experiences, human factors, sedation, consent and medicolegal issues. Anaesthesia 2014, 69, 1102–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veselis, R.A. Memory: A guide for anaesthetists. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Fan, K.M.; Qiao, Y.N.; Xu, J.H.; Qiu, L.J.; Li, X.; Liu, Y.; Qian, Z.Q.; Wei, C.L.; Han, J.; et al. Hippocampal µ-opioid receptors on GABAergic neurons mediate stress-induced impairment of memory retrieval. Mol. Psychiatry 2020, 25, 977–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, T.D. Are opioids indispensable for general anaesthesia? Br. J. Anaesth. 2019, 122, e127–e135. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Memory and Brain Systems: 1969–2009. J. Neurosci. 2009, 29, 12711–12716. [Google Scholar] [CrossRef] [PubMed]
- Fahy, B.G.; Chau, D.F. The Technology of Processed Electroencephalogram Monitoring Devices for Assessment of Depth of An-esthesia. Anesth. Analg. 2018, 126, 111–117. [Google Scholar] [CrossRef]
- Squire, L.R.; DeDe, A.J. Conscious and Unconscious Memory Systems. Cold Spring Harb. Perspect. Biol. 2015, 7, a021667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkire, M.T.; Gruver, R.; Miller, J.; McReynolds, J.R.; Hahn, E.L.; Cahill, L. Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc. Natl. Acad. Sci. USA 2008, 105, 1722–1727. [Google Scholar] [CrossRef] [Green Version]
- Veselis, R.A.; Reinsel, R.; Feshchenko, V.A.; Wronski, M. The Comparative Amnestic Effects of Midazolam, Propofol, Thiopental, and Fentanyl at Equisedative Concentrations. Anesthesiology 1997, 87, 749–764. [Google Scholar] [CrossRef]
- Chortkoff, B.S.; Bennett, H.L.; Eger, E.I. Subanesthetic concentrations of isoflurane suppress learning as defined by the cate-gory-example task. Anesthesiology 1993, 79, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pryor, K.; Root, J.; Mehta, M.; Stern, E.; Pan, H.; Veselis, R.; Silbersweig, D. Effect of propofol on the medial temporal lobe emotional memory system: A functional magnetic resonance imaging study in human subjects. Br. J. Anaesth. 2015, 115, i104–i113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perouansky, M.; Rau, V.; Ford, T.; Oh, S.I.; Perkins, M.; Eger, E.I. Slowing of the hippocampal θ-rhythm correlates with anesthetic-induced amnesia. Anesthesiology 2010, 113, 1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, K.; MacIver, M.B. Agent-selective effects of volatile anesthetics on GABAA receptor-mediated synaptic inhibition in hippocampal interneurons. Anesthesiology 2001, 94, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Aberg, K.C.; Albrecht, E.; Tartaglia, E.M.; Farron, A.; Soom, P.; Herzog, M.H. Anesthesia prevents auditory perceptual learning. Anesthesiology 2009, 111, 1010–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Implicit Memory | |||||
|---|---|---|---|---|---|---|
| Yes | No | OR | L95%CI | U95%CI | p-Value | |
| Age | ||||||
| ≤50 years | 31 | 58 | ||||
| >50 years | 11 | 10 | 2.06 | 0.79 | 5.38 | 0.22 |
| ASA | ||||||
| I–II | 23 | 56 | ||||
