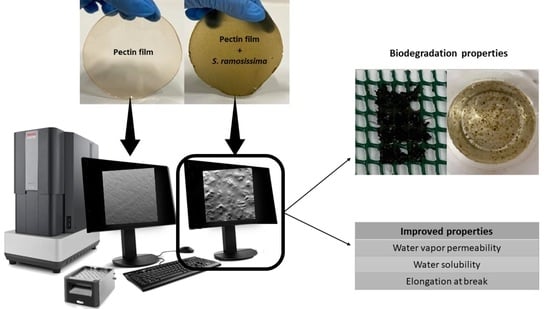
Development and Characterization of Pectin Films with Salicornia ramosissima: Biodegradation in Soil and Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Films
2.3. Thickness and Water Vapor Permeability (WVP)
2.4. Color and Opacity
2.5. Solubility and Moisture Content
2.6. Texture Measurements
2.7. FTIR
2.8. Scanning Electron Microscopy (SEM)
2.9. Biodegradation Tests
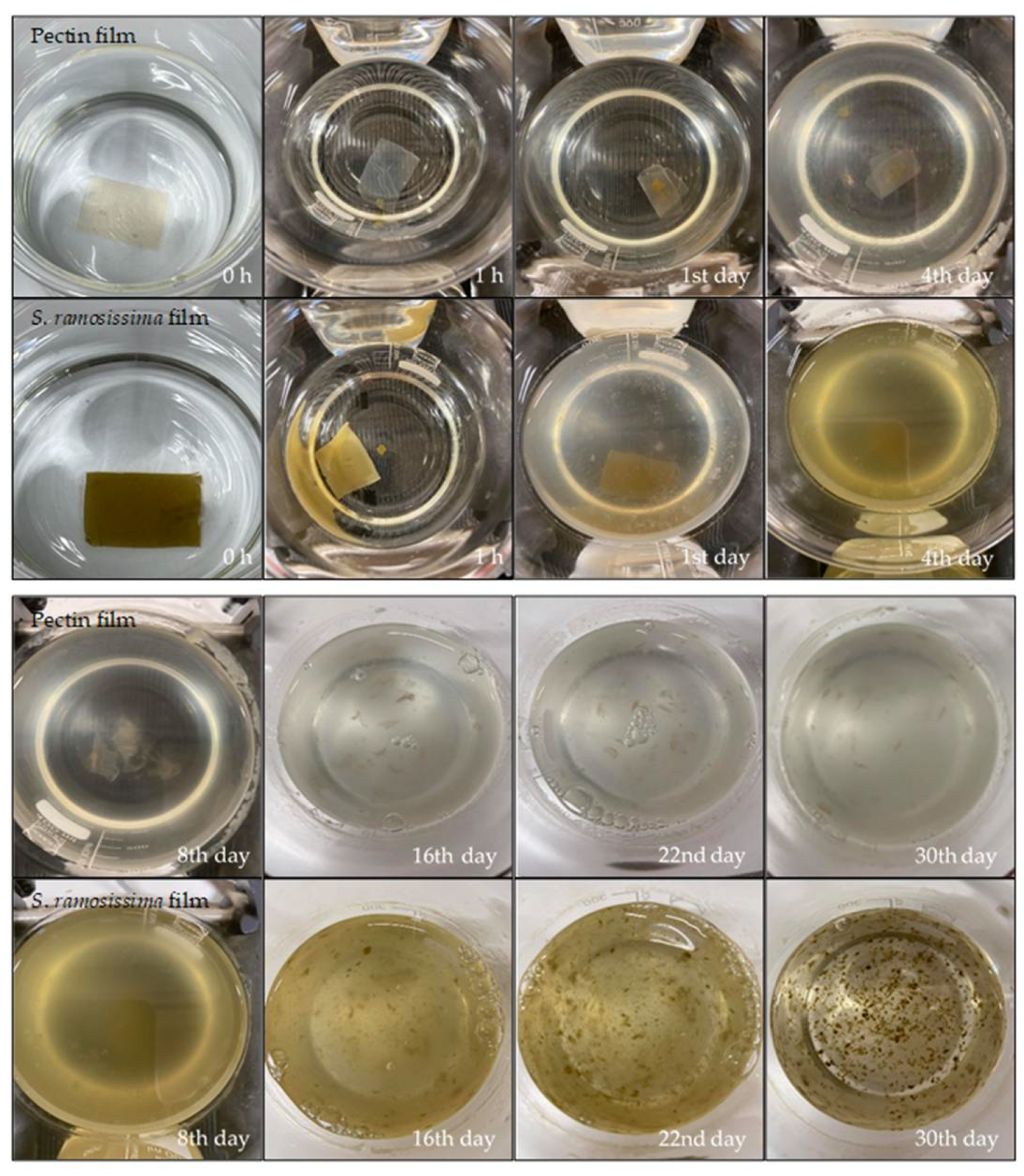
2.9.1. Seawater
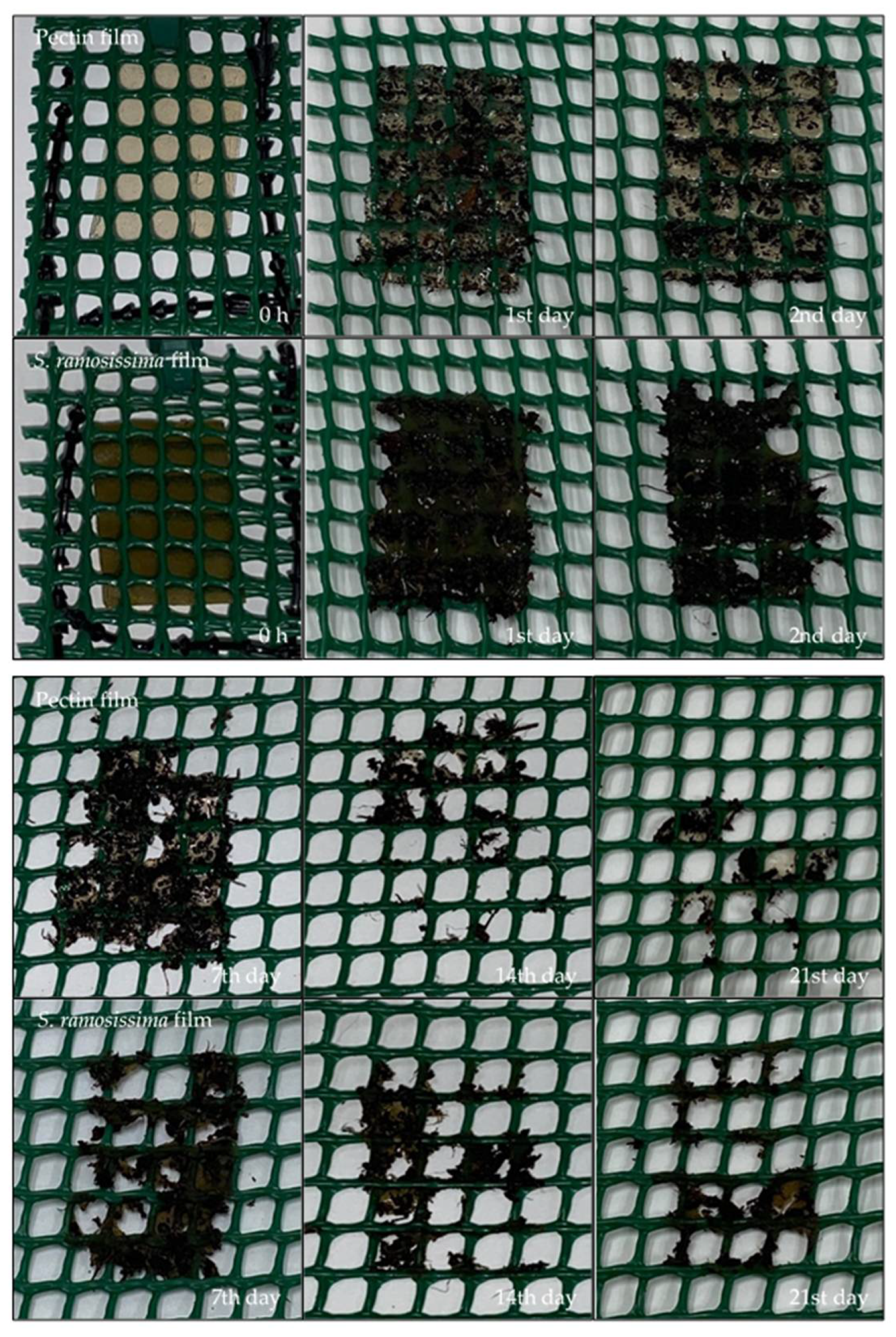
2.9.2. Soil
2.10. Statistical Analyses
3. Results and Discussion
3.1. Physicomechanical Properties
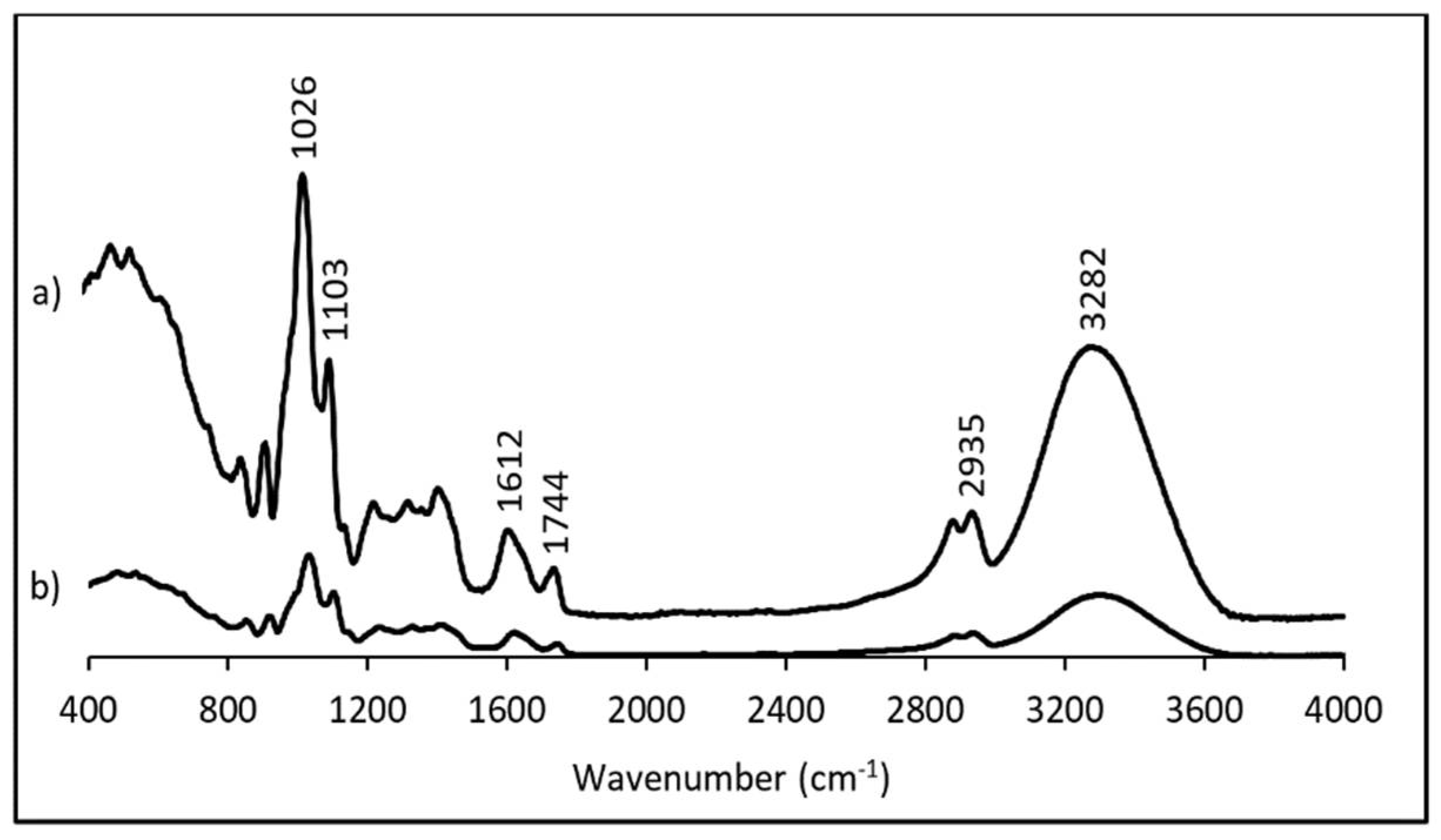
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
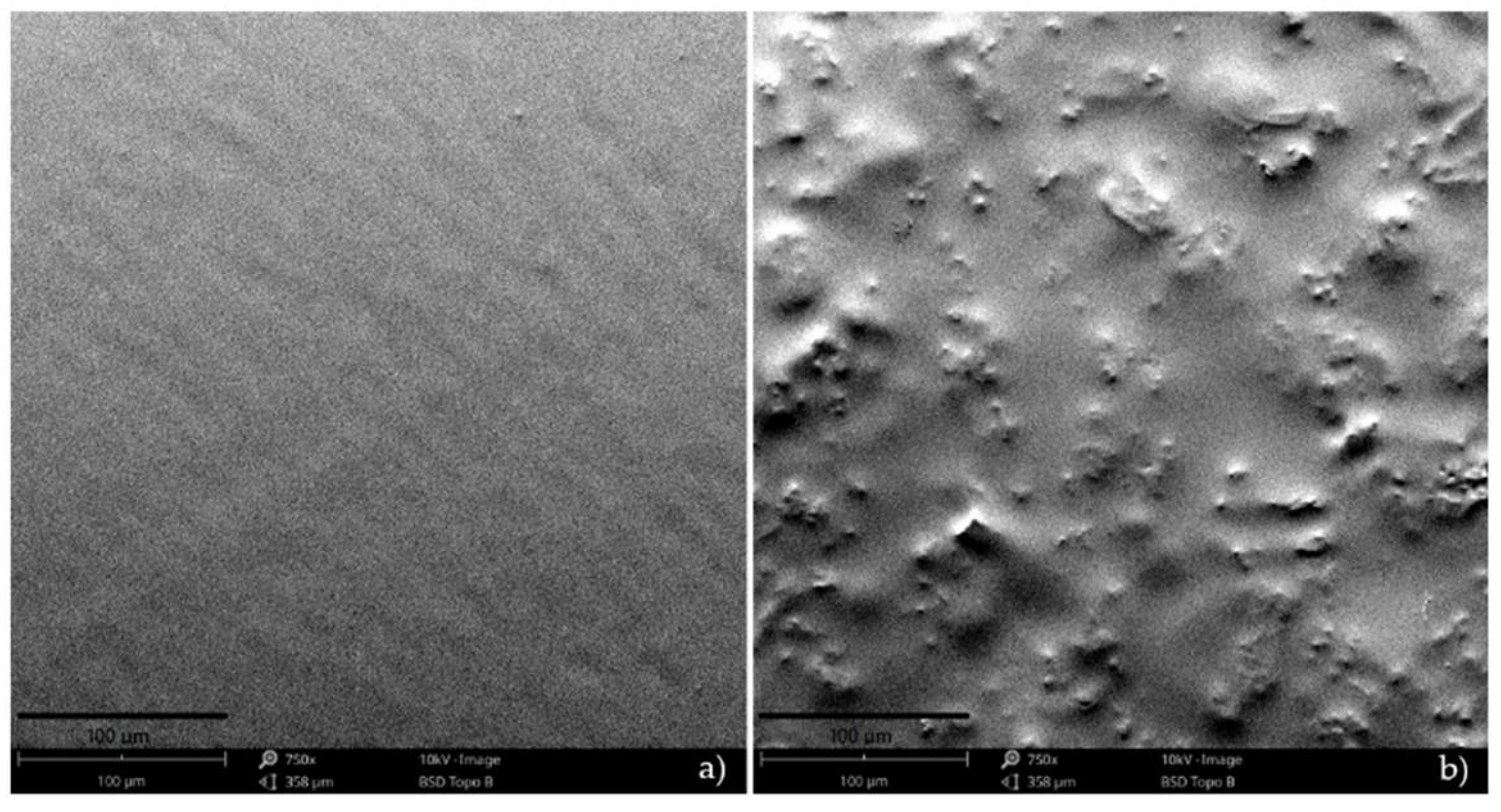
3.3. Scanning Electron Microscopy (SEM)
3.4. Biodegradation Properties
3.4.1. Seawater
3.4.2. Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch–chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef] [PubMed]
