Microbiota Gut–Brain Axis in Ischemic Stroke: A Narrative Review with a Focus about the Relationship with Inflammatory Bowel Disease
Abstract
:1. Introduction: The Gut–Brain Microbiota Axis
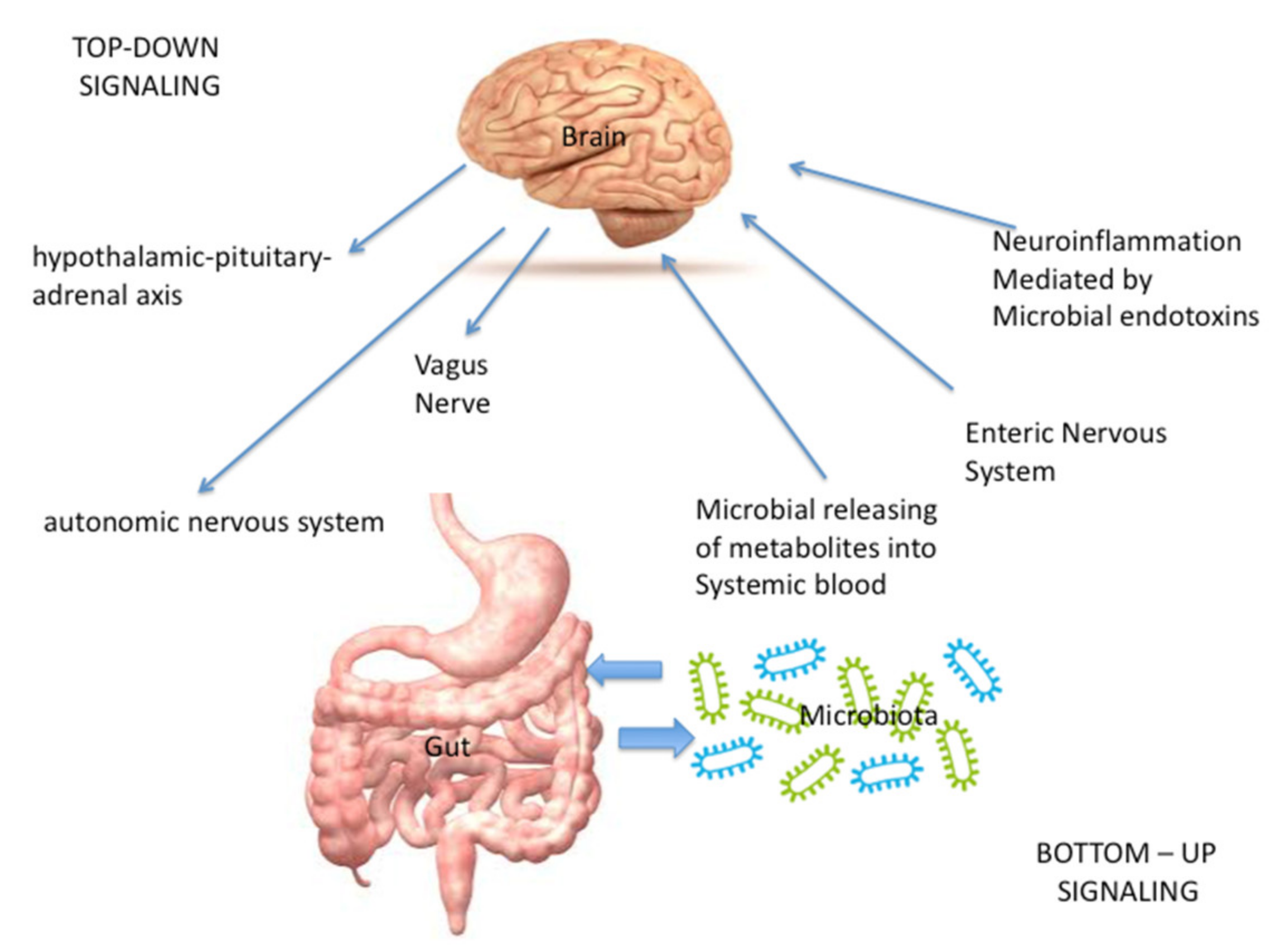
- Directly, through the vagus nerve (VN), whose stimulation is mediated by microbial metabolites and neuro-hormones [1] released from the enteric nervous system (ENS), that controls bowel function even though it is completely separate from the CNS; the ENS is made up of interneurons, sensory neurons, motor neurons, and neurotransmitters [2]. Furthermore, neuroinflammation could be caused also by the production of immunogenic microbial endotoxins (such as LPS), that act both through a direct damage or through the activation of immune cells [1,5,6].
