Crystal Growth on Cenospheres from High-Calcium Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cenospheres Separation
2.2. Material Characterization
3. Results
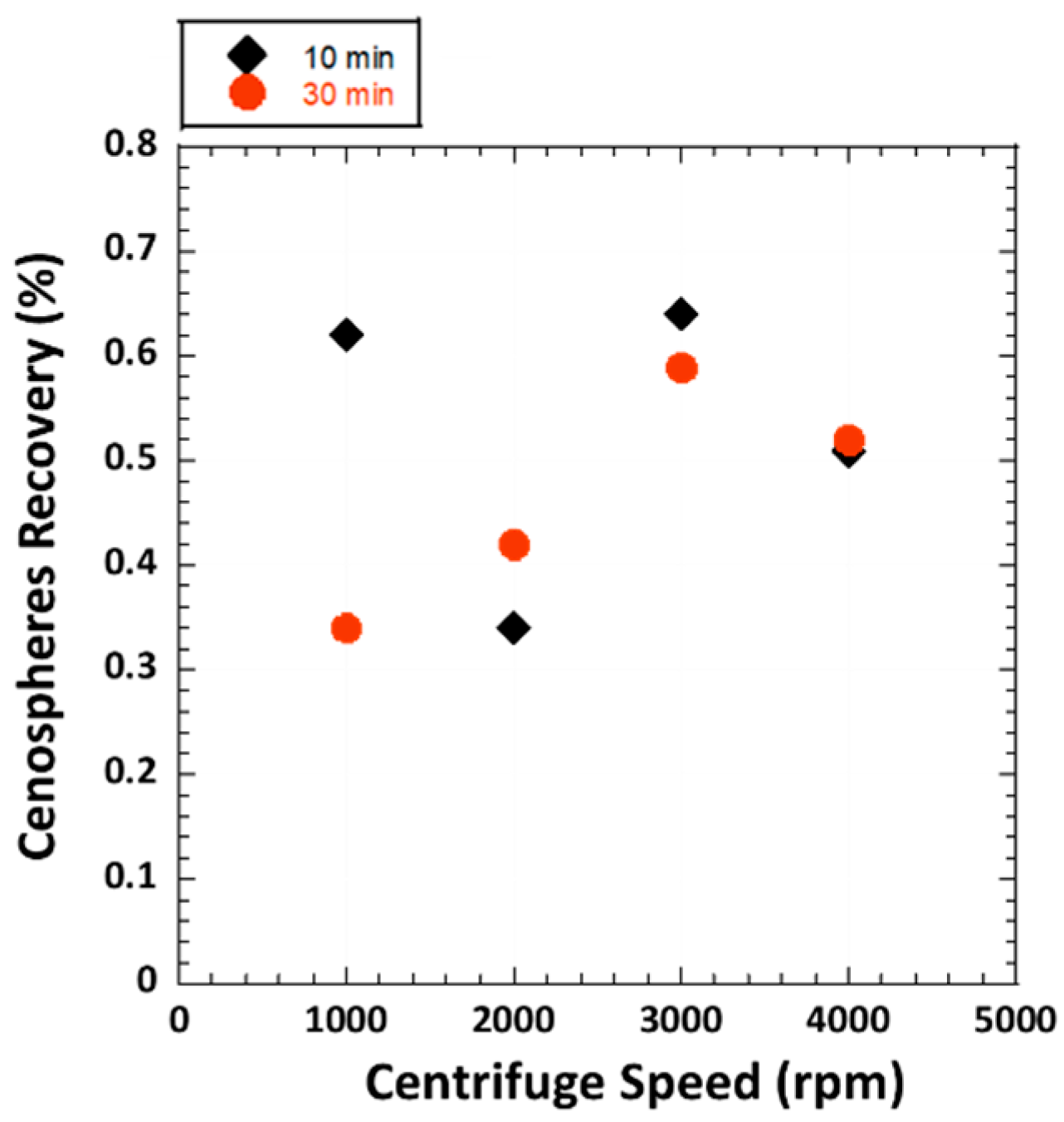
3.1. Cenospheres Recovery by Centrifugal Method
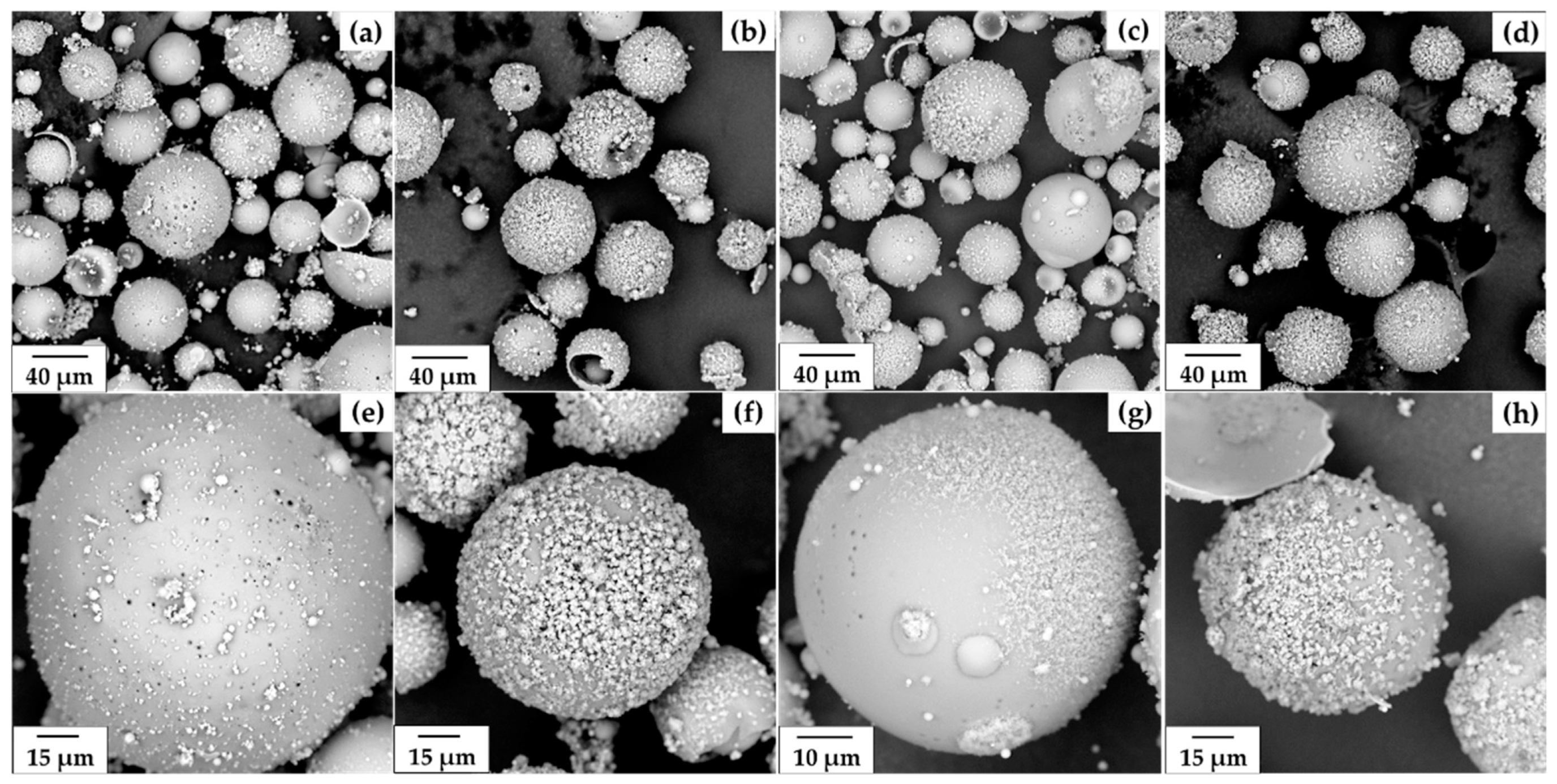
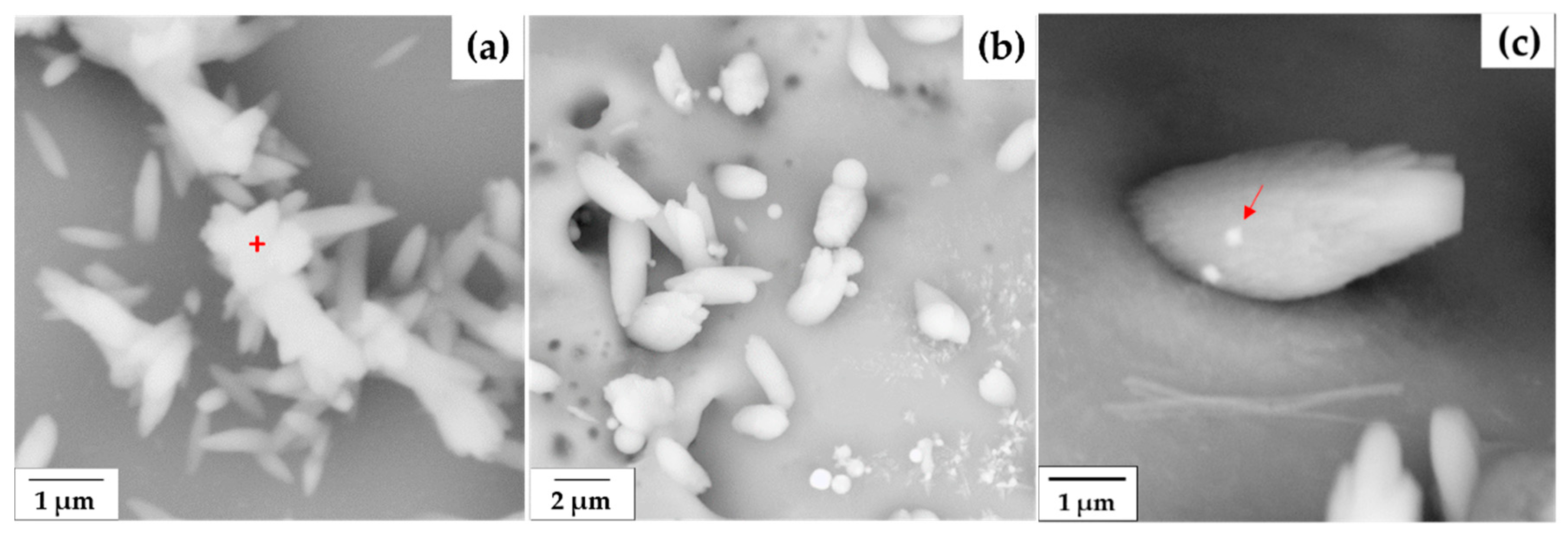
3.2. Morphology
3.3. Chemical Composition
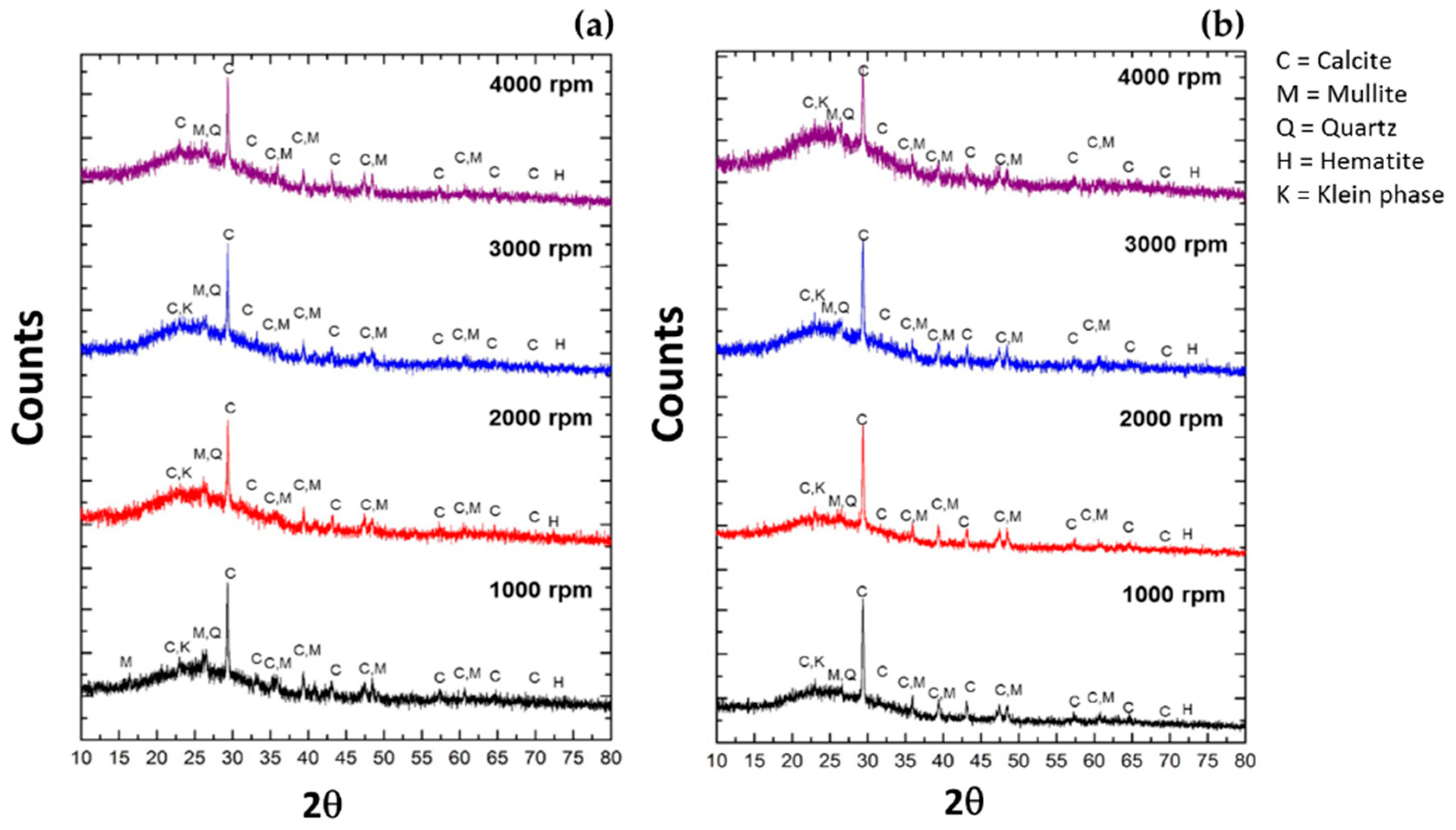
3.4. Minerallogical Phase Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danish, A.; Mosaberpanah, M.A. Formation mechanism and applications of cenospheres: A review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A Review of the Multi-Component Utilization of Coal Fly Ash. Fuel 2012, 97, 1. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Acar, I.; Atalay, M.U. Recovery Potentials of Cenospheres from Bituminous Coal Fy Ashes. Fuel 2016, 180, 97. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Mishra, K.K.; Mazumder, A. Characterization of Cenospheres Collected from Ash-pond of a Super Thermal Power Plant. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 30, 271–283. [Google Scholar] [CrossRef]
- Kolay, P.; Bhusal, S. Recovery of hollow spherical particles with two different densities from coal fly ash and their characterization. Fuel 2014, 117, 118–124. [Google Scholar] [CrossRef]
- Drozhzhin, V.S.; Shpirt, M.; Danilin, L.D.; Kuvaev, M.D.; Pikulin, I.V.; Potemkin, G.A.; Redyushev, S.A. Formation processes and main properties of hollow aluminosilicate microspheres in fly ash from thermal power stations. Solid Fuel Chem. 2008, 42, 107–119. [Google Scholar] [CrossRef]
