Thermodynamic Description of Dilution and Dissolution Processes in the MgCl2−CsCl−H2O Ternary System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples’ Preparation Details
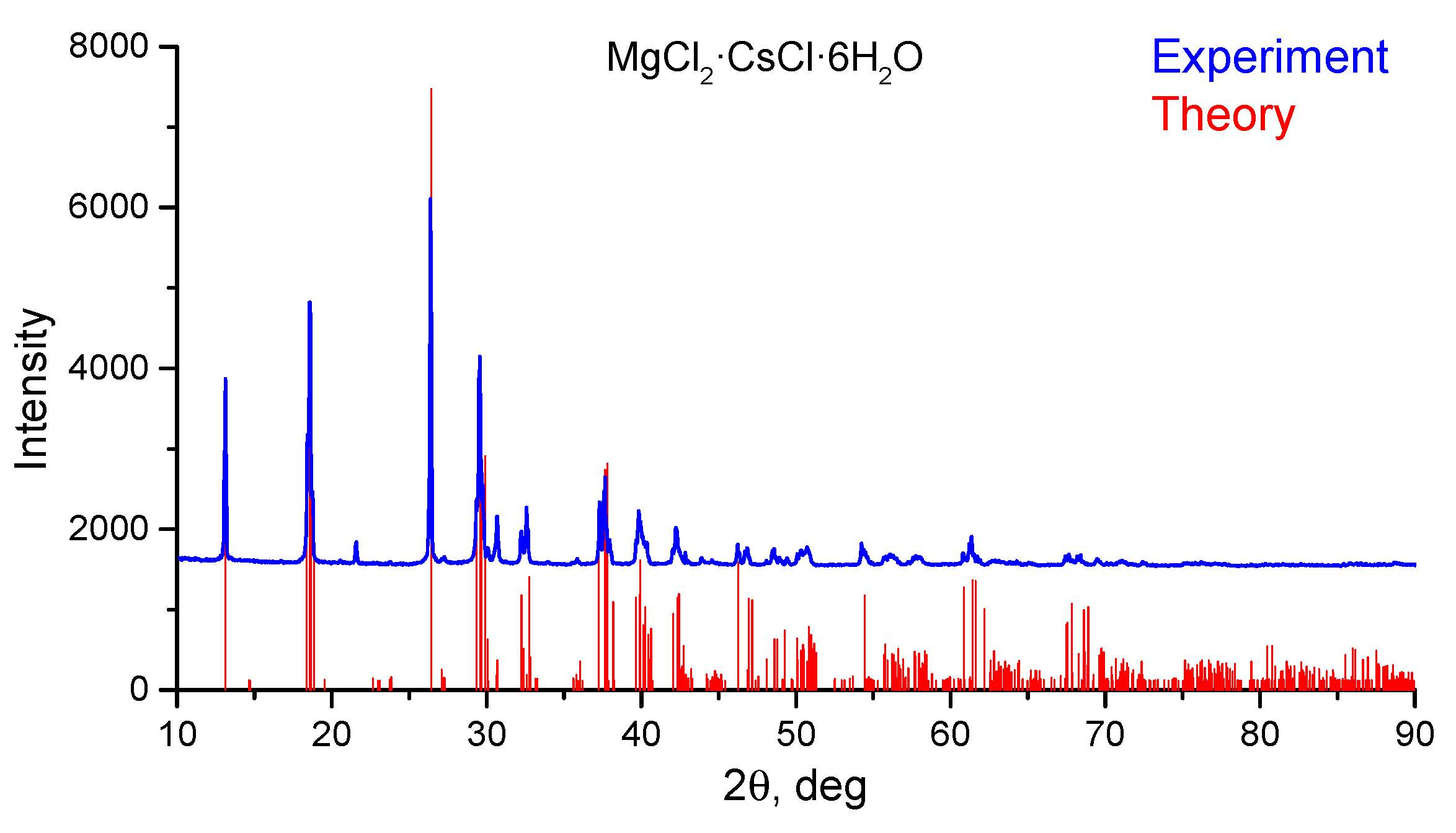
2.2. X-ray Crystallography and Powder Diffraction
2.3. Dissolution Calorimetry
2.4. Dilution Calorimetry
3. Results and Discussion
3.1. X-ray Crystallography and Powder Diffraction
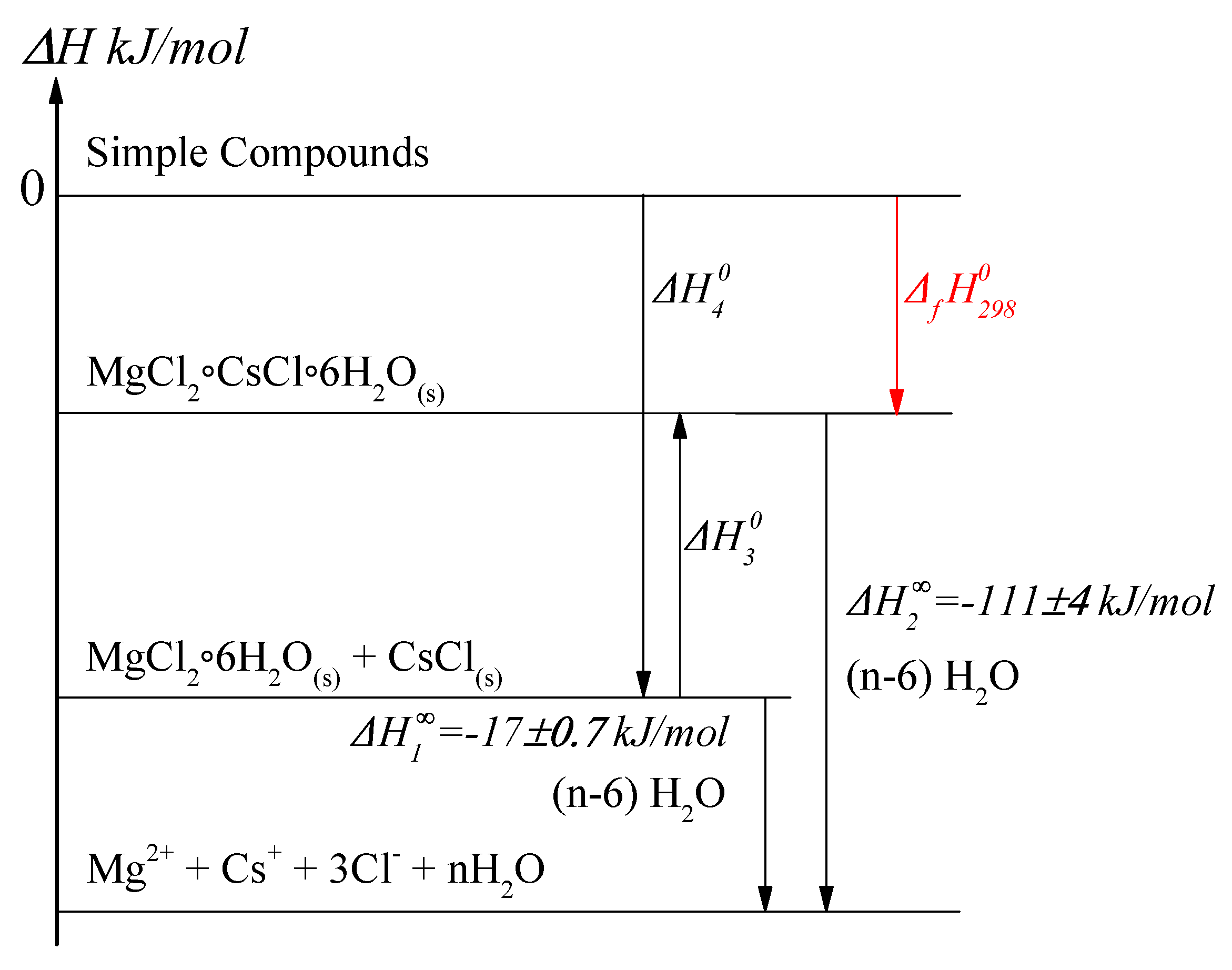
3.2. Dissolution Calorimetry
- is dissolution enthalpy of salt mixture kJ/mol
- is double salt dissolution enthalpy kJ/mol
- is enthalpy of [MgCl2·6H2O]s+ [CsCl]s = [MgCl2·CsCl·6H2O]s reaction
- is the sum of the standard formation enthalpies of [MgCl2·6H2O]s and [CsCl]s salts
- is the standard formation enthalpy of the solid double salt
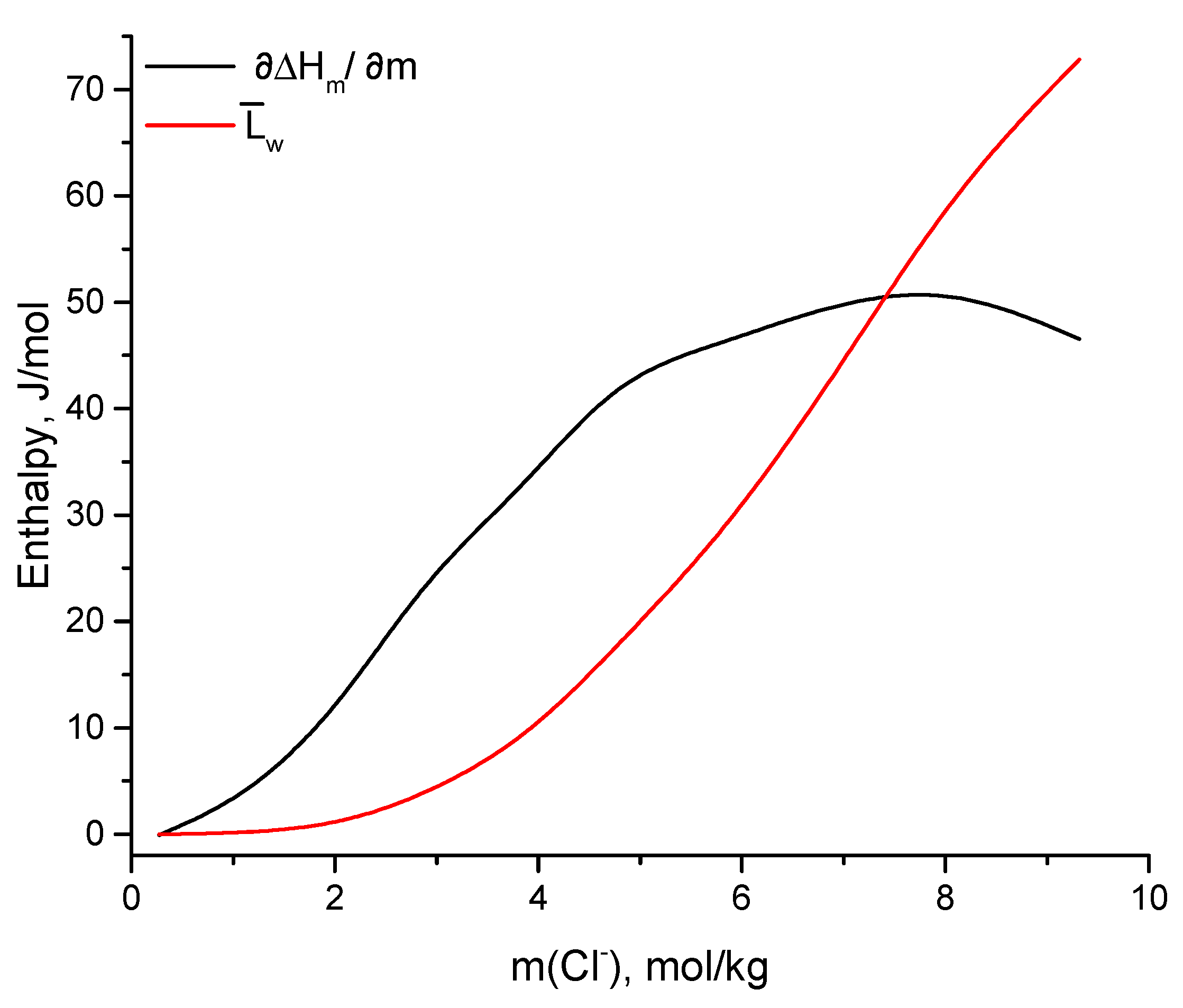
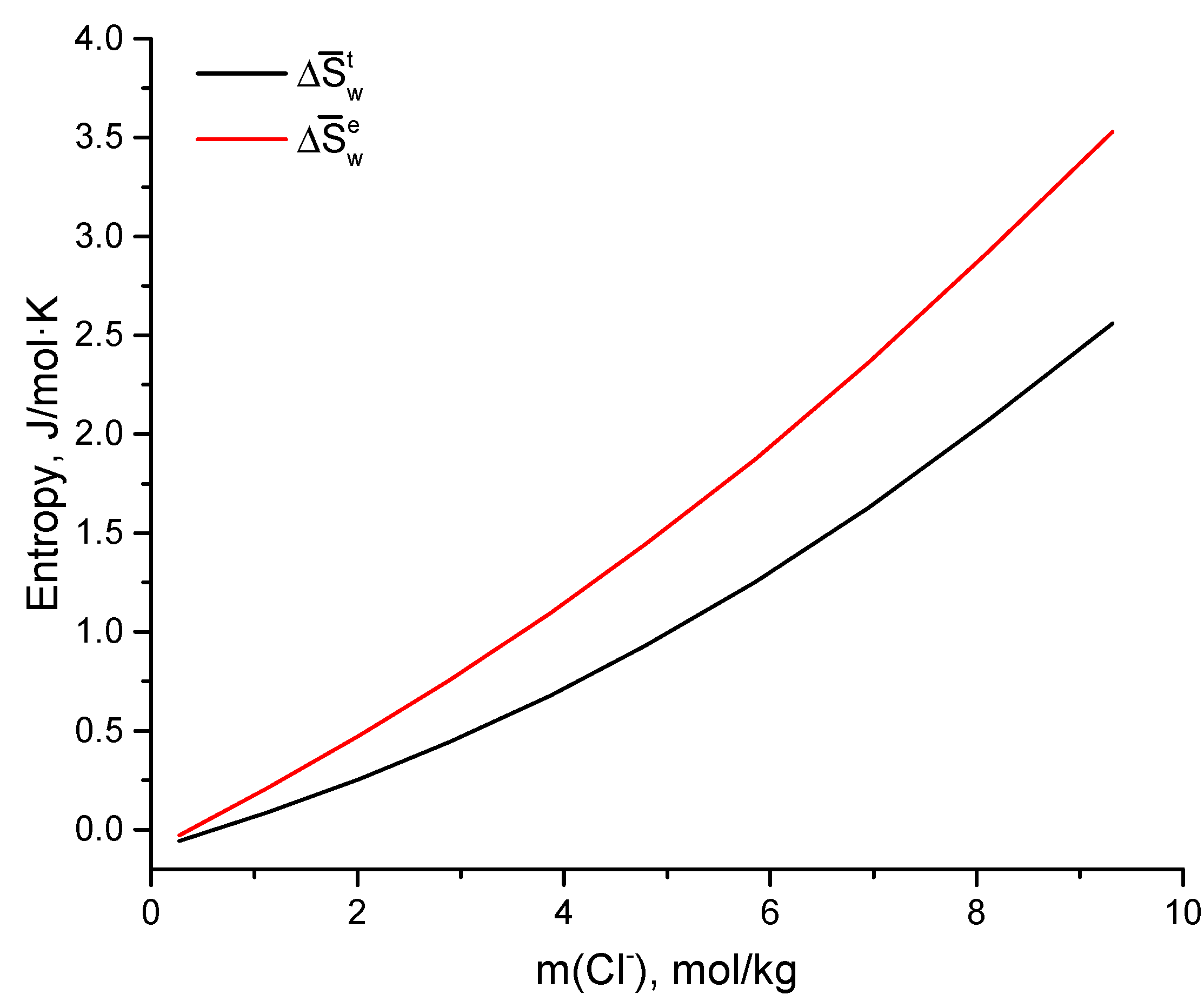
3.3. Dilution Calorimetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRC | X-ray crystallography |
| The dissolution enthalpy | |
| The standard formation enthalpy | |
| The Gibbs energy change of the reaction | |
| The entropy change of the reaction | |
| The enthalpy change of the reaction | |
| The relative partial molal enthalpy of the solvent (water) | |
| The molar differential dilution enthalpy | |
| The total relative partial molar entropy | |
| The ideal relative partial molar entropy | |
| The excess relative partial molar entropy |
Appendix A
| Mass, g | , mol | S | , kJ | K | Error |
|---|---|---|---|---|---|
| 0.0273 | 3.66 × 10 | 1.644 | 6.32 | 0.260 | 0.008 |
| 0.0232 | 3.11 × 10 | 1.468 | 5.37 | 0.274 | −0.005 |