- Indirectly, through the microbial releasing of metabolites such as short-chain fatty acids (SCFA), bile acids, indoles and neurotransmitters that, after entering the systemic blood, travel to the brain in order to modulate the function of neurons, microglia, astrocytes, and the blood brain barrier [1,7,8,9].
- In the “top-down” signaling, the gut microbiota receives input:
- -
- Indirectly, from the enteric nervous system (ENS). In this context, also the neuroendocrine signaling network mediated by the hypothalamic–pituitary–adrenal (HPA) axis, activated by the integrative reactions of specific centres in the CNS, plays a pivotal role; in fact, it represents a central integrative system essential for the successful physiological adaptation of our organism to stress [2].
- -
- Directly, through the autonomic nervous system (ANS), the pivotal modulator of the ENS [2].
2. The Role of Microbiota Gut–Brain Axis in Ischemic Stroke
2.1. The Role of Gut Dysbiosis inInfluencing Stroke Risk Factors
2.1.1. Aging
2.1.2. Metabolic Diseases
2.1.3. Arterial Hypertension and Vascular Dysfunction
2.2. The Effect of Gut Dysbiosis in Influencing Stroke Outcomes
3. The Role of the Gut–Brain Microbiota Axis in the Occurrence of Gastrointestinal Complications after Ischemic Stroke, and the Focus about the Link between Inflammatory Bowel Disease and Ischemic Stroke
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durgan, D.J.; Lee, J.; McCullough, L.D.; Bryan, R.M., Jr. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 2019, 50, 2270–2277. [Google Scholar] [CrossRef]
- Sinagra, E.; Utzeri, E.; Morreale, G.C.; Fabbri, C.; Pace, F.; Anderloni, A. Microbiota-gut-brain axis and its affect inflammatory bowel disease: Pathophysiological concepts and insights for clinicians. World J. Clin. Cases 2020, 8, 1013–1025. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [Green Version]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ling, Z.; Wang, F.; Chen, W.; Li, H.; Jin, J.; Zhang, H.; Pang, M.; Yu, J.; Liu, J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 2016, 613, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Ling, Z.; Yu, X.; Chen, W.; Li, H.; Jin, J.; Pang, M.; Zhang, H.; Yu, J. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016, 1642, 180–188. [Google Scholar] [CrossRef]
- Winek, K.; Dirnagl, U.; Meisel, A. The Gut Microbiome as Therapeutic Target in Central Nervous System Diseases: Implications for Stroke. Neurotherapeutics 2016, 13, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Quigley, E.M.M. The Gut-Brain Axis and the Microbiome: Clues to Pathophysiology and Opportunities for Novel Management Strategies in Irritable Bowel Syndrome (IBS). J. Clin. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cai, Y.; Meng, C.; Ding, X.; Huang, J.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. The role of the microbiome in diabetes mellitus. Diabetes Res. Clin. Pract. 2021, 172, 108645. [Google Scholar] [CrossRef]
- Song, B.C.; Bai, J. Microbiome-gut-brain axis in cancer treatment-related psychoneurological toxicities and symptoms: A systematic review. Supportive Care Cancer 2021, 29, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.Y. The Association between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Bai, F.; Yu, Y. Spinal cord injury and gut microbiota: A review. Life Sci. 2021, 266, 118865. [Google Scholar] [CrossRef]
