Bioevaluation of Pheretima vulgaris Antithrombotic Extract, PvQ, and Isolation, Identification of Six Novel PvQ-Derived Fibrinolytic Proteases
Abstract
:1. Introduction
2. Results
2.1. Enrichment of Active Extract
2.2. Determination of Fibrinolytic Activity
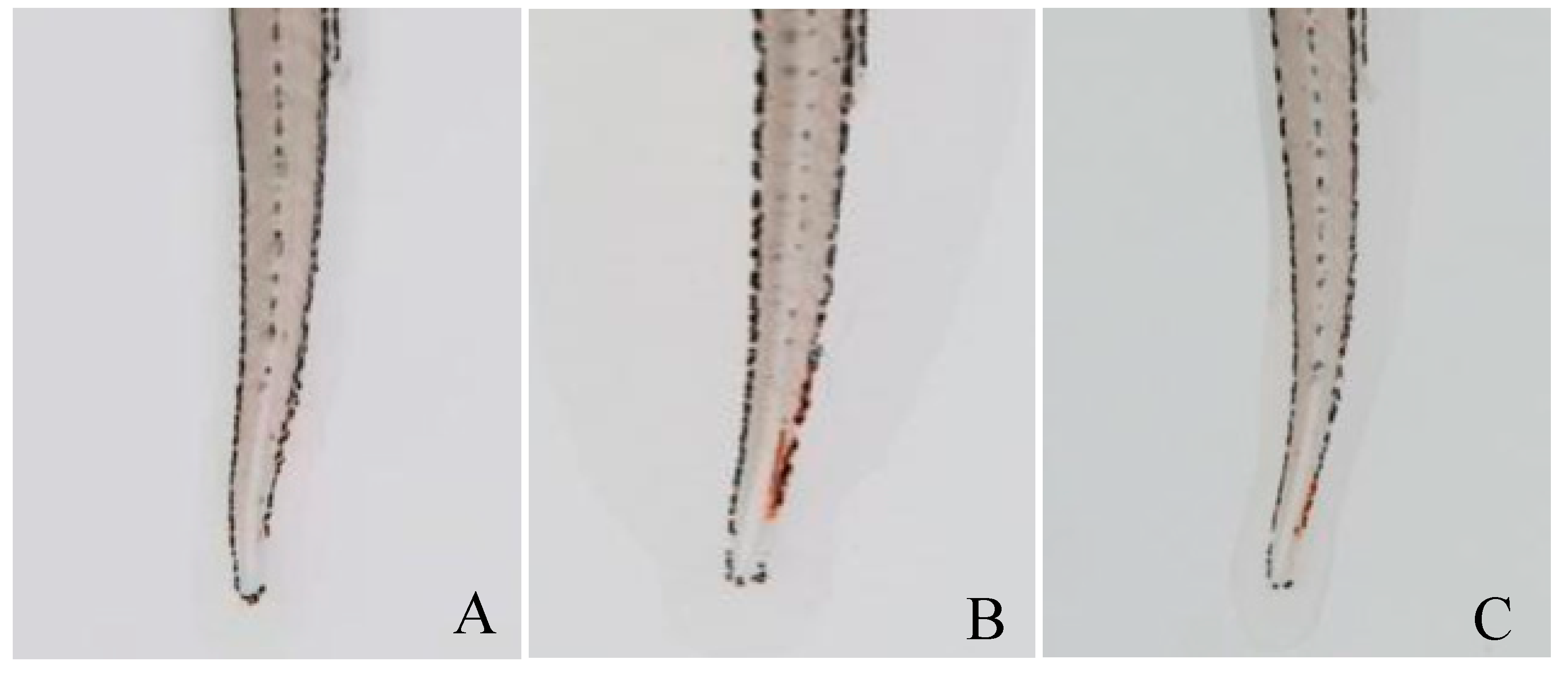
2.3. Evaluation of Thrombolytic Activity In Vivo
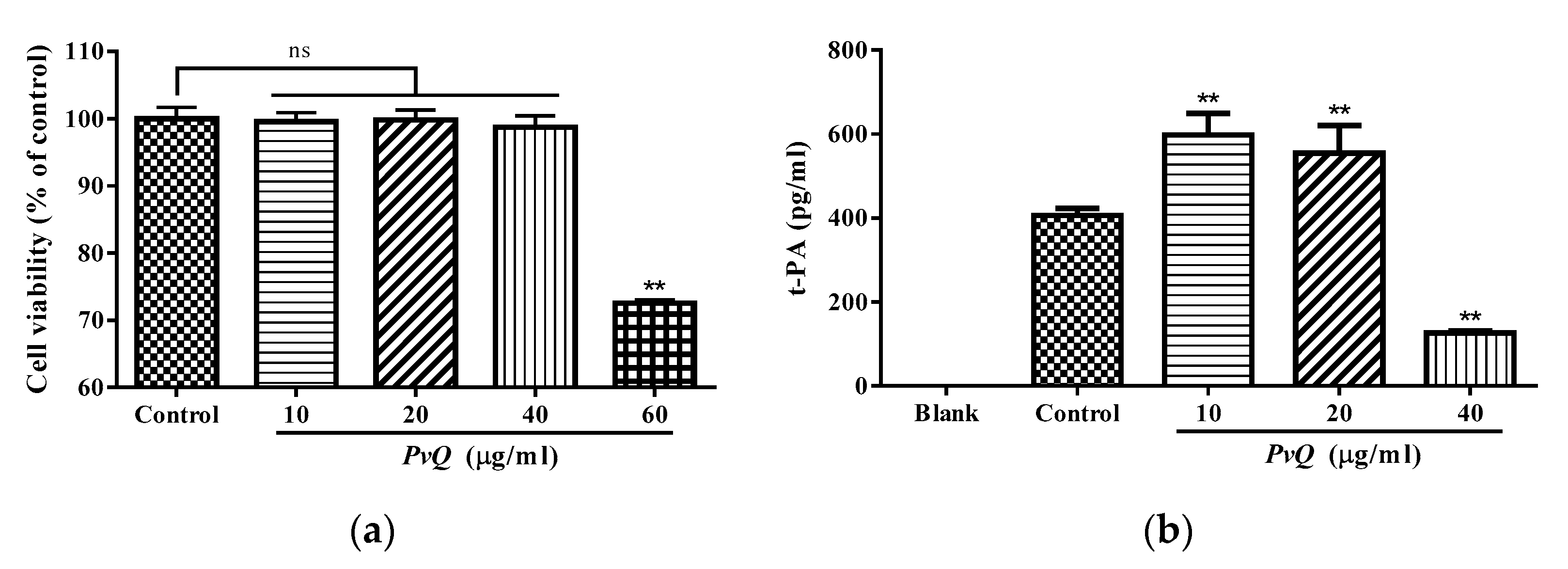
2.4. Determination of t-PA Content
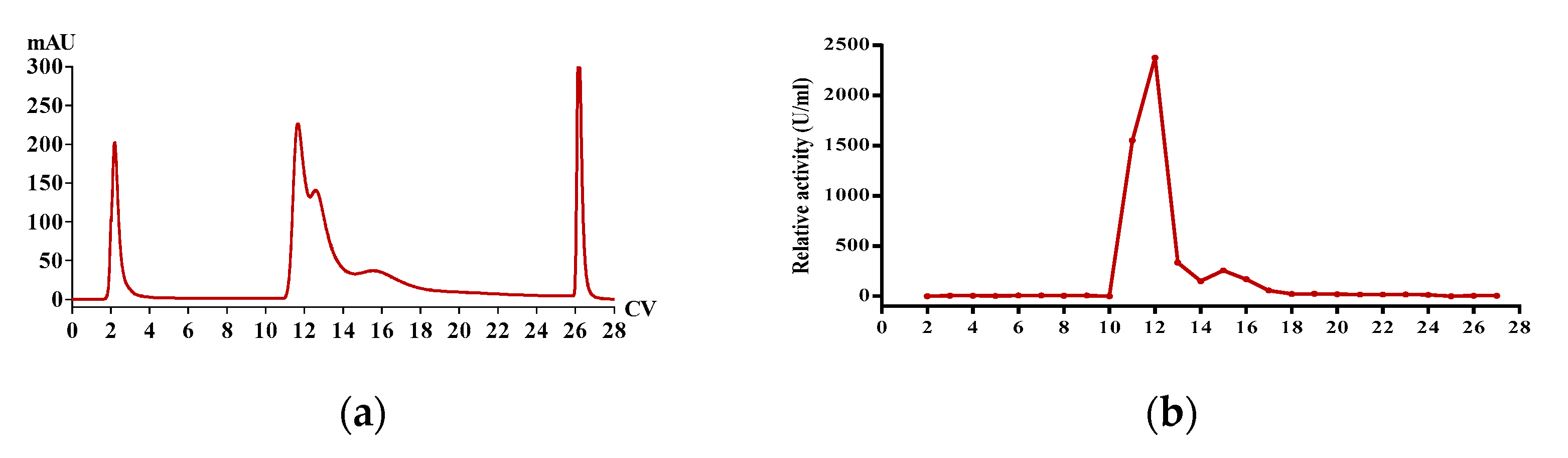
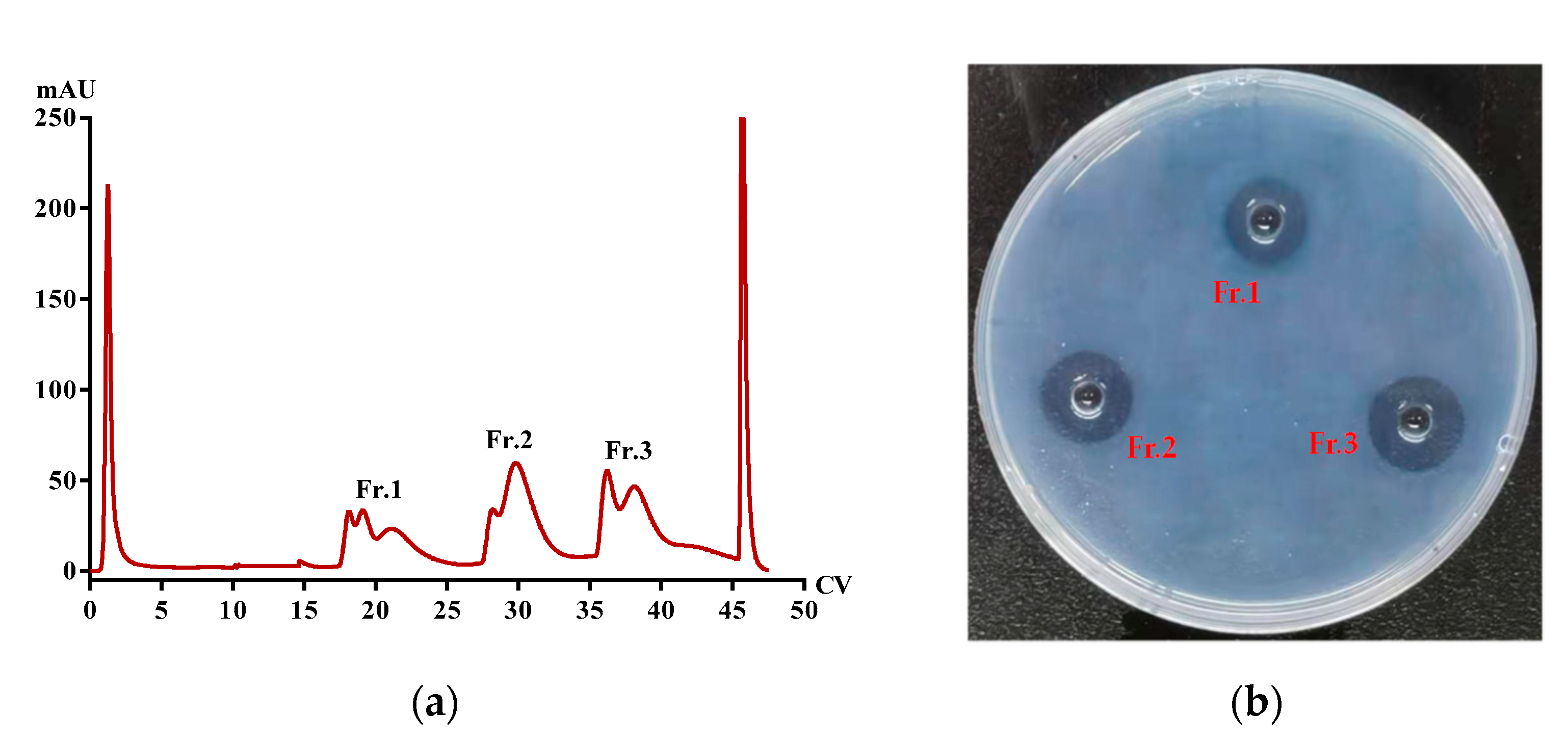
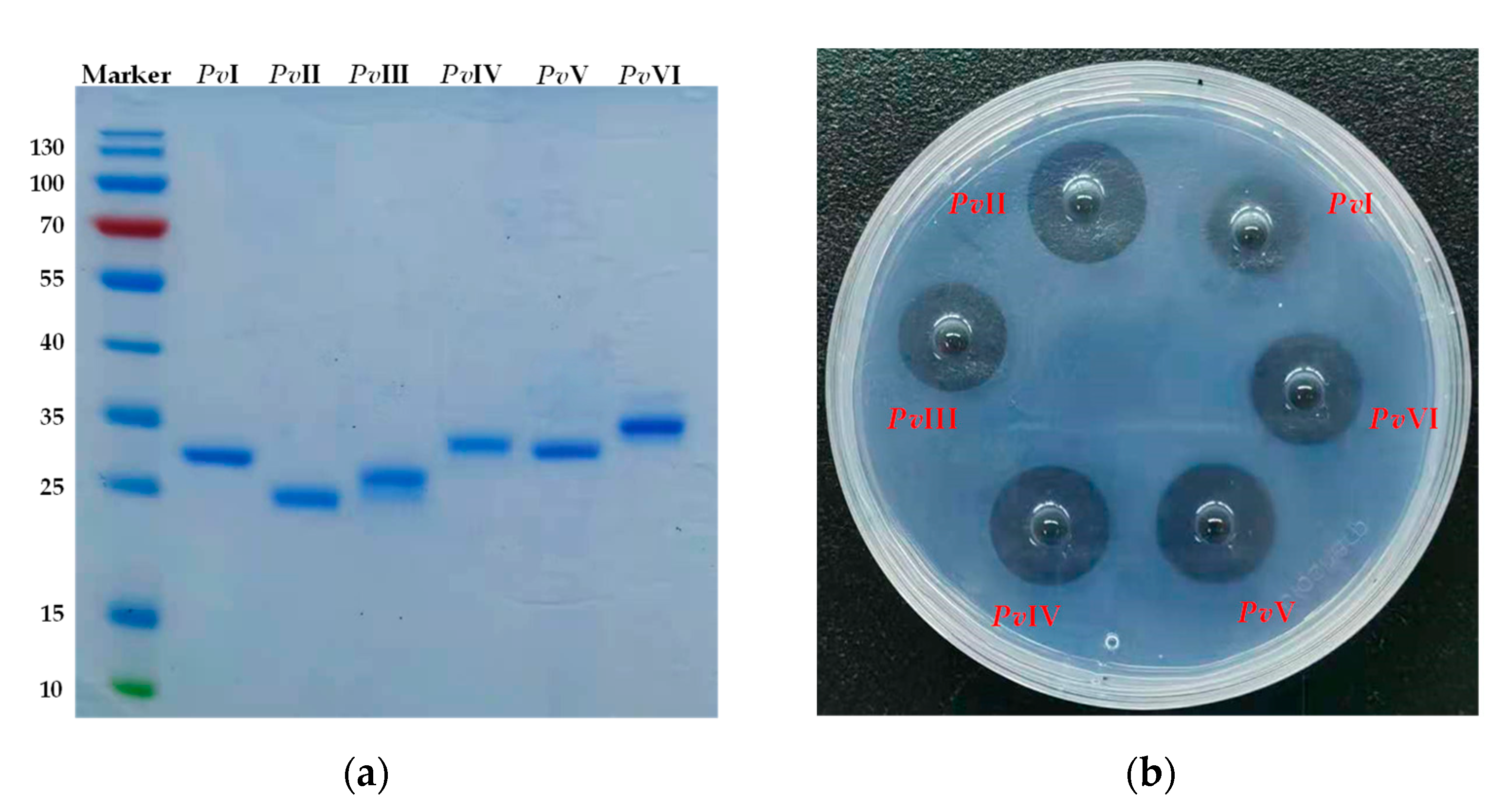
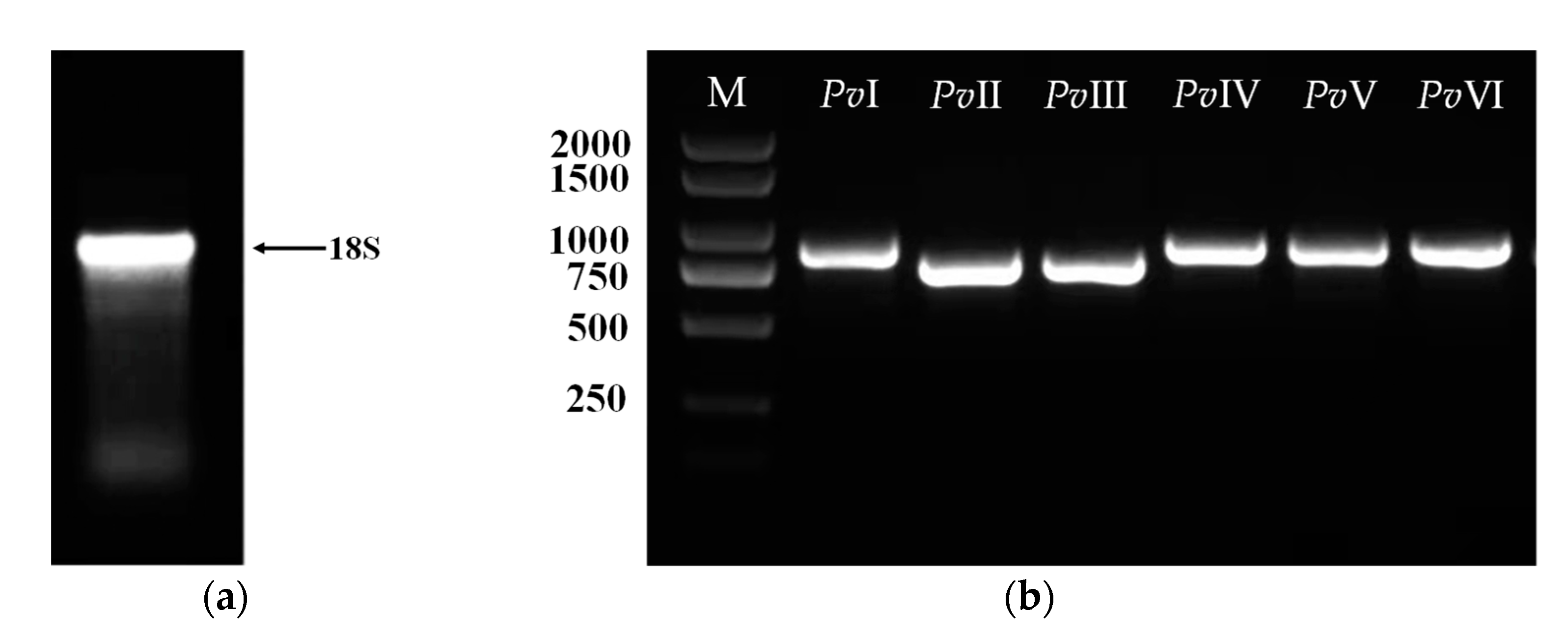
2.5. Purification of Functional Proteins from PvQ
2.6. Structural Identification of PvI–PvVI
2.6.1. The Bottom-Up Proteomics and N-Terminal Sequence Analysis
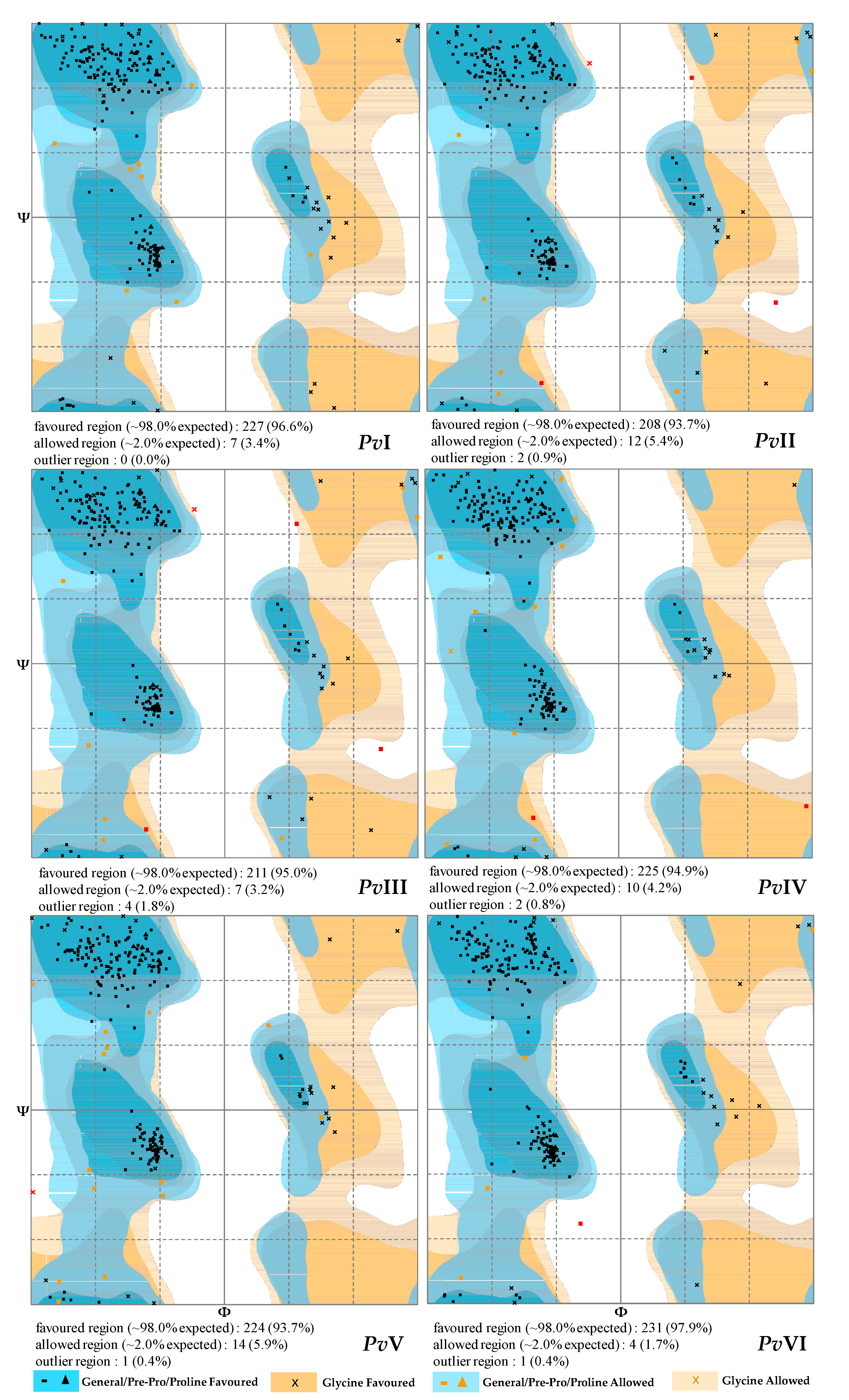
2.6.2. Secondary-Structure Prediction
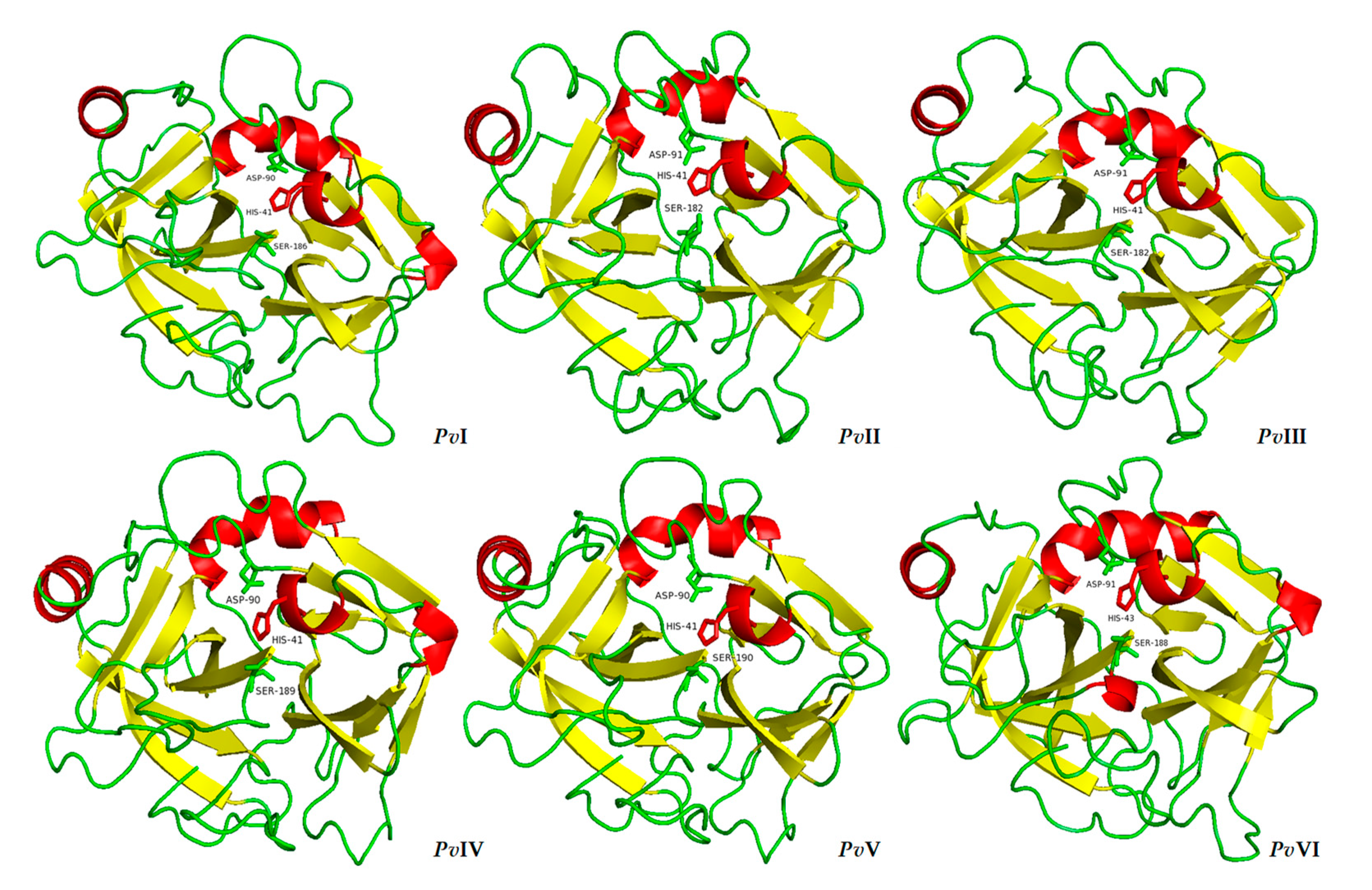
2.6.3. Tertiary-Structure Prediction