- Tiwari, V.; Shukla, A.; Bose, A. Acoustic properties of cenosphere reinforced cement and asphalt concrete. Appl. Acoust. 2004, 65, 263–275. [Google Scholar] [CrossRef]
- Blanco, F.; García, P.; Mateos, P.; Ayala, J. Characteristics and Properties of Lightweight Concrete Mmanufactured with Cenospheres. Cem. Concr. Res. 2000, 30, 1715–1722. [Google Scholar] [CrossRef]
- Hanif, A.; Lu, Z.; Li, Z. Utilization of fly ash cenosphere as lightweight filler in cement-based composites—A review. Constr. Build. Mater. 2017, 144, 373–384. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, K.; Tang, Y.; Hu, C. Preparation of a Cenosphere Curing Agent and Its Application to Foam Concrete. Adv. Mater. Sci. Eng. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Brooks, A.L. Thermal and mechanical properties of structural lightweight concrete containing lightweight aggregates and fly-ash cenospheres. Constr. Build. Mater. 2019, 198, 512–526. [Google Scholar] [CrossRef]
- Wasekar, P.A.; Kadam, P.G.; Mhaske, S.T. Effect of Cenosphere Concentration on the Mechanical, Thermal, Rheological and Morphological Properties of Nylon 6. J. Miner. Mater. Charact. Eng. 2012, 11, 807–812. [Google Scholar] [CrossRef]
- Kruger, R.A. The Use of Cenospheres in Refractories. Energeia 1996, 7, 1–5. [Google Scholar]
- Sahu, P.K.; Mahanwar, P.A.; Bambole, V.A. Effect of Hollow Glass Microspheres and Cenospheres on Insulation Properties of Coatings. Pigment Resin Technol. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Braszczyńska-Malik, K.N.; Kamieniak, J. The Role of Ni-P Coating Structure on Fly Ash Cenospheres in the Formation of Magnesium Matrix Composites. Metall. Mater. Trans. A 2017, 48, 5649–5657. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.; Shukla, A.; Bose, A. Effect of Particle Size and Surface Treatment on Constitutive Properties of Polyester-Cenosphere Composites. J. Mater. Sci. 2002, 37, 603–613. [Google Scholar] [CrossRef]
