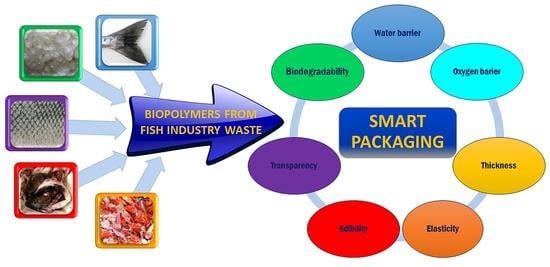
Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging
Abstract
1. Introduction
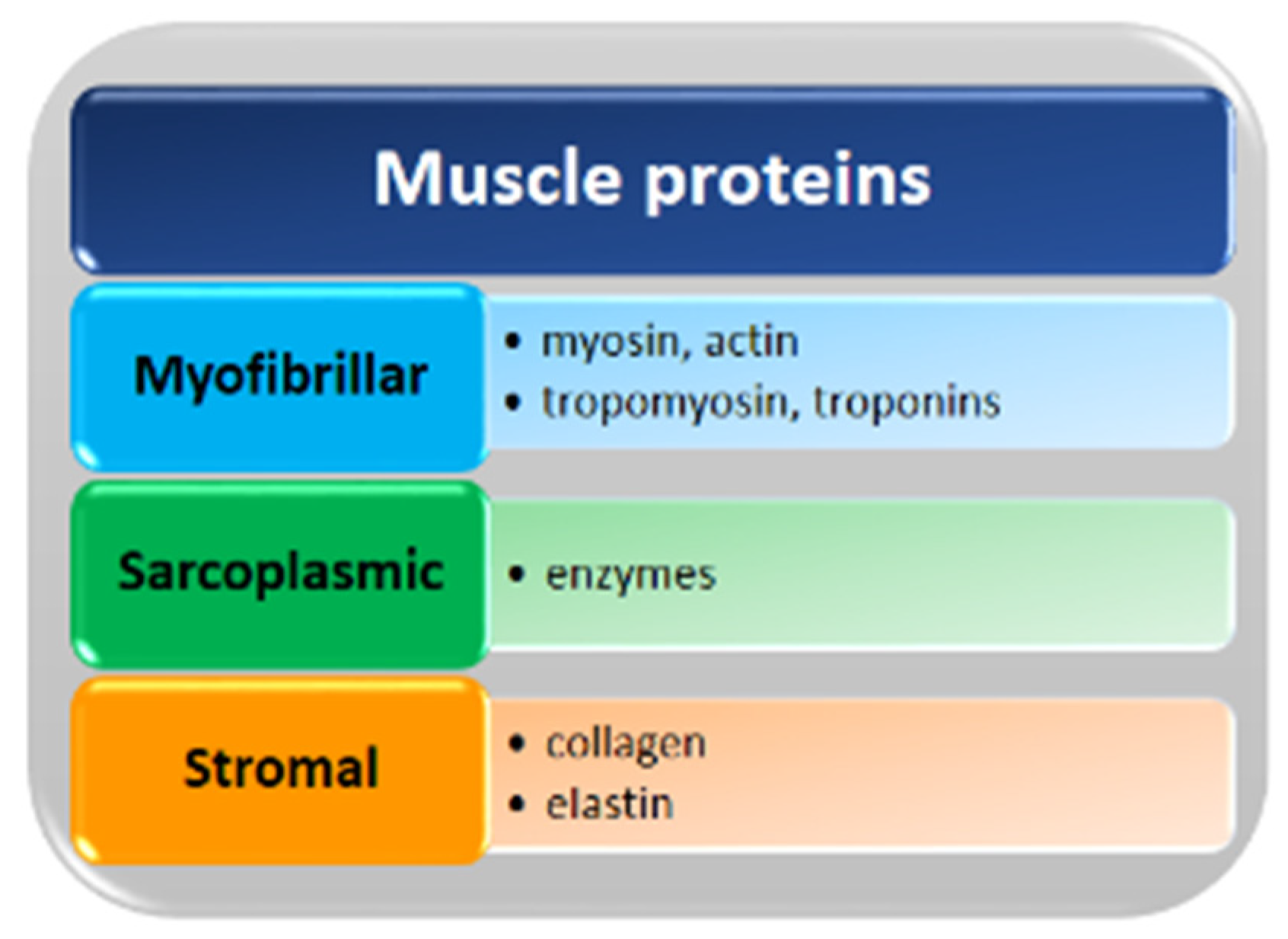
2. Muscle Proteins
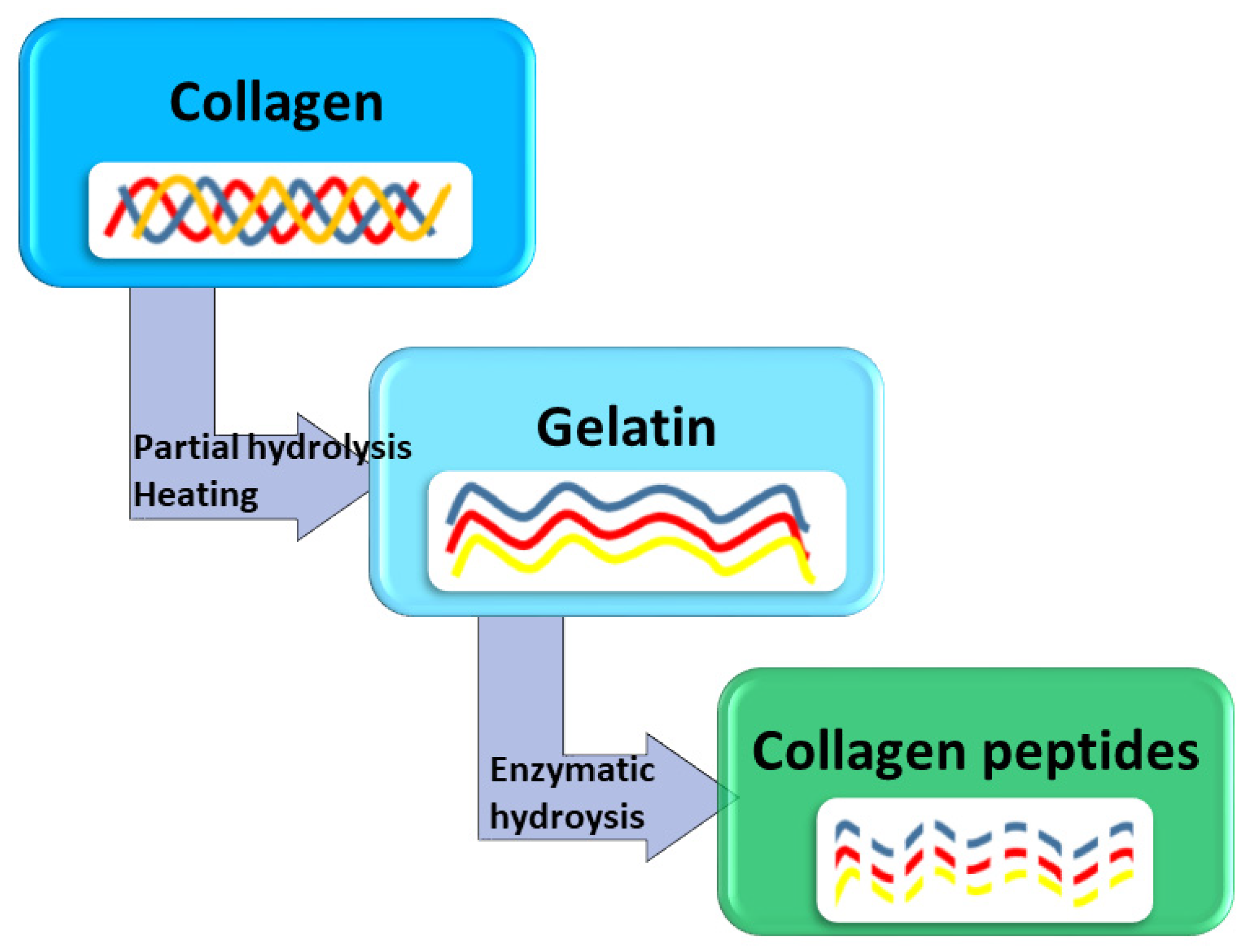
3. Marine Collagen
4. Fish Gelatin
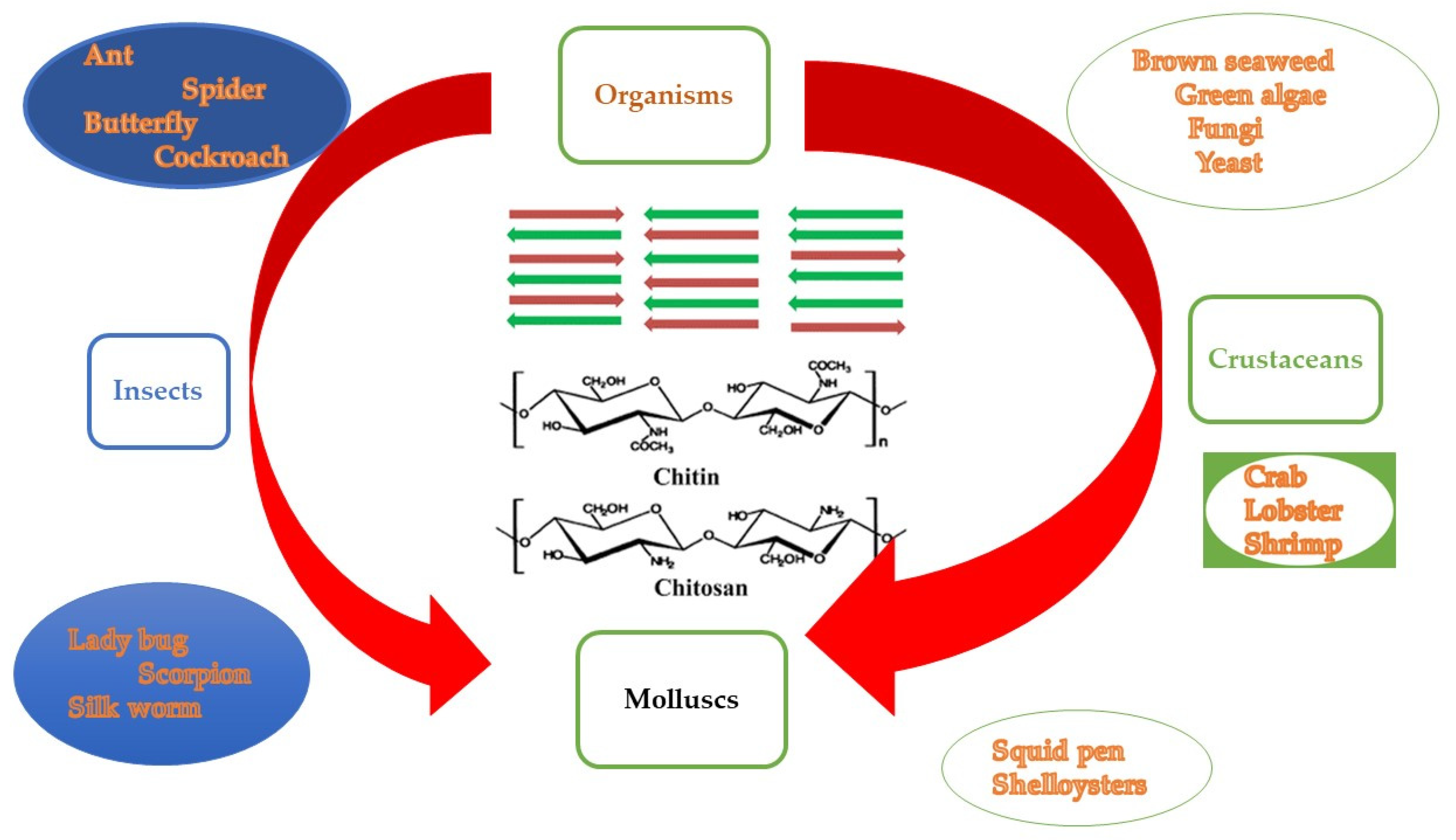
5. Chitin and Chitosan
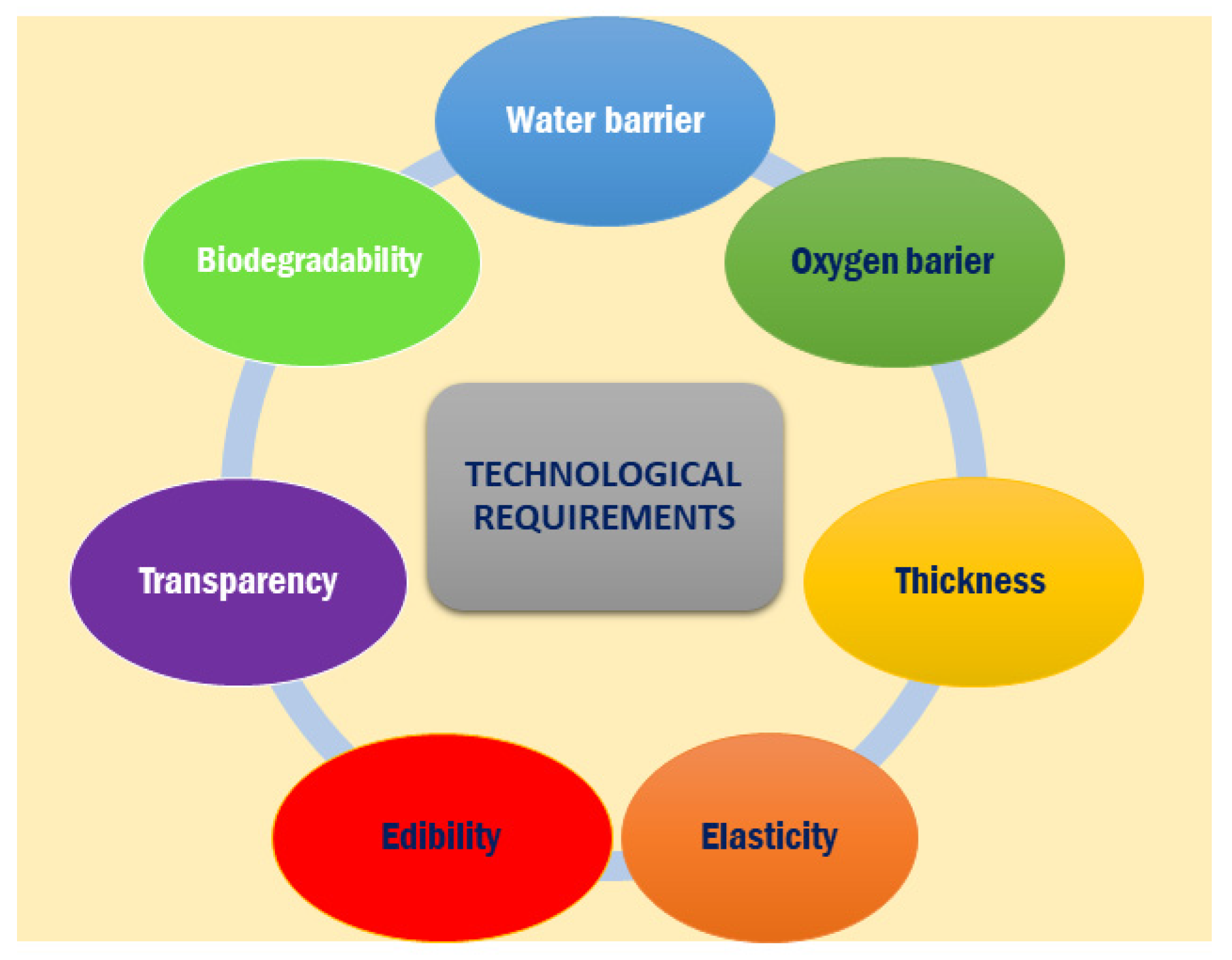
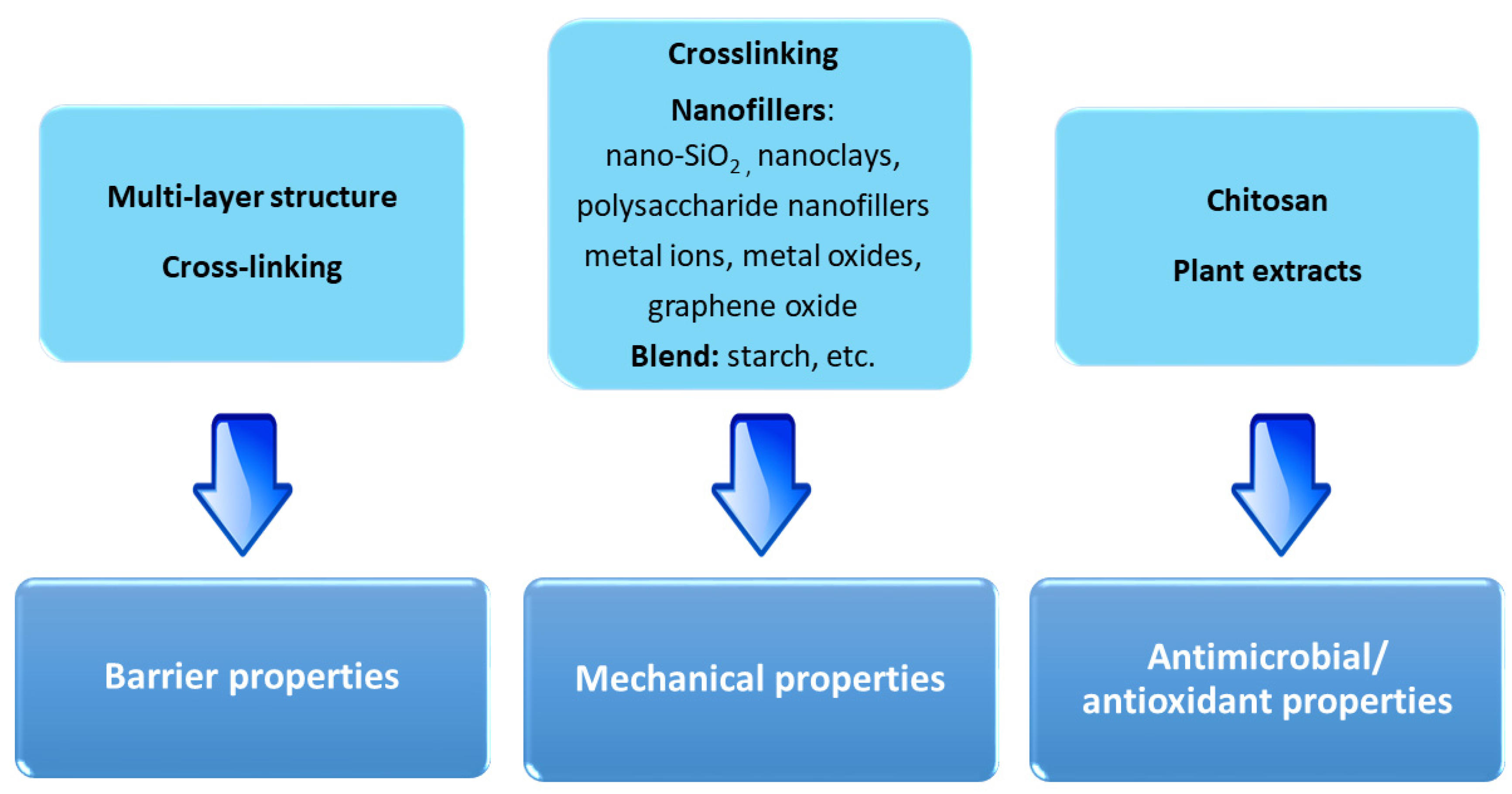
6. Technological Properties of Biopolymers Derived from Fish Waste for Food Packaging