| 0.0232 | 3.11 × 10 | 1.408 | 5.37 | 0.262 | 0.006 |
| 0.0282 | 3.78 × 10 | 1.652 | 6.53 | 0.253 | 0.015 |
| 0.0315 | 4.23 × 10 | 1.949 | 7.29 | 0.267 | 0.001 |
| 0.0218 | 2.92 × 10 | 1.428 | 5.04 | 0.283 | −0.015 |
| 0.0244 | 3.27 × 10 | 1.559 | 5.65 | 0.276 | −0.008 |
| 0.0203 | 2.72 × 10 | 1.266 | 4.70 | 0.270 | −0.001 |
| 0.268 | ±0.021 | ||||
| Mass, g | , mol | S | , kJ | , kJ | Error |
|---|---|---|---|---|---|
| 0.0235 | 6.32 × 10 | 1.905 | 7.11 | −112 | 1.79 |
| 0.0370 | 9.95 × 10 | 2.928 | 10.93 | −110 | −0.88 |
| 0.0302 | 8.12 × 10 | 2.350 | 8.77 | −108 | −2.71 |
| 0.0392 | 1.05 × 10 | 3.678 | 11.86 | −112 | 1.80 |
| −128 | ±4.07 | ||||
| m(MgCl2·6H2O), g | m(CsCl), g | S | , kJ | , kJ | Error |
|---|---|---|---|---|---|
| 0.0264 | 0.0224 | 1.179 | 4.04 | −16.73 | −0.52 |
| 0.0275 | 0.0259 | 1.355 | 5.06 | −17.49 | 0.24 |
| 0.0135 | 0.0119 | 0.626 | 2.34 | −17.05 | −0.20 |
| 0.0135 | 0.0112 | 0.622 | 2.32 | −17.45 | 0.20 |
| −17.25 | ±0.66 | ||||
| Parameter | Value |
|---|---|
| Formula sum | Cl3 H12 Cs Mg O6 |
| Formula weight | 371.69 g/mol |
| Crystal system | monoclinic |
| Space-group | C 1 2/c 1 (15) |
| Cell parameters | a = 9.4134(3) Å b = 9.6425(3) Å c = 13.4708(3) Å |
| Cell ratio | a/b = 0.9762 b/c = 0.7158 c/a = 1.4310 |
| Cell volume | 1222.71(6) Å |
| Z | 4 |
| Calc. density | 2.01891 g/cm |
| RAll | 0.0322 |
| Pearson code | mC100 |
| Formula type | NO2P4Q6R12 |
| Wyckoff sequence | f12e |
References
- Høy-Petersen, N.; Rizley, J.H. Magnesium Processing; Encyclopædia Britannica Inc.: Chicago, IL, USA, 2020. [Google Scholar]