2.7. Gene Cloning of Fibrinolytic Proteins
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Enrichment of the Fibrinolytic Extract, PvQ
4.3. Fibrin Plate Assay
4.4. The Effect of PvQ on Zebrafish Thrombotic Model of Vascular Damage
4.5. The Determination of t-PA Content
4.6. Purification of Fibrinolytic Proteases (PvI–PvVI) from PvQ
4.7. Identification of Functional Proteins, PvI–PvVI
4.7.1. Proteins Sequencing Using LC-MS/MS
4.7.2. N-Terminal Sequence Analysis
4.7.3. Secondary Structure Determination
4.7.4. Three-Dimensional Protein Structure Prediction
4.8. Cloning of the Six Genes Proteins Gene
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Weitz, J.I.; Eikelboom, J.W. Advances in thrombosis and hemostasis. Circ. Res. 2016, 118, 1337–1339. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Chen, J.; Jin, F.; Wu, X.; Zhu, Z.; Zhang, J.; Zhao, Y. Animal drugs in treatment of cerebral ischemia and their mechanisms. Int. J. Pharma Sci. Res. 2015, 6, 15–22. [Google Scholar]
- Wu, Y.; Hu, S.; Ma, Y.; Zhao, B.; Yang, W.; Lu, Y.; Li, P.; Du, S. Novel Pheretima guillelmi-derived antithrombotic protein DPf3: Identification, characterization, in vitro evaluation and antithrombotic mechanisms investigation. Int. J. Biol. Macromol. 2020, 154, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Marc, V. Third-generation thrombolytic drugs. Am. J. Med. 2000, 109, 52–58. [Google Scholar]
- Wang, Y.H.; Li, S.A.; Huang, C.H.; Su, H.H.; Chen, Y.H.; Chang, J.T.; Huang, S.S. Sirt1 activation by post-ischemic treatment with lumbrokinase protects against myocardial ischemia-reperfusion injury. Front. Pharmacol. 2018, 9, 636. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Fan, S.C.; Chen, Y.; Ma, X.F.; He, R.Q. Earthworm protease in anti-thrombosis and