7. Applications in Food Packaging
7.1. Applications of Biopolymers from Muscle Proteins in Food Packaging
| Fish Waste Source (Starting Material) | Protein Type and Content (%) | T (mm) | TS (MPa) | EAB (%) | WVP (× 10−11 g m−1 s−1 Pa−1) | S (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Whitemouth croaker | myofibrillar | 0.132 | 5.41 | 251 | 2.5 | 31 | [41] |
| Yellow stripe trevally | protein isolate/gelatin blend | 0.036 | 13.98 | 64 | 3.3 | 42 | [42] |
| Argentine anchovy | protein isolate | 0.113 | 0.6 | 28 | 11.6 | 45 | [45] |
| King weakfish | myofibrillar/gelatin blend | 0.106 | 6.5 | 384 | 2.7 | 27 | [144] |
| Gilded catfish | myofibrillar proteins | 0.033 | 4.9 | 178 | 6.4 | 19 | [12] |
| Sardine | proteins from bones, heads, guts, and fins | 0.21 | 0.34 | - | - | [145] | |
| Tilapia | myofibrillar protein/sorbitol | 0.014 | 12.5 | 66 | 3.0 | 63 | [48] |
| Catfish | 0.17 | 1.27 | 88 | 7.7 | 15 | [4] | |