- Chalivendra, V.B.; Shukla, A.; Bose, A.; Parameswaran, V. Processing and mechanical characterization of lightweight polyurethane composites. J. Mater. Sci. 2003, 38, 1631–1643. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, D.; Zhou, Y.; Liu, B. Novel Method for Fabrication of Silicon Nitride/Silicon Oxynitride Composite Ceramic Foams Using Fly Ash Cenosphere as a Pore-Forming Agent. J. Am. Ceram. Soc. 2008, 91, 3781–3785. [Google Scholar] [CrossRef]
- Rugele, K.; Lehmhus, D.; Hussainova, I.; Peculevica, J.; Lisnanskis, M.; Shishkin, A. Effect of Fly-Ash Cenospheres on Properties of Clay-Ceramic Syntactic Foams. Materials 2017, 10, 828. [Google Scholar] [CrossRef] [Green Version]
- Ferdous, W.; Manalo, A.; Van Erp, G.; Aravinthan, T.; Ghabraie, K. Evaluation of an Innovative Composite Railway Sleeper for a Narrow-Gauge Track under Static Load. J. Compos. Constr. 2018, 22, 04017050. [Google Scholar] [CrossRef]
- Anand, R.L.; Gaitonde, V.N.; Mokashi, P. Delaminatino Analysis in Epoxy/Cenosphere Composite Drilling. Mater. Today Proc. 2018, 5, 23499–23507. [Google Scholar] [CrossRef]
- Ghosal, S.; Self, S.A. Particle Size-Density Relation and Cenosphere Content of Coal Fly Ash. Fuel 1995, 74, 522–529. [Google Scholar] [CrossRef]
- Goodarzi, F.; Hower, J.C. Classification of carbon in Canadian fly ashes and their implications in the capture of mercury. Fuel 2008, 87, 1949–1957. [Google Scholar] [CrossRef]