- Moradi, K.; Ashraf-Ganjouei, A.; Tavolinejad, H.; Bagheri, S.; Akhondzadeh, S. The interplay between gut microbiota and autism spectrum disorders: A focus on immunological pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110091. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Mohd Ismail, N.I.; Wei, L.K. Microbiome and ischemic stroke: A systematic review. PLoS ONE 2021, 16, e0245038. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 2016, 94, 634A. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.K.; Hu, B. Brain-gut axis after stroke. Brain Circ. 2018, 4, 165–173. [Google Scholar] [CrossRef]
- Battaglini, D.; Pimentel-Coelho, P.M.; Robba, C.; Dos Santos, C.C.; Cruz, F.F.; Pelosi, P.; Rocco, P.R.M. Gut Microbiota in Acute Ischemic Stroke: From Pathophysiology to Therapeutic Implications. Front. Neurol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef]
- Jandzinski, M. Manipulation of the Microbiome and Its Impact on Functional Recovery Following Ischemic Stroke. Honors Scholar Theses. University of Connecticut. 2015. Available online: https://opencommons.uconn.edu/cgi/viewcontent.cgi?referer=&httpsredir=1&article=1444&context=srhonors_theses (accessed on 18 July 2021).
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.R.; Haeusler, R.A. Bile acids in glucose metabolism and insulin signalling-mechanisms and research needs. Nat. Rev. Endocrinol. 2019, 15, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Karbach, S.H.; Schönfelder, T.; Brandão, I.; Wilms, E.; Hörmann, N.; Jäckel, S.; Schüler, R.; Finger, S.; Knorr, M.; Lagrange, J.; et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J. Am. Heart Assoc. 2016, 5, e003698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluznick, J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol. Renal. Physiol. 2013, 305, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, C.; Barata, P.; Fernandes, R. The influence of gut microbiota in cardiovascular diseases-a brief review. Porto Biomed. J. 2021, 6, e106. [Google Scholar] [CrossRef]
- Ma, F.X.; Zhou, B.; Chen, Z.; Ren, Q.; Lu, S.H.; Sawamura, T.; Han, Z.C. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J. Lipid. Res. 2006, 47, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subah Packer, C. Estrogen protection, oxidized LDL, endothelial dysfunction and vasorelaxation in cardiovascular disease: New insights into a complex issue. Cardiovasc. Res. 2007, 73, 6–7. [Google Scholar] [CrossRef]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Tonomura, S.; Ihara, M.; Friedland, R.P. Microbiota in cerebrovascular disease: A key player and future therapeutic target. J. Cereb. Blood Flow Metab. 2020, 40, 1368–1380. [Google Scholar] [CrossRef]
- Crapser, J.; Ritzel, R.; Verma, R.; Venna, V.R.; Liu, F.; Chauhan, A.; Koellhoffer, E.; Patel, A.; Ricker, A.; Maas, K.; et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging. 2016, 8, 1049–1063. [Google Scholar] [CrossRef] [Green Version]
- Haak, B.W.; Westendorp, W.F.; van Engelen, T.S.R.; Brands, X.; Brouwer, M.C.; Vermeij, J.D.; Hugenholtz, F.; Verhoeven, A.; Derks, R.J.; Giera, M.; et al. Disruptions of Anaerobic Gut Bacteria Are Associated with Stroke and Post-stroke Infection: A Prospective Case-Control Study. Transl. Stroke Res. 2020. [Google Scholar] [CrossRef]
- Tan, C.; Wu, Q.; Wang, H.; Gao, X.; Xu, R.; Cui, Z.; Zhu, J.; Zeng, X.; Zhou, H.; He, Y.; et al. Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Acute Ischemic Stroke and the Subsequent Risk for Poor Functional Outcomes. JPEN J. Parenter. Enteral. Nutr. 2021, 45, 518–529. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Pan, D.; Liu, Y.; Yan, X.; Tang, Y.; Tao, M.; Gong, L.; Zhang, T.; Woods, C.R.; et al. Dysbiosis characteristics of gut microbiota in cerebral infarction patients. Transl. Neurosci. 2020, 11, 124–133. [Google Scholar] [CrossRef]