- Tavares, J.; Moura, L.; Bernardo, A.; Giulietti, M. Crystallization and separation of KCl from carnallite ore: Process development, simulation, and economic feasibility. Chem. Ind. Chem. Eng. Q. 2018, 24, 239–249. [Google Scholar] [CrossRef]
- Prokhorov, A.; Knunyants, I. Khimicheskaia Entsiklopedia: V Piati Tomakh (Chemical Encyclopedia in 5 Volumes); Soviet Encyclopedia: Moscow, Russia, 1988; pp. 654–656. [Google Scholar]
- Mamani, V.; Gutiérrez, A.; Fernández, A.; Ushak, S. Industrial carnallite-waste for thermochemical energy storage application. Appl. Energy 2020, 265, 114738. [Google Scholar] [CrossRef]
- Gutierrez, A.; Ushak, S.; Mamani, V.; Vargas, P.; Barreneche, C.; Cabeza, L.F.; Grágeda, M. Characterization of wastes based on inorganic double salt hydrates as potential thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 170, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, A.; Ushak, S.; Linder, M. High Carnallite-Bearing Material for Thermochemical Energy Storage: Thermophysical Characterization. ACS Sustain. Chem. Eng. 2018, 6, 6135–6145. [Google Scholar] [CrossRef]
- Emons, H.H. Mechanism and kinetics of formation and decomposition of carnallitic double salts. J. Therm. Anal. 1988, 33, 113–120. [Google Scholar] [CrossRef]
- Emons, H.; Brand, P.; Pohl, T.; Köhnke, K. Crystal chemistry of MX·MgX2·6H2O type compounds. ZAAC J. Inorg. Gen. Chem. 1988, 563, 180–184. [Google Scholar] [CrossRef]
- Emons, H.H.; Naumann, R.; Pohl, T.; Voigt, H. Thermoanalytical investigations on the decomposition of double salts. J. Therm. Anal. 1984, 29, 571–579. [Google Scholar] [CrossRef]