- Kalateh-Seifari, F.; Yousefi, S.; Ahari, H.; Hosseini, S.H. Corn starch-chitosan nanocomposite film containing nettle essential oil nanoemulsions and starch nanocrystals: Optimization and characterization. Polymers 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- Oliver-Ortega, H.; Tresserras, J.; Julian, F.; Alcalà, M.; Bala, A.; Espinach, F.X.; Méndez, J.A. Nanocomposites materials of PLA Reinforced with nanoclays using a masterbatch technology: A study of the mechanical performance and its sustainability. Polymers 2021, 13, 2133. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent applications of biopolymers derived from fish industry waste in food packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/gelatin/silver nanoparticles composites films for biodegradable food packaging applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A review of the applications and biodegradation of polyhydroxyalkanoates and poly(lactic acid) and its composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef]
- Brito, T.B.; Carrajola, J.F.; Gonçalves, E.C.B.A.; Martelli-Tosi, M.; Ferreira, M.S.L. Fruit and vegetable residues flours with different granulometry range as raw material for pectin-enriched biodegradable film preparation. Food Res. Int. 2019, 121, 412–421. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Incorporation of spray dried and freeze-dried blackberry particles in edible films: Morphology, stability to pH, sterilization and biodegradation. Food Packag. Shelf Life 2019, 20, 100313. [Google Scholar] [CrossRef]
- Zarandona, I.; Barba, C.; Guerrero, P.; de la Caba, K.; Maté, J. Development of chitosan films containing β-cyclodextrin inclusion complex for controlled release of bioactives. Food Hydrocoll. 2020, 104, 105720. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; de Oliveira, R.A. Bioactive films of arrowroot starch and blackberry pulp: Physical, mechanical and barrier properties and stability to pH and sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and physical characteristics of edible films, based on κ- and ι-carrageenans with the addition of lapacho tea extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.; Wang, L. A pH-sensing film from tamarind seed polysaccharide with Litmus lichen extract as an indicator. Polymers 2017, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.B.S.; Lee, S.M.; Solouk, A.; Pasbakhsh, P. Safely