| Silver carp | myofibrillar/glycerol/tannic acid | 0.06 | 3.9 | 94 | 15 | 2 | [44] |
| Whitemouth croaker | 10% | 0.114 | 4.2 | 28 | 8.6 | 100 | [139] |
| PVC film | - | 0.010 | 46.9 | 268 | 3.1 | - | [46] |
7.2. Applications of Marine Collagen in Food Packaging
| Fish Waste Source | T (mm) | TS (MPa) | EAB (%) | WVP (g m−1 s−1 Pa−1) | S (%) | Ref. |
|---|---|---|---|---|---|---|
| Starry triggerfish A. stellatus (skin) | [57] | |||||
| acid solubilized | 29 | 47 | 28 | 4.8 × 10−10 | ||
| pepsin solubilized | 29 | 34 | 40 | 6.6 × 10−10 | ||
| Unicorn leatherjacket Aluterus Monoceros (skin) | 21 | 25 | 15 | 3.0 × 10−10 | [147] | |
| Blend with chitosan CG/CH (8:2) | 31 | 20 | 24 | 4.5 × 10−10 | ||
| Blend with soy protein isolate CG/SPI (8:2) | 28 | 40 | 8 | 2.4 × 10−10 | ||
| Smooth-hound Mustelus mustelus (skin) Collagen-chitosan film 25:75 | 16 | 66 | 4 | 18 | [148] | |
| Fish skin collagen (Shanghai Yuanye Bio-Technology Co) Collagen/sodium alginate (10:2) | 32 | 26 | 65 | 1.7 × 10−10 | [129] |
7.3. Applications of Fish Gelatin in Food Packaging
7.4. Applications of Chitosan in Food Packaging
7.5. Applications of Biopolymers from Fish Scales in Food Packaging
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbandeh, M. Fish Production Worldwide 2002–2019. Available online: https://www.statista.com/statistics/264577/total-world-fish-production-since-2002/ (accessed on 4 November 2020).