- Balarew, C.; Tepavitcharova, S. Co-Crystallization in systems with carnallite-type double salts. Z. Für Anorg. Und Allg. Chem. 1990, 583, 186–194. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, W.; Li, S.; Zhai, Q.; Jiang, Y. Thermodynamic investigation of a ternary mixed electrolyte (CsCl/MgCl2/H2O) system using electromotive force measurement at 298.15 K. J. Chem. Eng. Data 2009, 54, 2023–2027. [Google Scholar] [CrossRef]
- Guo, L.; Tu, L.; Wang, Y.; Li, J. Water Activity and Solubility Measurements and Model Simulation of the CsCl−MgCl2−H2O Ternary System at 323.15 K. J. Chem. Eng. Data 2018, 63, 483–487. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Tu, L.; Li, J. Thermodynamics and Phase Equilibrium of the System CsCl−MgCl2−H2O at 298.15 K. J. Chem. Eng. Data 2017, 62, 1397–1402. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M.; Holler, F.J. Fundamentals of Analytical Chemistry, 7th ed.; Saunders Golden Sunburst Series; Saunders College Pub.: Fort Worth, TX, USA, 1996. [Google Scholar]
- Pestova, O.; Bukesova, V.; Kondrat’ev, Y.; Khripun, V.; Baranauskaite, V. Energy Characteristics of Lithium–Cesium Binary Chloride Dissolution in Water. Russ. J. Gen. Chem. 2018, 88, 596–597. [Google Scholar] [CrossRef]
- Kilday, M. The enthalpy of solution of SRM 1655 (KCl) in H2O. J. Res. Natl. Bur. Stand. 1980, 85, 467. [Google Scholar] [CrossRef]
- Waizumi, K.; Masuda, H.; Ohtaki, H.; Scripkin, M.Y.; Burkov, K.A. Crystallographic investigations of [Mg(H2O)6]XCl3 double salts (X+ = K+, Rb+, Cs+, NH4+): Crystal structure of [Mg(H2O)6]CsCl3. Am. Mineral. 1991, 76, 1884–1888. [Google Scholar]