- Atla, S.B.; Huang, Y.-H.; Yang, J.; Chen, H.-J.; Kuo, Y.-H.; Hsu, C.-M.; Lee, W.-C.; Chen, C.-C.; Hsu, D.-W.; Chen, C.-Y. Hydrophobic Calcium Carbonate for Cement Surface. Crystals 2017, 7, 371. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Martinez-Tarazona, M. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Yoriya, S.; Tepsri, P. Separation Process and Microstructure-Chemical Composition Relationship of Cenospheres from Lignite Fly Ash Produced from Coal-Fired Power Plant in Thailand. Appl. Sci. 2020, 10, 5512. [Google Scholar] [CrossRef]
- Alcalá, J.F.C.; Dávila, R.M.; Quintero, R.L. Recovery of Cenospheres and magnetite from coal burning power plant fly ash. Trans. Iron Steel Inst. Jpn. 1987, 27, 531–538. [Google Scholar] [CrossRef]
- Yoriya, S.; Intana, T.; Tepsri, P. Separation of Cenospheres from Lignite Fly Ash Using Acetone–Water Mixture. Appl. Sci. 2019, 9, 3792. [Google Scholar] [CrossRef] [Green Version]
- Manocha, L.; Ram, K.; Manocha, S. Separation of Cenospheres from Fly Ashes by Floatation Method. Eurasian Chem. J. 2010, 13, 89–95. [Google Scholar] [CrossRef]
- Kolay, P.; Singh, D. Physical, chemical, mineralogical, and thermal properties of cenospheres from an ash lagoon. Cem. Concr. Res. 2001, 31, 539–542. [Google Scholar] [CrossRef]
- Elford, W.J. Centrifugation Studies: I. Critical Examination of a New Method as Applied to the Sedimentation of Bacteria, Bacteriophages and Proteins. Br. J. Exp. Pathol. 1936, 17, 399–422. [Google Scholar]
- Ramme, B.W.; Noegel, J.J.; Rohatgi, P.K. Separation of Cenospheres from Fly Ash. U.S. Patent 807,480,4B2, 13 December 2011. [Google Scholar]
- Vassilev, S.; Menendez, R.; Alvarez, D.; Diaz-Somoano, M.; Martinez-Tarazona, M. Phase-Mineral and Chemical Composition of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 1. Characterization of Feed Coals and Fly Ashes. Fuel 2003, 82, 1793–1811. [Google Scholar] [CrossRef]