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio (nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Kakatkar, A.S.; Karani, M.N. Development of protein-based biodegradable films from fish processing waste. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 878–888. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gautam, R.K.; Kumar, V.; Kakatkar, A.S.; Chatterjee, S. Synthesis of Biodegradable Films Using Gamma Irradiation from Fish Waste. Waste Biomass Valorization 2020, 12, 2247–2257. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Araújo, C.S.; Rodrigues, A.M.C.; Joele, M.R.S.P.; Araújo, E.A.F.; Lourenço, L.F.H. Optimizing process parameters to obtain a bioplastic using proteins from fish byproducts through the response surface methodology. Food Packag. Shelf Life 2018, 16, 23–30. [Google Scholar] [CrossRef]
- Discarding in Fisheries. Available online: https://ec.europa.eu/oceans-and-fisheries/fisheries/rules/discarding-fisheries_en (accessed on 30 June 2021).
- Putro, S.P.; Sharani, J.; Adhy, S. Biomonitoring of the Application of Monoculture and Integrated Multi-Trophic Aquaculture (IMTA) Using Macrobenthic Structures at Tembelas Island, Kepulauan Riau Province, Indonesia. J. Mar. Sci. Eng. 2020, 8, 942. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, H.-J.; Moon, C.-H. Eutrophication Driven by Aquaculture Fish Farms Controls Phytoplankton and Dinoflagellate Cyst Abundance in the Southern Coastal Waters of Korea. J. Mar. Sci. Eng. 2021, 9, 362. [Google Scholar] [CrossRef]
- Blanco, M.; Sotelo, C.G.; Pérez-Martín, R.I. New strategy to cope with common fishery policy landing obligation: Collagen extraction from skins and bones of undersized hake (Merluccius merluccius). Polymers 2019, 11, 1485. [Google Scholar] [CrossRef]
- Goossens, Y.; Schmidt, T.G.; Kuntscher, M. Evaluation of food waste prevention measures—The use of fish products in the food service sector. Sustainability 2020, 12, 6613. [Google Scholar] [CrossRef]
- Resolution, A. RES/70/1. Transforming our world: The 2030 agenda for sustainable development. In Proceedings of the Seventieth United Nations General Assembly, New York, NY, USA, 15 September 2015; Volume 25. [Google Scholar]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable Sources from Aquatic Organisms for Cosmeceuticals Ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Lucarini, M.; Zuorro, A.; Di Lena, G.; Lavecchia, R.; Durazzo, A.; Benedetti, B.; Lombardi-Boccia, G. Sustainable management of secondary raw materials from the marine food-chain: A case-study perspective. Sustainability 2020, 12, 8997. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Aldawsari, H.M.; Hosny, K.M.; Ahmad, J.; Akhter, S.; Kammoun, A.K.; Alghaith, A.F.; Asfour, H.Z.; Al-Rabia, M.W.; Md, S. Formulation design and pharmacokinetic evaluation of docosahexaenoic acid containing self-nanoemulsifying drug delivery system for oral administration. Nanomater. Nanotechnol. 2020, 10, 1847980420950988. [Google Scholar] [CrossRef]
- Hülsey, M.J. Shell biorefinery: A comprehensive introduction. Green Energy Environ. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Ferrari, F.; Striani, R.; Visconti, P.; Greco, A. An innovative green process for the stabilization and valorization of organic fraction of municipal solid waste (OFMSW): Optimization of the curing process II part. Appl. Sci. 2019, 9, 3702. [Google Scholar] [CrossRef]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- What Are Bioplastics? Available online: https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_bioplastics.pdf (accessed on 5 December 2020).