- Huang, L.; Wang, T.; Wu, Q.; Dong, X.; Shen, F.; Liu, D.; Qin, X.; Yan, L.; Wan, Q. Analysis of microbiota in elderly patients with Acute Cerebral Infarction. PeerJ 2019, 7, e6928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, G.H.; You, C.; Gao, X.X.; Zeng, X.L.; Zhu, J.J.; Xu, K.Y.; Tan, C.H.; Xu, R.T.; Wu, Q.H.; Zhou, H.W.; et al. Stroke Dysbiosis Index (SDI) in Gut Microbiome Are Associated with Brain Injury and Prognosis of Stroke. Front. Neurol. 2019, 10, 397. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Yao, X.; Cheng, X.; Zhu, Y. The characteristics analysis of intestinal microecology on cerebral infarction patients and its correlation with apolipoprotein E. Medicine 2018, 97, e12805. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sadler, R.; Heindl, S.; Llovera, G.; Roth, S.; Benakis, C.; Liesz, A. The gut microbiome primes a cerebroprotective immune response after stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1293–1298. [Google Scholar] [CrossRef]
- Stanley, D.; Moore, R.J.; Wong, C.H.Y. An insight into intestinal mucosal microbiota disruption after stroke. Sci. Rep. 2018, 8, 568. [Google Scholar] [CrossRef]
- Boaden, E.; Lyons, M.; Singhrao, S.K.; Dickinson, H.; Leathley, M.; Lightbody, C.E.; McLoughlin, A.; Khan, Z.; Crean, S.; Smith, C.; et al. Oral flora in acute stroke patients: A prospective exploratory observational study. Gerodontology 2017, 34, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar] [CrossRef]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota with Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, e002699. [Google Scholar] [CrossRef] [Green Version]
- Vermeij, J.D.; Westendorp, W.F.; van de Beek, D.; Nederkoorn, P.J. Post-stroke infections and preventive antibiotics in stroke: Update of clinical evidence. Int. J. Stroke 2018, 13, 913–920. [Google Scholar] [CrossRef]
- Santos Samary, C.; Pelosi, P.; Leme Silva, P.; Rieken Macedo Rocco, P. Immunomodulation after ischemic stroke: Potential mechanisms and implications for therapy. Crit. Care 2016, 20, 391. [Google Scholar] [CrossRef] [Green Version]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018, 56, 1–15. [Google Scholar] [CrossRef]
- Skagen, K.; Trøseid, M.; Ueland, T.; Holm, S.; Abbas, A.; Gregersen, I.; Kummen, M.; Bjerkeli, V.; Reier-Nilsen, F.; Russell, D.; et al. The Carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016, 247, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Hénaut, L.; Grissi, M.; Brazier, F.; Assem, M.; Poirot-Leclercq, S.; Lenglet, G.; Boudot, C.; Avondo, C.; Boullier, A.; Choukroun, G.; et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci. Rep. 2019, 9, 6432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, J.; Ng, K.P.; Sims, D.; Gill, P.; Cockwell, P.; Ferro, C. Incidence and impact on outcomes of acute kidney injury after a stroke: A systematic review and meta-analysis. BMC Nephrol. 2018, 19, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.B.; Tian, J.; Zhao, J.X.; Song, D.B.; Tian, J.K. Study on the clinical epidemiological features of acute cerebral stroke inducing systemic inflammatory response syndrome and multiple organ dysfunction syndrome. Zhonghua Liu Xing Bing Xue Za Zhi 2008, 29, 294–296. [Google Scholar] [PubMed]
- Toyoda, K.; Ninomiya, T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014, 13, 823–833. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhu, Y.; Chuai, J. Changes in serum ghrelin and small intestinal motility in rats with ischemic stroke. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 307–312. [Google Scholar] [CrossRef]