- Tepsri, P.; Chumphu, A.; Yoriya, S. High-Calcium Fly Ash Recovery from Wet-Stored Condition and Its Properties. Mater. Res. Express 2018, 5, 115506. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Vassileva, C.G. Mineralogy of combustion wastes from coal-fired power stations. Fuel Process. Technol. 1996, 47, 261–280. [Google Scholar] [CrossRef]
- Zhou, Y.; Rahaman, M. Hydrothermal synthesis and sintering of ultrafine CeO2 powders. J. Mater. Res. 1993, 8, 1680–1686. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Vasilieva, N.G.; Mikhaylova, O.A.; Rogovenko, E.S.; Zhizhaev, A.M.; Anshits, A.G. Characterization of Fly Ash Cenospheres Produced from the Combustion of Ekibastuz Coal. Energy Fuels 2015, 29, 5390–5403. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Song, H.; Rao, Y.; Wang, X.; Zhu, G.; Wang, Q.; Qi, Y.; Zhu, G.; Gao, D.; Liu, J. Effect of Calcium Hydroxide Concentration and Stirring Rate on the Crystallization of the Calcium Carbonate on the Surface of Fly Ash. BioResources 2018, 13, 7017–7025. [Google Scholar]
- Fomenko, E.; Anshits, N.; Solovyov, L.; Mikhaylova, O.A.; Anshits, A. Composition and Morphology of Fly Ash Cenospheres Produced from the Combustion of Kuznetsk Coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Agrawal, U.S.; Wanjari, S.P. Physiochemical and Engineering Characteristics of Cenosphere and Its Application as a Light Weight Construction Material—A Review. Mater. Today Proc. 2017, 4, 9797–9802. [Google Scholar] [CrossRef]