- Ahtee, M. Lattice constants of some binary alkali halide solid solutions. Ann. Acad. Sci. Fenn. Ser. A Phys. 1969, 313, 1–11. [Google Scholar]
- Agron, P.A.; Busing, W.R. Magnesium dichloride hexahydrate, MgCl2·6H2O, by neutron diffraction. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1985, 41, 8–10. [Google Scholar] [CrossRef]
- Andress, K.R.; Carpenter, C. Kristallhydrate. Z. Krist. Cryst. Mater. 1934, 87. [Google Scholar] [CrossRef]
- Khripun, M.; Karavan, S.; Bulgakov, S. Vzaimosvjaz’ struktury i stroenija v koncentrirovannyh rastvorah jelektrolitov. (The correlation of the structure and the order in the concentrated electrolyte solutions). Probl. Sovrem. Him. Koord. Soedin. Quest. Contemp. Chem. Complex Compd. 1987, 8, 123–141. [Google Scholar]
- Naumov, G.B.; Ryzhenko, B.N.; Khodakovski, I.L.; Barnes, I.; Speltz, V. Handbook of Thermodynamic Data; N.T.I.S: Menlo Park, CA, USA, 1974.
- Fiziko-Chimiceskie Svojstva Galurgiceskich Rastvorov i Solej: Chloridy Natrija, Kalija i Magnija; Spravocnik; Chimija: Sankt-Peterburg, Russia, 1997; p. 172.
- Waizumi, K.; Masuda, H.; Ohtaki, H.; Burkov, K.A.; Scripkin, M.Y. Structure of MgCl2·RbCl·6H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 251–254. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [Green Version]
- Shomate, C.H.; Huffman, E.H. Heats of Formation of MgO, MgCl2, MgCl2·H2O, MgCl2·2H2O, MgCl2·4H2O, and MgCl2·6H2O. J. Am. Chem. Soc. 1943, 65, 1625–1629. [Google Scholar] [CrossRef]
- Johnson, G.K.; Gayer, K.H. The enthalpies of solution and formation of the chlorides of cesium and rubidium. J. Chem. Thermodyn. 1979, 11, 41–46. [Google Scholar] [CrossRef]