- Hang, C.H.; Shi, J.X.; Li, J.S.; Wu, W.; Yin, H.X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J. Gastroenterol. 2003, 9, 2776–2781. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.; Kou, L.; Chaogang, T.; Ruxun, H.; Zhong, P.; Zhendong, L. Ischemic stroke damages the intestinal mucosa and induces alteration of the intestinal lymphocytes and CCL19 mRNA in rats. Neurosci. Lett. 2017, 658, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Houlden, A.; Goldrick, M.; Brough, D.; Vizi, E.S.; Lénárt, N.; Martinecz, B.; Roberts, I.S.; Denes, A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 2016, 57, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Herbrüggen, O.; Quarcoo, D.; Meisel, A.; Meisel, C. Differential affection of intestinal immune cell populations after cerebral ischemia in mice. Neuroimmunomodulation 2009, 16, 213–218. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Marcoionni, A.M.; Dempsey, E.R.; Woenig, J.A.; Turner, R.J. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int. J. Mol. Sci. 2017, 18, 1788. [Google Scholar]
- Barbara, G.; Stanghellini, V.; Brandi, G.; Cremon, C.; di Nardo, G.; de Giorgio, R.; Corinaldesi, R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am. J. Gastroenterol. 2005, 100, 2560–2568. [Google Scholar] [CrossRef]
- Huh, J.R.; Veiga-Fernandes, H. Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol. 2019, 20, 217–228. [Google Scholar] [CrossRef]
- Kohoutova, D.; Moravkova, P.; Kruzliak, P.; Bures, J. Thromboembolic complications in inflammatory bowel disease. J. Thromb. Thrombolysis 2015, 39, 489–498. [Google Scholar] [CrossRef]
- Bollen, L.; Casteele, N.C.; Ballet, V. Thromboembolism as an important complication of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-S.; Tseng, C.-H.; Chen, P.-C.; Tsai, C.-H.; Lin, C.-L.; Sung, F.-C.; Kao, C.-H. Inflammatory bowel diseases increase future ischemic stroke risk: A Taiwanese population-based retrospective cohort study. Eur. J. Intern. Med. 2014, 25, 561–565. [Google Scholar] [CrossRef]
- Ana Maria, F.; Lucian, N.; Michelle, D.; Andreea, C.; Carmen, M.P.; Dragos, V. Cardiovascular involvement in inflammatory bowel disease: Dangerous liaisons. World J. Gastroenterol. 2015, 21, 9688–9692. [Google Scholar]
- Jacob, R.E.; Kevin, H.; Elaine, N.M.; Manvir, K.; Colin, R.; Kim, E.B.; Mélanie, G.G. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, 989–998. [Google Scholar]
- Lee, H.J.; Jeong, J.J.; Han, M.J.; Kim, D.H. Lactobacillus plantarum c29 alleviates TNBS-induced memory impairment in mice. J. Microbiol. Biotechnol. 2018, 28, 175–179. [Google Scholar] [CrossRef]
- Jang, S.-E.; Lim, S.-M.; Jeong, J.-J.; Jang, H.-M.; Lee, H.-J.; Han, M.J.; Kim, D.-H. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018, 11, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Role of the Gut–Brain Microbiota Axis in the Occurrence of Gastrointestinal Complications after Ischemic Stroke | ||||
|---|---|---|---|---|
| Author | Year of Publication | Type of Study | Population | Results |
| Hang C.H. et al. [64] | 2003 | Pre-clinical study | Mice subjected to experimental stroke | Severe mucosal atrophy and disruption of gut epithelial cell tight junctions after few hours from stroke to 7 days |
| Xu X. et al. [63] | 2012 | Pre-clinical study | Mice subjected to experimental stroke | Decreased gastrointestinal motility and damage to the intestinal mucosa existed in rats with experimental stroke |