- Joseph, K.V.; Francis, F.; Chacko, J.; Das, P.; Hebbar, G.S. Fly Ash Cenosphere Waste Formation in Coal Fired Power Plants and Its Application as a Structural Material—A Review. Int. J. Eng. Res. Technol. 2013, 2, 1236–1260. [Google Scholar]
- Sokol, E.; Maksimova, N.; Volkova, N.; Nigmatulina, E.; Frenkel, A. Hollow silicate microspheres from fly ashes of the Chelyabinsk brown coals (South Urals, Russia). Fuel Process. Technol. 2000, 67, 35–52. [Google Scholar] [CrossRef]
- Diamon, S. On the Glass Present in Low-Ca and High-Ca Fly Ash. Cem. Concr. Res. 1983, 13, 459–464. [Google Scholar] [CrossRef]
- Hooton, R.; Tishmack, J.; Olek, J.; Diamond, S. Characterization of High-Calcium Fly Ashes and Their Potential Influence on Ettringite Formation in Cementitious Systems. Cem. Concr. Aggreg. 1999, 21, 82. [Google Scholar] [CrossRef]
- Bischoff, W.D.; Bishop, F.C.; Mackenzie, F.T. Biogenically Produced Magnesian Calcite Inhomogeneities in Chemical and Physical-Properties Comparison with Synthesis Phases. Am. Mineral. 1983, 68, 1183–1188. [Google Scholar]
- Kutchko, B.; Kim, A. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Sawada, K. The mechanisms of crystallization and transformation of calcium carbonates. Pure Appl. Chem. 1997, 69, 921–928. [Google Scholar] [CrossRef]
- Cizer, O.; Rodrigues-Navarro, C.; Ruiz-Agudo, E.; Elsen, J.; Gemert, D.V.; Balen, K.V. Phase and Morphology Evolution of Calcium Carbonate Precipitated by Carbonation of Hydrated Lime. J. Mater. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, G.J.; Solem-Tishmack, J.K. Hydration Mineralogy of Cementitious Coal Combustion byproducts. In Advances in Cement and Concrete, Proceedings of the Engineering Foundation Conference, Materials Engineering Division, Durham, NH, USA, 24–29 July 1994; ASCE: New York, NY, USA, 1994. [Google Scholar]