- Li, D.; Zeng, D.; Yin, X.; Gao, D.; Fan, Y. Phase diagrams and thermochemical modeling of salt lake brine systems. IV. Thermodynamic framework and program implementation for multicomponent systems. Calphad 2020, 71, 101806. [Google Scholar] [CrossRef]
- Balarew, C.; Christov, C.; Valyashko, V.; Petrenko, S. Thermodynamics of formation of carnallite type double salts. J. Solut. Chem. 1993, 22, 173–181. [Google Scholar] [CrossRef]
- Pepple, G.W. Relative Apparent Molal Heat Contents of Some Aqueous Rare-Earth Chloride Solutions at 25 C. Ph.D. Thesis, Iowa State University, Iowa, ON, Canada, 1967. [Google Scholar] [CrossRef]
- Mishchenko, K.P.; Poltoratskiy, G.M. Termodinamika i Stroenie Vodnyh i Nevodnyh Rastvorov Elektrolitov (Thermodynamics and Structure of the Water and Non-Water Electrolyte Solutions), 2nd ed.; Chimiya (Chemistry): Leningrad, Russia, 1976; p. 186. [Google Scholar]
- Belysheva, M.; Pestova, O.; Baranauskaite, V.; Anufrikov, Y. Dissolution and Dilution Enthalpies of the Ternary System MgCl2−CsCl−H2O at 298,15 K. In Proceedings of the Mendeleev 2019 Conference, Saint Petersburg, Russia, 9–13 September 2019; p. 42. [Google Scholar]
- Enderby, J.E.; Neilson, G.W. The structure of electrolyte solutions. Rep. Prog. Phys. 1981, 44, 593–653. [Google Scholar] [CrossRef]
- Copestake, A.P.; Neilson, G.W.; Enderby, J.E. The structure of a highly concentrated aqueous solution of lithium chloride. J. Phys. C Solid State Phys. 1985, 18, 4211–4216. [Google Scholar] [CrossRef]