| Liu Y. et al. [65] | 2017 | Pre-clinical study | Mice subjected to experimental stroke | Ischemic stroke significantly damaged the intestinal epithelium and activated intestinal immunity. |
| Houlden A. et al. [66] | 2016 | Pre-clinical study | Mice subjected to experimental stroke | Noradrenaline release with alteration of cecal mucoprotein production, goblet cell numbers, composition of cecal microbiota, with specific changes in Peptococcaceae and Prevotellaceae |
| Haak B.W. et al. [42] | 2020 | Prospective case–control study | Stroke patients and controls | Altered microbiota composition in adults with stroke with higher level of bacteria implicated in trimethylamine-N-oxide (TMAO) production and a loss of butyrate-producing bacteria. |
| Tan C. et al. [43] | 2021 | Prospective case–control study | Stroke patients and controls | Dysbiosis of SCFAs-producing bacteria and SCFAs in AIS patients increased the subsequent risk for poor functional outcomes |
| Li H. et al. [44] | 2020 | Prospective case–control study | Stroke patients and controls | The abundance and functions ofbutyrate-producing bacteria in stroke patients were significantly decreased while lactic acid bacteria were increased. |
| Schulte-Herbrüggen O. et al. [67] | 2009 | Pre-clinical study | Mice subjected to experimental stroke | Peyer’s patches revealed a significant reduction of T and B cell counts after cerebral ischemia. |
| Link between inflammatory bowel disease and ischemic stroke | ||||
| Bollen L. et al. [73] | 2016 | retrospective monocentric cohort study | IBD patients with a history of TE | 83% of patients developed a venous TE. At the time of TE, 71% patients were diagnosed with active disease |
| Huang W.S. et al. [45] | 2014 | population-based retrospective cohort study | IBD adult patients and IBD-free controls | The risk of ischemic stroke was 1.12-fold higher among the IBD cohort than among the non-IBD cohort. The risk of developing ischemic stroke significantly increased with the increased frequency of IBD exacerbation and hospitalization. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinagra, E.; Pellegatta, G.; Guarnotta, V.; Maida, M.; Rossi, F.; Conoscenti, G.; Pallio, S.; Alloro, R.; Raimondo, D.; Pace, F.; et al. Microbiota Gut–Brain Axis in Ischemic Stroke: A Narrative Review with a Focus about the Relationship with Inflammatory Bowel Disease. Life 2021, 11, 715. https://doi.org/10.3390/life11070715
Sinagra E, Pellegatta G, Guarnotta V, Maida M, Rossi F, Conoscenti G, Pallio S, Alloro R, Raimondo D, Pace F, et al. Microbiota Gut–Brain Axis in Ischemic Stroke: A Narrative Review with a Focus about the Relationship with Inflammatory Bowel Disease. Life. 2021; 11(7):715. https://doi.org/10.3390/life11070715
Chicago/Turabian StyleSinagra, Emanuele, Gaia Pellegatta, Valentina Guarnotta, Marcello Maida, Francesca Rossi, Giuseppe Conoscenti, Socrate Pallio, Rita Alloro, Dario Raimondo, Fabio Pace, and et al. 2021. "Microbiota Gut–Brain Axis in Ischemic Stroke: A Narrative Review with a Focus about the Relationship with Inflammatory Bowel Disease" Life 11, no. 7: 715. https://doi.org/10.3390/life11070715
APA StyleSinagra, E., Pellegatta, G., Guarnotta, V., Maida, M., Rossi, F., Conoscenti, G., Pallio, S., Alloro, R., Raimondo, D., Pace, F., & Anderloni, A. (2021). Microbiota Gut–Brain Axis in Ischemic Stroke: A Narrative Review with a Focus about the Relationship with Inflammatory Bowel Disease. Life, 11(7), 715. https://doi.org/10.3390/life11070715