dissolvable and healable active packaging films based on alginate and pectin. Polymers 2019, 11, 1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of clindamycin hydrochloride loaded de-esterified low-methoxyl mango peel pectin film used as a topical drug delivery system. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of incorporating plant-derived bioactive compounds into films made with agro-based polymers for application as food packaging: A brief review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; Pinheiro, A.C.M.; Oliveira, J.E.; Mattoso, L.H.C. Characterization of pectin films integrated with cocoa butter by continuous casting: Physical, thermal and barrier properties. J. Polym. Environ. 2020, 28, 2905–2917. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Souza, B.W.S.; Carmo Avides, M.d.; Vicente, A.A. Shelf life extension of ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against Listeria monocytogenes. J. Agric. Food Chem. 2010, 58, 1884–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- ASTM D 882. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Accinelli, C.; Saccà, M.L.; Mencarelli, M.; Vicari, A. deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere 2012, 89, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Heaton, J.W.; Marangoni, A.G. Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food Sci. Technol. 1996, 7, 8–15. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Alim, A.; Iqbal, M.; Yang, X.; Sun, L.; Guo, Y. Citrus pectin films enriched with thinned young apple polyphenols for potential use as bio-based active packaging. CyTA J. Food 2019, 17, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Sganzerla, W.G.; Paes, B.B.; Azevedo, M.; Ferrareze, J.; Da Rosa, C.G.; Nunes, M.R.; De Lima Veeck, A.P. Bioactive and biodegradable films packaging incorporated with Acca sellowiana extracts: Physicochemical and antioxidant characterization. Chem. Eng. Trans. 2019, 75, 445–450. [Google Scholar] [CrossRef]
- Dias, A.B. Desenvolvimento e caracterização de filmes biodegradáveis obtidos de amido e de farinha de arroz. Univ. Fed. St. Catarina 2008, 1–116. Available online: http://repositorio.ufsc.br/xmlui/handle/123456789/92138 (accessed on 10 February 2021).