anti-fibrosis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Ru, B.G. Purification and characterization of an SDS-activated fibrinolytic enzyme from Eisenia fetida. Comp. Biochem. Physiol. Part B 1997, 118, 623–631. [Google Scholar] [CrossRef]
- Matausic-Pisl, M.; Tomicic, M.; Micek, V.; Grdisa, M. Influences of earthworm extract G-90 on haematological and haemostatic parameters in Wistar rats. Eur. Rev. Med. Pharmcol. 2011, 15, 71–78. [Google Scholar]
- Joyia, F.A.; Zia, M.A.; Mustafa, G.; Faheem, A.; Raana, H.T.; Khan, M.S. Isolation, purification and functional characterization of fibrinolytic protease from an earthworm Eisenia foetida. Pure Appl. Biol. 2018, 7, 906–909. [Google Scholar] [CrossRef]
- Cho, I.H.; Choi, E.S.; Lim, H.G.; Lee, H.H. Purification and characterization of six fibrinolvtic serine-proteases from earthworm Lumbricus rubellus. J. Biochem. Mol. Biol. 2004, 37, 199–205. [Google Scholar]
- Trisina, J.; Sunardi, F.; Suhartono, M.T.; Tjandrawinata, R.R. DLBS1033, A protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activities. J. Biomed. Biotechnol. 2011, 2011, 519652. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.; Ta, T.; Nguyen, D.; Broek, L.; Duong, G. Purification and characterization of novel fibrinolytic proteases as potential antithrombotic agents from earthworm Perionyx excavatus. AMB Express 2011, 1, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Liu, C.; Liang, Z.; Gong, M.; Hu, D. Studies on separation and properties of lumbrokinase in Pheretima praepinguis. J. Bangladesh. Pharmacol. 2016, 11, 106–109. [Google Scholar]
- Verma, M.K.; Pulicherla, K.K. Broad substrate affinity and catalytic diversity of fibrinolytic enzyme from Pheretima posthumous-purification and molecular characterization study. Int. J. Biol. Macromol. 2017, 95, 1011–1021. [Google Scholar] [CrossRef]
- Mihara, H.; Sumi, H.; Yoneta, T.; Mizumoto, H.; Ikeda, R.; Seiki, M.; Maruyama, M. A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn. J. Physiol. 1991, 41, 461–472. [Google Scholar] [CrossRef]
- Tjandrawinata, R.R.; Trisina, J.; Rahayu, P.; Prasetya, L.A.; Hanafiah, A.; Rachmawati, H. Bioactive protein fraction DLBS1033 containing lumbrokinase isolated from Lumbricus rubellus: Ex vivo, in vivo, and pharmaceutic studies. Drug Des. Dev. Ther. 2014, 8, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Ma, Y.; Hu, S.; Zhao, B.; Yang, W.; Sun, Z.; Zhu, B.; Lu, Y.; Li, P.; Du, S. Transcriptomic-proteomics-anticoagulant bioactivity integrated study of Pheretima guillemi. J. Ethnopharmacol. 2019, 243, 112101. [Google Scholar] [CrossRef]
- State Food and Drug Administration. National Drug Standard; China Medical Science Press: Beijing, China, 2010; pp. 113–114.