- Podolsky, R.J. The Structure of Water and Electrolyte Solutions. Circulation 1960, 21, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Efimov, A.Y.; Khripun, M.K.; Myund, L.A.; Pestova, O.N. Mobile nanostructures (cybotactic groups) as a basis of generalised phenomenological model of aqueous electrolyte solutions. Int. J. Nanotechnol. 2016, 13, 95. [Google Scholar] [CrossRef]
- Pestova, O.N.; Efimov, A.Y.; Myund, L.A.; Kudrev, A.G.; Khripun, V.D.; Davidian, A.G.; Baranauskaite, V.E. Structural Inhomogeneity in Electrolyte Solutions: The Calcium Perchlorate–Water System. J. Solut. Chem. 2017, 46, 1854–1870. [Google Scholar] [CrossRef]
- Pham, V.T.; Fulton, J.L. Contact ion-pair structure in concentrated cesium chloride aqueous solutions: An extended X-ray absorption fine structure study. J. Electron Spectrosc. Relat. Phenom. 2018, 229, 20–25. [Google Scholar] [CrossRef]
- Afanas’ev, V.N.; Ustinov, A.N. Hydration numbers and the state of water in hydration spheres of magnesium chloride and magnesium sulfate solutions. Russ. J. Inorg. Chem. 2012, 57, 1107–1122. [Google Scholar] [CrossRef]
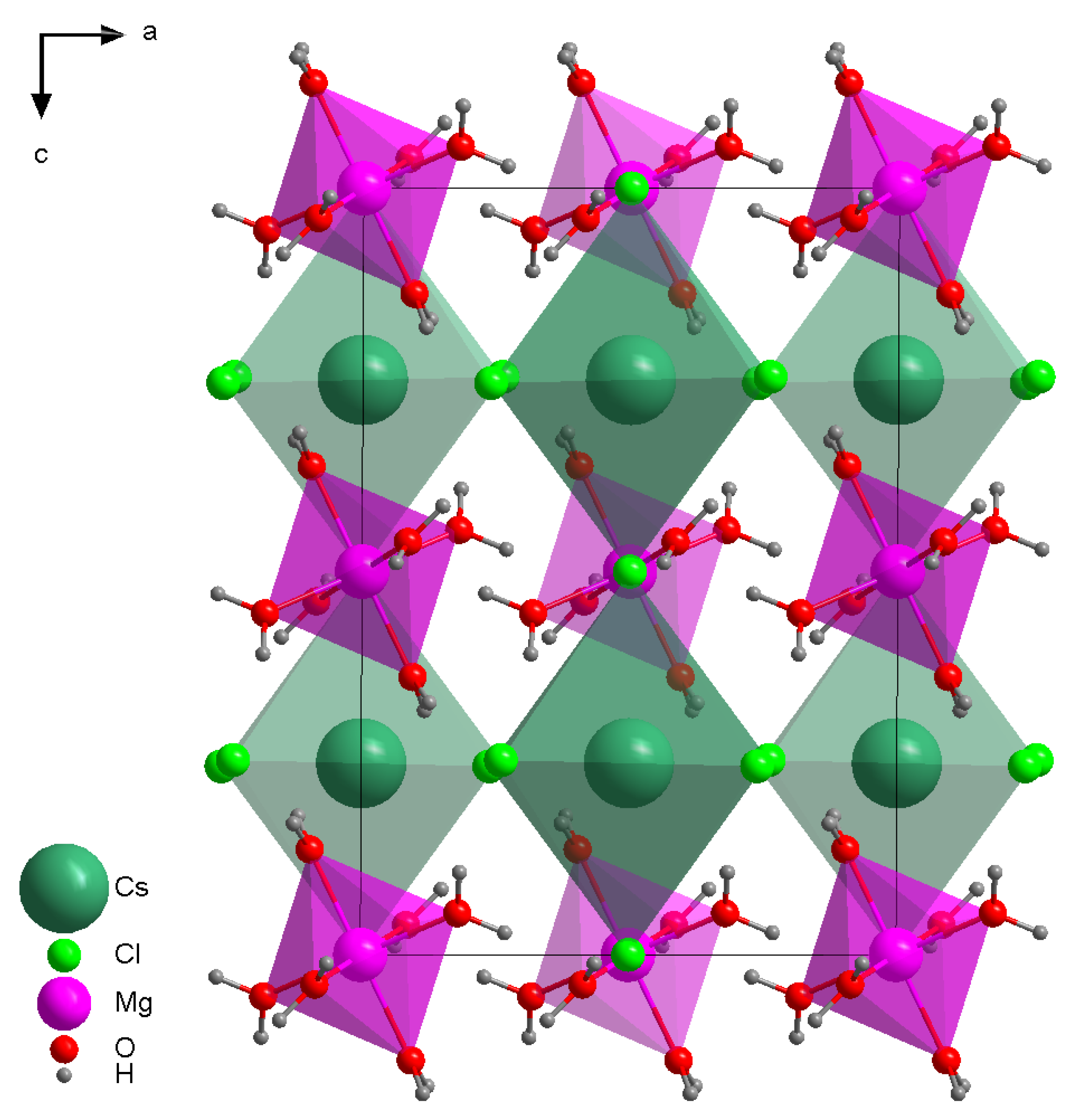


| Salt | d(Cs-Cs), Å | d(Cs-Cl), Å |
|---|---|---|
| MgCl2·CsCl·6H2O | 6.7275 | 3.3686 |
| CsCl | 4.1150 | 3.5637 |
| Salt | d(Mg-Mg), Å | d(Mg-O), Å |
|---|---|---|
| MgCl2·CsCl·6H2O | 6.7258 | 2.051–2.071 |
| MgCl2·6H2O | 6.0737 | 2.0573–2.0620 |
| MgCl2 | CsCl | Cl− | ||||||
|---|---|---|---|---|---|---|---|---|
| mol/kg | J/mol | J/molK | ||||||
| 2.45 | 4.41 | 9.32 | 0.67 | 46.55 | 72.81 | 0.97 | 2.56 | 3.53 |
| 2.13 | 3.84 | 8.11 | 0.72 | 51.47 | 60.92 | 0.85 | 2.07 | 2.92 |
| 1.83 | 3.29 | 6.95 | 0.77 | 50.13 | 43.63 | 0.73 | 1.63 | 2.36 |