- Meneguin, A.; Cury, B.; Evangelista, R. Films from resistant starch-pectin dispersions intended for colonic drug delivery. Carbohydr. Polym. 2014, 99, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Chen, H. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Yehuala, G.A.; Emire, S.A. Antimicrobial activity, physicochemical and mechanical properties of Aloe (Aloe debrana) based packaging films. BJAST 2014, 3, 1257–1275. [Google Scholar] [CrossRef]
- Shaw, N.B.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Physical properties of WPI films plasticized with glycerol, xylitol, or sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Gonçalves, M.P.; Souza, H.K.S. A New approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Meerasri, J.; Sothornvit, R. Characterization of bioactive film from pectin incorporated with gamma-aminobutyric acid. Int. J. Biol. Macromol. 2020, 147, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jo, C.; Lee, N.; Kwon, J.; Byun, M. A Combination of gamma irradiation and CaCl immersion for a pectin-based biodegradable film. Carbohydr. Polym. 2005, 60, 547–551. [Google Scholar] [CrossRef]
- Cerna, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Copikova, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; Moura, M.R.d.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high and low-methyl pectin Films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Zhu, Z.; Wen, Y.; Su, C.; Jiang, L.; He, S.; Shao, W. Facile and green preparation of pectin/cellulose composite films with enhanced antibacterial and antioxidant behaviors. Polymers 2019, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syntsya, A.; Čpíková, J.; Marounek, M.; Mlčochová, P.; Sihelková, L.; Blafková, P.; Tkadlecová, M.; Havlíček, J. Preparation of N-alkylamides of highly methylated (HM) citrus pectin. Czech J. Food Sci. 2011, 21, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gelling properties of krueo ma noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Yu, H.; Peng, C.; Li, F.-C.; Yu, P. Effect of chloride salt type on the physicochemical, mechanical and morphological properties of fish gelatin film. Mater. Res. Express 2019, 6, 126414. [Google Scholar] [CrossRef]
- Ju, A.; Song, K.B. Active biodegradable films based on water soluble polysaccharides from white jelly mushroom (Tremella fuciformis) containing roasted peanut skin extract. LWT Food Sci. Technol. 2020, 126, 109293. [Google Scholar] [CrossRef]
- Alvarez-Zeferino, J.C.; Beltrán-Villavicencio, M.; Vázquez-Morillas, A. Degradation of plastics in seawater in laboratory. Open J. Polym. Chem. 2015, 05, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, A.; Yamano, N.; Kawasaki, N. Biodegradation in seawater of aliphatic polyesters. Polym. Degrad. Stab. 2019, 166, 290–299. [Google Scholar] [CrossRef]