- Fan, J.J.; Li, F.R.; Zhao, C.J.; Xu, K.X.; Yang, R.; Qiao, Y.H.; Ni, Y.Y.; Wang, Y.T.; Ma, Z.Q.; Lin, R.C. Optimization and adaptability of the zebrafish thrombosis model induced by adrenaline hydrochloride. Glob. Tradit. Chin. Med. 2017, 10, 663–668. [Google Scholar]
- Fan, J.J.; Qiao, Y.H.; Zhao, C.J.; Ni, Y.Y.; Yang, R.; Feng, Y.R.; Ma, Z.Q.; Lin, R.C. Applicability of zebrafish thrombosis model in antithrombotic activity screening of Chinese materia medica. Chin. J. Inf. TCM 2017, 24, 58–61. [Google Scholar]
- Zhao, J.; Pan, R.; He, J.; Liu, Y.; Li, D.F.; He, R.Q. Eisenia fetida protease-III-1 functions in both fibrinolysis and fibrogenesis. J. Biomed. Biotechnol. 2007, 2007, 97654. [Google Scholar] [CrossRef]
- Nakajima, N.; Sugimoto, M.; Ishihara, K. Earthworm-serine protease: Characterization, molecular cloning, and application of the catalytic functions. J. Mol. Catal. B-Enzym. 2003, 23, 191–212. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Refregiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Liang, D.; Jiang, T.; Zhang, J.; Gui, L.; Chang, W. Crystal structure of earthworm fibrinolytic enzyme component A: Revealing the structural determinants of its dual fibrinolytic activity. J. Mol. Biol. 2002, 321, 57–68. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ishihara, K.; Nakajima, N. Structure and function of an isozyme of earthworm proteases as a new biocatalyst. J. Mol. Catal. B-Enzym. 2003, 23, 405–409. [Google Scholar] [CrossRef]
- Cho, H.; Choi, E.; Lee, H.H. Molecular cloning, sequencing, and expression of a fibrinolytic serine-protease gene from the earthworm Lumbricus rubellus. J. Biochem. Mol. Biol. 2004, 37, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Ge, T.; Sun, Z.J.; Fu, S.H.; Liang, G.D. Cloning of thrombolytic enzyme (lumbrokinase) from earthworm and its expression in the yeast Pichia pastoris. Protein Expr. Purif. 2005, 42, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xiao, R.; He, J.; Pan, R.; Fan, R.; Wu, C.; Liu, X.; Liu, Y.; He, R.Q. In situ localization and substrate specificity of earthworm protease-II and protease-III-1 from Eisenia fetida. Int. J. Biol. Macromol. 2007, 40, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Jin, H.; Zhang, G.; Xu, G. Changes in coagulation and tissue plasminogen activator after the treatment of cerebral infarction with lumbrokinase. Clin. Hemorheol. Microcirc. 2000, 23, 213–218. [Google Scholar]
- Grdisa, M.; Mikecin, A.; Hrzenjak, T. Earthworms as a source of bioactive molecules. Curr. Bioact. Compd. 2009, 5, 155–159. [Google Scholar] [CrossRef]
- Zhao, G.; Li, H.; Xu, W.; Luo, J.; Xu, R.A. An overview of the fibrinolytic enzyme from earthworm. Chin. J. Nat. Med. 2010, 8, 301–307. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, C.; Shan, Y.; Zhao, Z.; Chen, J. Expression and characterization of earthworm Eisenia foetida Lumbrokinase-3 in Pichia pastoris. Prep. Biochem. Biotechnol. 2006, 36, 273–279. [Google Scholar] [CrossRef]
- Ueda, M.; Hirano, Y.; Fukuhara, H.; Naka, Y.; Nakazawa, M.; Sakamoto, T.; Ogata, Y.; Tamada, T. Gene cloning, expression, and X-ray crystallographic analysis of a β-mannanase from Eisenia fetida. Enzym. Microb. Technol. 2018, 117, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.Q.; Wang, K.Y.; Li, D.H.; Wang, N.; Liu, D.H.; Jitesh, P. Cloning, expression and characterization of a gene from earthworm Eisenia fetida encoding a blood-clot dissolving protein. PLoS ONE 2012, 12, e53110. [Google Scholar] [CrossRef]
- Li, D.; Tong, W.; Yang, Y. Functional expression of an earthworm fibrinolytic enzyme in Escherichia coli. World J. Microb. Biotechnol. 2008, 24, 613–618. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Gui, Q.; Zhang, L.; Hu, L. Expression, purification, and characterization of recombinant lumbrokinase PI239 in Escherichia coli. Protein Expr. Purif. 2010, 69, 198–203. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.L.; Xu, J.P.; Lu, W.; Wang, J. Cloning and expression of earthworm fibrinolytic enzyme PM246 in Pichia pastoris. Protein Expr. Purif. 2005, 43, 18–25. [Google Scholar] [CrossRef]
- Ge, T.; Fu, S.H.; Xu, L.H.; Tang, Q.; Wang, H.Y.; Guan, K.P.; Liang, G.D. High density fermentation and activity of a recombinant lumbrokinase (PI239) from Pichia pastoris. Protein Expr. Purif. 2007, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, Z.B.; Guo, Y.D.; Song, C.C.; Hu, Y.C.; Wang, B.C.; Jin, J.J. Research on the method of fibrinogen-thrombin time by coagulometer for quality control of hirudo. Chin. J. Pharm. Anal. 2014, 34, 1802–1806. [Google Scholar]