| III–IV | 10 | 7 | 3.48 | 1.18 | 10.25 | 0.04 |
| Type of anesthesia | ||||||
| General anesthesia | 42 | 61 | ||||
| Deep sedation | 1 | 15 | 0.10 | 0.01 | 0.76 | 0.017 |
| Duration | ||||||
| ≤60 min | 4 | 16 | ||||
| >60 min | 25 | 39 | 2.56 | 0.77 | 8.56 | 0.20 |
| Premedication | ||||||
| No benzodiazepines | 34 | 52 | ||||
| Benzodiazepines | 7 | 23 | 0.47 | 0.18 | 1.20 | 0.16 |
| Induction | ||||||
| No benzodiazepines | 40 | 70 | ||||
| Benzodizepines | 3 | 6 | 0.88 | 0.14 | 4.37 | 1.0 |
| Maintenance | ||||||
| Intravenous | 29 | 42 | ||||
| Inhalational | 14 | 34 | 1.68 | 0.77 | 3.67 | 0.26 |
| No N2O during maintenance | 21 | 46 | ||||
| N2O during maintenance | 22 | 30 | 1.61 | 0.76 | 3.42 | 0.30 |
| No benzodiazepines | 42 | 74 | ||||
| Benzodiazepines | 1 | 2 | 0.88 | 0.02 | 17.42 | 1.0 |
| No opioids | 6 | 23 | ||||
| Opioids | 37 | 53 | 2.68 | 0.99 | 7.21 | 0.08 |
| No NMBA | 4 | 18 | ||||
| NMBA | 39 | 58 | 3.02 | 0.95 | 9.62 | 0.09 |
| No light anesthetic regimen | 29 | 52 | ||||
| Light anesthetic regimen | 14 | 24 | 1.05 | 0.47 | 2.33 | 1.0 |
| No deep anesthetic regimen | 14 | 25 | ||||
| Deep anesthetic regimen | 29 | 51 | 1.02 | 0.46 | 2.25 | 1.0 |
| No light analgesic regimen | 25 | 41 | ||||
| Light analgesic regimen | 18 | 35 | 0.84 | 0.40 | 1.80 | 0.80 |
| No deep analgesic regimen | 18 | 25 | ||||
| Deep analgesic regimen | 25 | 51 | 1.02 | 0.46 | 2.25 | 1.0 |
| Monitoring | ||||||
| No ABM monitoring | 24 | 48 | ||||
| ABM monitoring | 15 | 28 | 1.07 | 0.48 | 2.38 | 1.0 |
| No AMB-guided anesthesia | 36 | 57 | ||||
| ABM-guided anesthesia | 7 | 18 | 0.62 | 0.23 | 1.62 | 0.45 |
| Listening to the auditory task | ||||||
| No during surgical stimulation | 5 | 19 | ||||
| During surgical stimulation | 38 | 57 | 2.53 | 0.87 | 7.37 | 0.13 |
| No during all the maintenance period | 31 | 52 | ||||
| During all the maintenance period | 7 | 16 | 0.73 | 0.27 | 1.98 | 0.71 |
| Timing of memory testing | ||||||
| ≤24 h | 31 | 54 | ||||
| >24 h | 12 | 20 | 1.05 | 0.45 | 2.42 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linassi, F.; Obert, D.P.; Maran, E.; Tellaroli, P.; Kreuzer, M.; Sanders, R.D.; Carron, M. Implicit Memory and Anesthesia: A Systematic Review and Meta-Analysis. Life 2021, 11, 850. https://doi.org/10.3390/life11080850
Linassi F, Obert DP, Maran E, Tellaroli P, Kreuzer M, Sanders RD, Carron M. Implicit Memory and Anesthesia: A Systematic Review and Meta-Analysis. Life. 2021; 11(8):850. https://doi.org/10.3390/life11080850
Chicago/Turabian StyleLinassi, Federico, David Peter Obert, Eleonora Maran, Paola Tellaroli, Matthias Kreuzer, Robert David Sanders, and Michele Carron. 2021. "Implicit Memory and Anesthesia: A Systematic Review and Meta-Analysis" Life 11, no. 8: 850. https://doi.org/10.3390/life11080850
APA StyleLinassi, F., Obert, D. P., Maran, E., Tellaroli, P., Kreuzer, M., Sanders, R. D., & Carron, M. (2021). Implicit Memory and Anesthesia: A Systematic Review and Meta-Analysis. Life, 11(8), 850. https://doi.org/10.3390/life11080850