| 1.54 | 2.77 | 5.85 | 0.81 | 46.30 | 28.57 | 0.62 | 1.25 | 1.87 |
| 1.26 | 2.27 | 4.79 | 0.85 | 43.23 | 17.88 | 0.51 | 0.93 | 1.44 |
| 1.02 | 1.84 | 3.88 | 0.88 | 32.83 | 8.89 | 0.42 | 0.68 | 1.10 |
| 0.76 | 1.37 | 2.88 | 0.91 | 24.14 | 3.62 | 0.31 | 0.44 | 0.75 |
| 0.53 | 0.96 | 2.03 | 0.94 | 11.59 | 0.86 | 0.22 | 0.26 | 0.48 |
| 0.30 | 0.54 | 1.13 | 0.97 | 3.50 | 0.08 | 0.12 | 0.09 | 0.21 |
| 0.07 | 0.13 | 0.27 | 1.00 | −0.10 | 0.00 | 0.03 | −0.06 | −0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranauskaite, V.; Belysheva, M.; Pestova, O.; Anufrikov, Y.; Skripkin, M.; Kondratiev, Y.; Khripun, V. Thermodynamic Description of Dilution and Dissolution Processes in the MgCl2−CsCl−H2O Ternary System. Materials 2021, 14, 4047. https://doi.org/10.3390/ma14144047
Baranauskaite V, Belysheva M, Pestova O, Anufrikov Y, Skripkin M, Kondratiev Y, Khripun V. Thermodynamic Description of Dilution and Dissolution Processes in the MgCl2−CsCl−H2O Ternary System. Materials. 2021; 14(14):4047. https://doi.org/10.3390/ma14144047
Chicago/Turabian StyleBaranauskaite, Valeriia, Maria Belysheva, Olga Pestova, Yuri Anufrikov, Mikhail Skripkin, Yuri Kondratiev, and Vassily Khripun. 2021. "Thermodynamic Description of Dilution and Dissolution Processes in the MgCl2−CsCl−H2O Ternary System" Materials 14, no. 14: 4047. https://doi.org/10.3390/ma14144047
APA StyleBaranauskaite, V., Belysheva, M., Pestova, O., Anufrikov, Y., Skripkin, M., Kondratiev, Y., & Khripun, V. (2021). Thermodynamic Description of Dilution and Dissolution Processes in the MgCl2−CsCl−H2O Ternary System. Materials, 14(14), 4047. https://doi.org/10.3390/ma14144047