- Errasti, M.E.; Prospitti, A.; Viana, C.A.; Gonzalez, M.M.; Ramos, M.V.; Rotelli, A.E.; Caffini, N.O. Effects on fibrinogen, fibrin, and blood coagulation of proteolytic extracts from fruits of Pseudananas macrodontes, Bromelia balansae, and B. hieronymi (Bromeliaceae) in comparison with bromelain. Blood Coagul. Fibrinolysis 2016, 27, 441–449. [Google Scholar] [CrossRef]
- Mustafa, A.; Thornqvist, P.O.; Roman, E.; Winberg, S. The aggressive spiegeldanio, carrying a mutation in the fgfr1a gene, has no advantage in dyadic fights with zebrafish of the AB strain. Behav. Brain Res. 2019, 370, 111942. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Shi, Y.M.; Yang, L.; Li, X.C.; Zhao, J.H.; Qu, Y.; Zhu, H.T.; Wang, D.; Cheng, R.R.; Yang, C.R.; et al. Lignans and aromatic glycosides from Piper wallichii and their antithrombotic activities. J. Ethnopharmacol. 2015, 162, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bern, M.; Kil, Y.J.; Becker, C. Byonic: Advanced peptide and protein identification software. Curr. Protoc. Bioinform. 2012, 40, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misconai, A.; Wien, F.; Bulyaki, E.; Kun, J.; Moussong, E.; Lee, Y.H.; Goto, Y.; Refregiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; Bakker, P.D.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα Geometry: Φ, Ψ and Cβ deviation. Proteins Struct. Funct. Bioinform. 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Bosco, K.H.; Brasseur, R. The Ramachandran plots of glycine and pre-proline. BMC Struct. Biol. 2005, 5, 14. [Google Scholar]







| No. | Specific Activity (U/μg) 1 | ||
|---|---|---|---|
| PvQ-1 | PvQ-2 | PvQ-3 | |
| 1 | 243.01 | 246.33 | 250.15 |
| 2 | 257.36 | 265.26 | 248.62 |
| 3 | 250.78 | 230.82 | 233.65 |
| Mean ± SD | 247.33 ± 10.74 | ||
| Group | Breeding Conditions | Staining Intensity |
|---|---|---|
| Normal | Culture medium | 2064.96 ± 152.12 |
| Model | Adrenaline hydrochloride solution (30 μM) | 2412.01 ± 131.94 ** |
| PvQ | PvQ 10 ng/fish | 2178.33 ± 186.91 *## |
| Sample | Coverage (%) | # of Spectra | Unique Peptides | Score |
|---|---|---|---|---|
| PvI | 26.87 | 470 | 6 | 81.46 |
| PvII | 24.84 | 507 | 7 | 90.87 |
| PvIII | 17.93 | 385 | 5 | 56.43 |
| PvIV | 8.78 | 53 | 3 | 19.73 |
| PvV | 34.12 | 407 | 10 | 70.53 |
| PvVI | 25.22 | 82 | 9 | 34.21 |
| Sample | N-terminal Amino Acid Sequences |
|---|---|
| PvI | Val-Ile-Gly-Gly-Ser-Asp-Ala-Thr-Arg-Gly |
| PvII | Ile-Val-Gly-Gly-Ala-Ala-Val-Ser-Ile-Ser |
| PvIII | Ile-Val-Gly-Gly-Ser-Ala-Val-Ser-Ile-Ser |
| PvIV | Ile-Leu-Gly-Gly-Gln-Asp-Ala-Ser-Pro-Gly |
| PvV | Ile-Leu-Gly-Gly-Thr-Glu-Ala-Arg-Val-Gly |
| PvVI | Ile-Val-Gly-Gly-Gln-Glu-Ala-Arg-Pro-Tyr |
| Sample | α-Helix (%) | β-Sheet (%) | Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| PvI | 11.8 | 35.0 | 15.2 | 38.0 |
| PvII | 9.4 | 38.0 | 17.4 | 35.2 |
| PvIII | 9.4 | 37.0 | 12.9 | 40.7 |
| PvIV | 10.9 | 35.6 | 16.3 | 37.2 |
| PvV | 10.0 | 34.0 | 14.5 | 41.5 |
| PvVI | 13.0 | 34.8 | 13.0 | 39.2 |
| Protein | Primer | Primer Sequence | Tm (°C) |
|---|---|---|---|
| PvI | PvI-F PvI-R | 5′-GTCATCGGTGGATCGGATGC-3′ 5′-TTAGGGACTGTTGTCAGCGATCC-3′ | 55.9 57.1 |
| PvII | PvII-F PvII-R | 5′-ATCGTCGGTGGAGCAGCCGTCAG-3′ 5′-TTATTGAATGATGATCCAGG-3′ | 62.4 45.6 |
| PvIII | PvIII-F PvIII-R | 5′-ATTGTTGGAGGATCGGCCGTCAG-3′ 5′-TTATTGAATGATGATCCAGG-3′ | 58.8 45.6 |
| PvIV | PvIV-F PvIV-R | 5′-ATTCTCGGAGGACAGGATGCCTC-3′ 5′-CTATTGGTTGTCGCTGATCCAT-3′ | 58.8 53.0 |
| PvV | PvV-F PvV-R | 5′-ATCCTTGGAGGAACGGAAGCCAG-3′ 5′-CTAGCGAATGTTGTCAATGATCC-3′ | 58.8 53.5 |
| PvVI | PvVI-F PvVI-R | 5′-ATCGTCGGAGGGCAGGAAGC-3′ 5′-TCAGTTGTTGTTAATGATGTTCG-3′ | 57.9 50.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wang, W.; Ma, Y.; Yang, Q.; Li, P.; Du, S. Bioevaluation of Pheretima vulgaris Antithrombotic Extract, PvQ, and Isolation, Identification of Six Novel PvQ-Derived Fibrinolytic Proteases. Molecules 2021, 26, 4946. https://doi.org/10.3390/molecules26164946
Yang W, Wang W, Ma Y, Yang Q, Li P, Du S. Bioevaluation of Pheretima vulgaris Antithrombotic Extract, PvQ, and Isolation, Identification of Six Novel PvQ-Derived Fibrinolytic Proteases. Molecules. 2021; 26(16):4946. https://doi.org/10.3390/molecules26164946
Chicago/Turabian StyleYang, Wanqing, Wenjie Wang, Yunnan Ma, Qilin Yang, Pengyue Li, and Shouying Du. 2021. "Bioevaluation of Pheretima vulgaris Antithrombotic Extract, PvQ, and Isolation, Identification of Six Novel PvQ-Derived Fibrinolytic Proteases" Molecules 26, no. 16: 4946. https://doi.org/10.3390/molecules26164946
APA StyleYang, W., Wang, W., Ma, Y., Yang, Q., Li, P., & Du, S. (2021). Bioevaluation of Pheretima vulgaris Antithrombotic Extract, PvQ, and Isolation, Identification of Six Novel PvQ-Derived Fibrinolytic Proteases. Molecules, 26(16), 4946. https://doi.org/10.3390/molecules26164946