- Kaufmann, G.; Dreybrodt, W. Calcite Dissolution Kinetics in the System CaCO3–H2O–CO2 at High Undersaturation. Geochim. Cosmochim. Acta 2007, 71, 1398–1410. [Google Scholar] [CrossRef]
- Fedyaeva, O.A.; Poshelyuzhnaya, E.G. Investigation of Interactions in the Cenospheres- Electrolyte System. Dyn. Syst. Mech. Mach. 2019, 7, 012116. [Google Scholar] [CrossRef]
- Koga, N.; Nakagoe, Y.; Tanaka, H. Crystallization of amorphous calcium carbonate. Thermochim. Acta 1998, 318, 239–244. [Google Scholar] [CrossRef]
- Usmany, Y.; Putranto, W.A.; Bayuseno, A.P.; Muryanto, S. Crystallization of calcium carbonate (CaCO3) in a flowing system: Influence of Cu2+ additives on induction time and crystalline phase transformation. In Proceedings of the 3rd International Conference on Advanced Materials Science and Technology (ICAMST 2015); AIP Publishing: Melville, NY, USA, 2016; Volume 1725, p. 020093. [Google Scholar]
- Nada, H. Importance of water in the control of calcite crystal growth by organic molecules. Polym. J. 2014, 47, 84–88. [Google Scholar] [CrossRef]


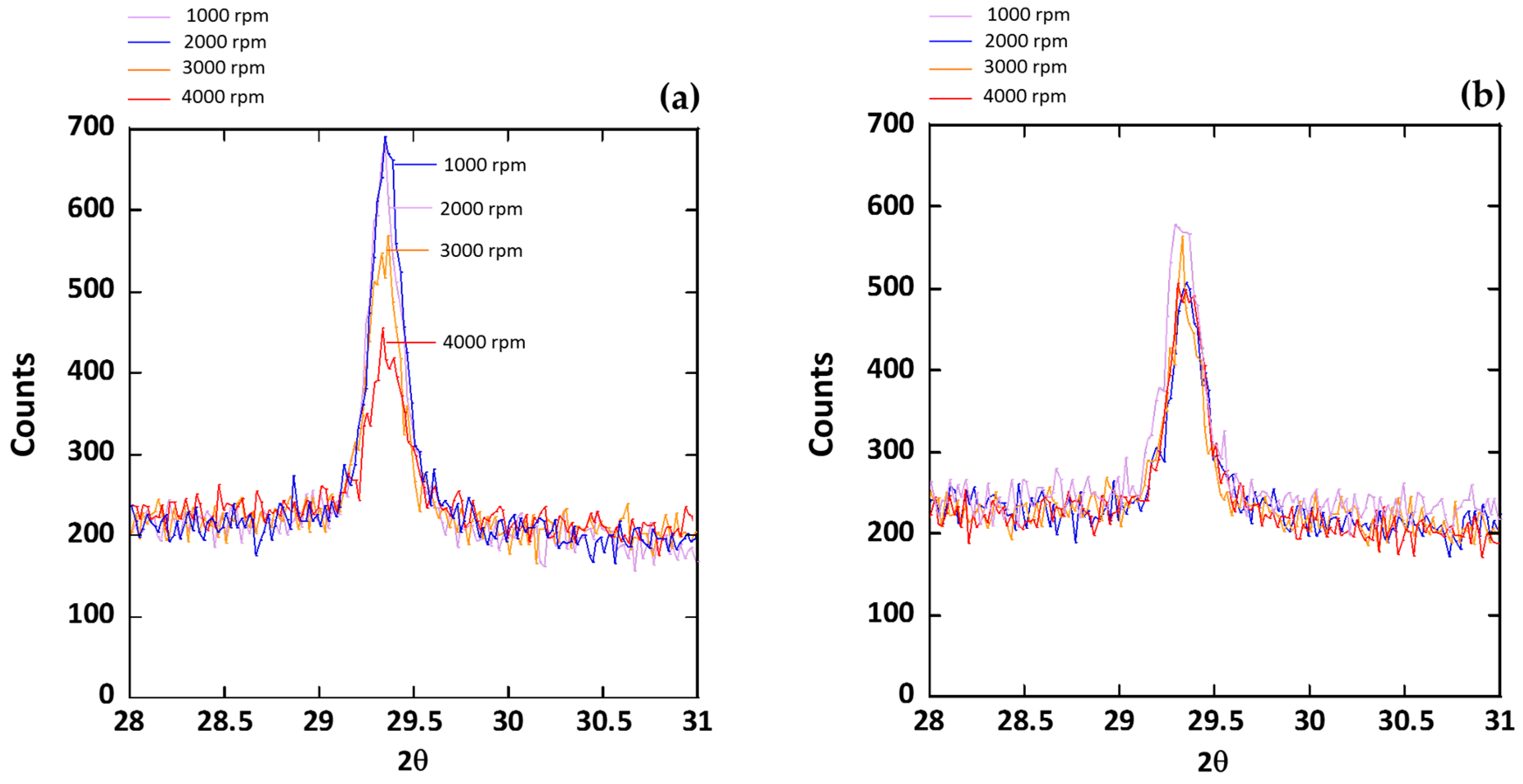
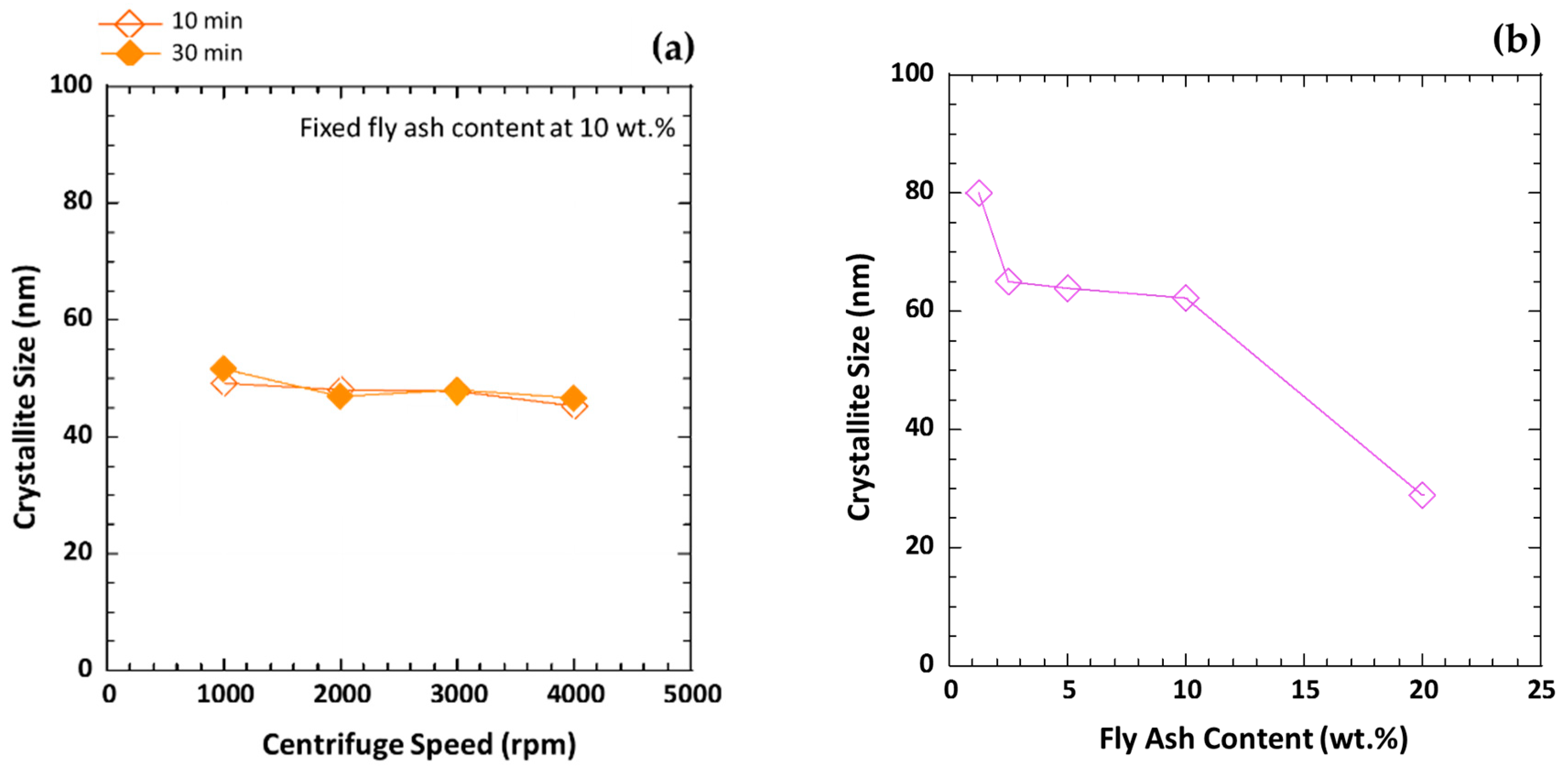
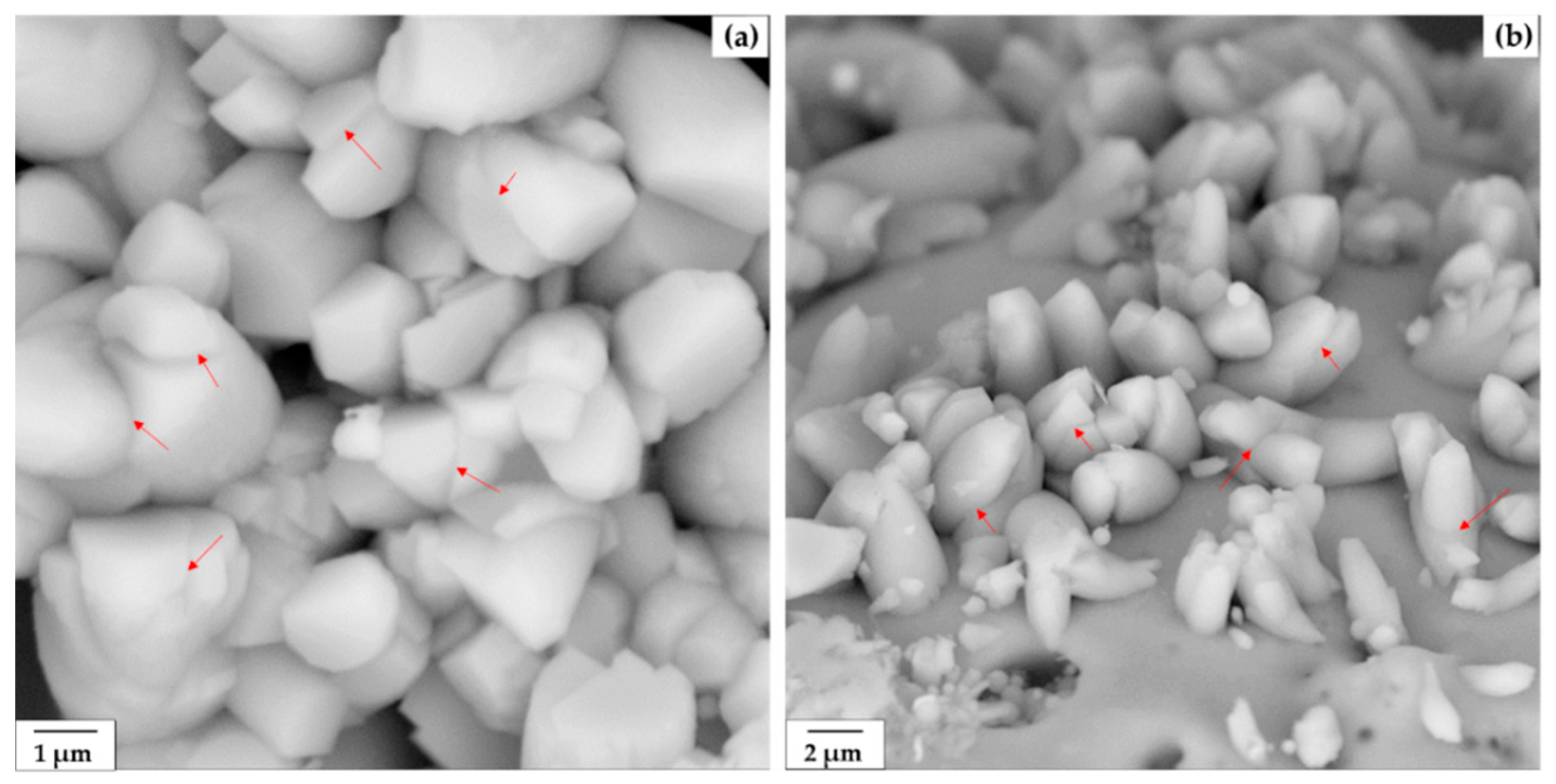
| Item | Composition (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | K2O | TiO2 | MnO | Cr2O3 | |
| Fly ash | 32.12 ± 0.29 | 13.82 ± 0.19 | 14.55 ± 0.27 | 24.49 ± 0.05 | 12.03 ± 0.26 | 2.31 ± 0.02 | 0.55 ± 0.02 | 0.55 ± 0.01 | 0.05 ± 0.00 |
| Cenospheres | |||||||||
| 10 min | |||||||||