- EN 13432:2000. Packaging—Requirements for Packaging Recoverable Through Composting and Biodegradation—Test. Scheme and Evaluation Criteria for the Final Acceptance of Packaging; European Committee for Standardization: Brussels, Belgium, 2000. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Rech, C.R.; da Silva Brabes, K.C.; Bagnara e Silva, B.E.; Bittencourt, P.R.S.; Koschevic, M.T.; da Silveira, T.F.S.; Martines, M.A.U.; Caon, T.; Martelli, S.M. Biodegradation of eugenol-loaded polyhydroxybutyrate films in different soil types. Case Stud. Chem. Environ. Eng. 2020, 2, 100014. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Olaosebikan, O.O.; Alo, M.N.; Ugah, U.I.; Olayemi, A.M. Environmental effect on biodegradability of plastic and paper bags. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 22–29. [Google Scholar]
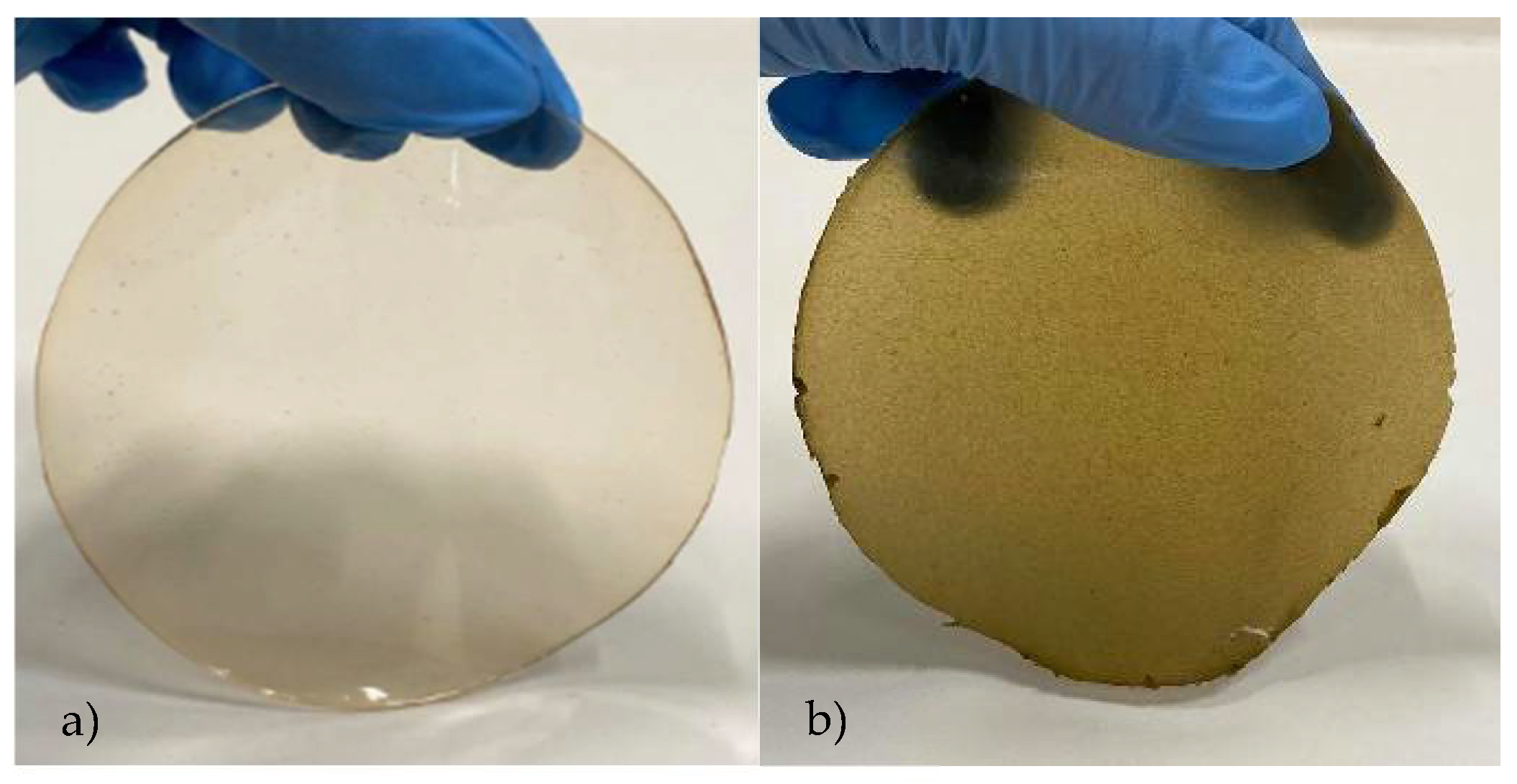
| Control Film | Film with S. ramosissima | |
|---|---|---|
| Thickness (mm) | 0.18 ± 0.01 a | 0.25 ± 0.01 b |
| Color | ||
| L* | 81.20 ± 0.70 a | 48.84 ± 1.60 b |
| a* | 3.29 ± 0.16 a | 4.96 ± 0.30 b |
| b* | 12.74 ± 0.75 a | 28.62 ± 0.51 b |
| Opacity (%) | 10.87 ± 0.05 a | 30.04 ± 1.49 b |
| Water vapor permeability (g/(m·s·Pa)) | 1.24 × 10−9 ± 6.58 × 10−11 a | 1.62 × 10−9 ± 1.09 × 10−10 b |
| Water solubility (%) | 11.56 ± 5.56 a | 50.50 ± 5.00 b |
| Moisture content (%) | 45.79 ± 0.76 a | 30.11 ± 4.41 b |
| Elongation at break (%) | 3.91 ± 0.62 a | 5.89 ± 0.29 b |
| Young’s modulus (MPa) | 0.93 ± 0.12 a | 0.01 ± 0 b |
| Tensile strength (MPa) | 4.19 ± 0.82 a | 0.13 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.G.M.; Vieira, J.M.; Vicente, A.A.; Cruz, R.M.S. Development and Characterization of Pectin Films with Salicornia ramosissima: Biodegradation in Soil and Seawater. Polymers 2021, 13, 2632. https://doi.org/10.3390/polym13162632
Pereira DGM, Vieira JM, Vicente AA, Cruz RMS. Development and Characterization of Pectin Films with Salicornia ramosissima: Biodegradation in Soil and Seawater. Polymers. 2021; 13(16):2632. https://doi.org/10.3390/polym13162632
Chicago/Turabian StylePereira, Daniela G. M., Jorge M. Vieira, António A. Vicente, and Rui M. S. Cruz. 2021. "Development and Characterization of Pectin Films with Salicornia ramosissima: Biodegradation in Soil and Seawater" Polymers 13, no. 16: 2632. https://doi.org/10.3390/polym13162632
APA StylePereira, D. G. M., Vieira, J. M., Vicente, A. A., & Cruz, R. M. S. (2021). Development and Characterization of Pectin Films with Salicornia ramosissima: Biodegradation in Soil and Seawater. Polymers, 13(16), 2632. https://doi.org/10.3390/polym13162632