| 1000 rpm | 43.78 ± 0.36 | 20.29 ± 0.33 | 9.06 ± 0.10 | 19.03 ± 0.63 | 2.28 ± 0.11 | 4.73 ± 0.04 | 0.72 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 2000 rpm | 41.02 ± 1.61 | 18.75 ± 0.69 | 9.69 ± 0.16 | 24.27 ± 2.38 | 1.39 ± 0.06 | 4.00 ± 0.03 | 0.78 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.00 |
| 3000 rpm | 45.77 ± 0.95 | 21.34 ± 0.43 | 7.43 ± 0.35 | 18.11 ± 1.37 | 1.41 ± 0.12 | 5.15 ± 0.06 | 0.70 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 4000 rpm | 38.17 ± 0.81 | 17.43 ± 0.31 | 9.60 ± 0.33 | 28.41 ± 1.31 | 1.62 ± 0.07 | 3.91 ± 0.05 | 0.76 ± 0.03 | 0.05 ± 0.00 | 0.06 ± 0.00 |
| 30 min | |||||||||
| 1000 rpm | 28.04 ± 2.42 | 12.71 ± 1.17 | 8.72 ± 0.60 | 45.38 ± 4.25 | 1.27 ± 0.03 | 3.14 ± 0.05 | 0.70 ± 0.05 | - | 0.06 ± 0.00 |
| 2000 rpm | 32.14 ± 1.28 | 15.02 ± 0.73 | 8.89 ± 0.53 | 38.27 ± 1.66 | 1.41 ± 0.09 | 3.51 ± 0.13 | 0.72 ± 0.01 | - | 0.06 ± 0.00 |
| 3000 rpm | 39.40 ± 1.24 | 18.36 ± 0.64 | 8.83 ± 0.40 | 27.37 ± 1.48 | 1.02 ± 0.07 | 4.17 ± 0.07 | 0.75 ± 0.02 | 0.04 ± 0.00 | 0.05 ± 0.00 |
| 4000 rpm | 40.45 ± 0.83 | 18.77 ± 0.44 | 8.62 ± 0.33 | 25.44 ± 1.23 | 1.64 ± 0.05 | 4.26 ± 0.08 | 0.72 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoriya, S.; Tepsri, P. Crystal Growth on Cenospheres from High-Calcium Fly Ash. Crystals 2021, 11, 919. https://doi.org/10.3390/cryst11080919
Yoriya S, Tepsri P. Crystal Growth on Cenospheres from High-Calcium Fly Ash. Crystals. 2021; 11(8):919. https://doi.org/10.3390/cryst11080919
Chicago/Turabian StyleYoriya, Sorachon, and Phattarathicha Tepsri. 2021. "Crystal Growth on Cenospheres from High-Calcium Fly Ash" Crystals 11, no. 8: 919. https://doi.org/10.3390/cryst11080919
APA StyleYoriya, S., & Tepsri, P. (2021). Crystal Growth on Cenospheres from High-Calcium Fly Ash. Crystals, 11(8), 919. https://doi.org/10.3390/cryst11080919