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef]
- Ferrari, F.; Striani, R.; Visconti, P.; Esposito Corcione, C.; Greco, A. Durability analysis of formaldehyde/solid urban waste blends. Polymers 2019, 11, 1838. [Google Scholar] [CrossRef]
- Ferrari, F.; Striani, R.; Minosi, S.; De Fazio, R.; Visconti, P.; Patrono, L.; Catarinucci, L.; Corcione, C.E.; Greco, A. An innovative IoT-oriented prototype platform for the management and valorisation of the organic fraction of municipal solid waste. J. Clean. Prod. 2020, 247, 119618. [Google Scholar] [CrossRef]
- Ferrari, F.; Esposito Corcione, C.; Montagna, F.; Maffezzoli, A. 3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter. Polymers 2020, 12, 1738. [Google Scholar] [CrossRef]
- Lionetto, F.; Maffezzoli, A.; Ottenhof, M.A.; Farhat, I.A.; Mitchell, J.R. Ultrasonic investigation of wheat starch retrogradation. J. Food Eng. 2006, 75, 258–266. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. An Overview of the Sorption Studies of Contaminants on Poly (Ethylene Terephthalate) Microplastics in the Marine Environment. J. Mar. Sci. Eng. 2021, 9, 445. [Google Scholar] [CrossRef]
- Lionetto, F.; López-Muñoz, R.; Espinoza-González, C.; Mis-Fernández, R.; Rodríguez-Fernández, O.; Maffezzoli, A. A Study on Exfoliation of Expanded Graphite Stacks in Candelilla Wax. Materials 2019, 12, 2530. [Google Scholar] [CrossRef]
- Lionetto, F.; Sannino, A.; Mensitieri, G.; Maffezzoli, A. Evaluation of the Degree of Cross-Linking of Cellulose-Based Superabsorbent Hydrogels: A Comparison between Different Techniques. Macromol. Symp. 2003, 200, 199–207. [Google Scholar] [CrossRef]
- Bioplastics Market Development Update 2020. Available online: https://docs.european-bioplastics.org/conference/Report_Bioplastics_Market_Data_2020_short_version.pdfv (accessed on 5 December 2020).
- Della Malva, A.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M.; Marino, R. Methods for extraction of muscle proteins from meat and fish using denaturing and non-denaturing solutions. J. Food Qual. 2018, 2018, 8478471. [Google Scholar]
- Medina, I.; Pazos, M. Oxidation and protection of fish. In Oxidation in Foods and Beverages and Antioxidant Applications. Volume 2: Management in Different Industry Sectors; Woodhead Publishing Ltd.: Cambridge, UK, 2010; pp. 91–120. [Google Scholar]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The potential of proteins for producing food packaging materials: A review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pereira, R.N.; Pastrana, L.M.; Vicente, A.A.; Cerqueira, M.A. Protein-based nanostructures for food applications. Gels 2019, 5, 9. [Google Scholar] [CrossRef]
- Zavareze, E.d.R.; El Halal, S.L.M.; Marques e Silva, R.; Dias, A.R.G.; Prentice-Hernández, C. Mechanical, Barrier and Morphological Properties of Biodegradable Films Based on Muscle and Waste Proteins from the W hitemouth Croaker (Micropogonias furnieri). J. Food Process. Preserv. 2014, 38, 1973–1981. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Osako, K. Development and characterisation of blend films based on fish protein isolate and fish skin gelatin. Food Hydrocoll. 2014, 39, 58–67. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Properties of biodegradable blend films based on fish myofibrillar protein and polyvinyl alcohol as influenced by blend composition and pH level. J. Food Eng. 2010, 100, 85–92. [Google Scholar] [CrossRef]
- Nie, X.; Zhao, L.; Wang, N.; Meng, X. Phenolics-protein interaction involved in silver carp myofibrilliar protein films with hydrolysable and condensed tannins. LWT Food Sci. Technol. 2017, 81, 258–264. [Google Scholar] [CrossRef]
- da Rocha, M.; Loiko, M.R.; Gautério, G.V.; Tondo, E.C.; Prentice, C. Influence of heating, protein and glycerol concentrations of film-forming solution on the film properties of Argentine anchovy (Engraulis anchoita) protein isolate. J. Food Eng. 2013, 116, 666–673. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Rawdkuen, S. Effect of protein concentrations on the properties of fish myofibrillar protein based film compared with PVC film. J. Food Sci. Technol. 2016, 53, 2083–2091. [Google Scholar] [CrossRef]
- Rostamzad, H.; Paighambari, S.Y.; Shabanpour, B.; Ojagh, S.M.; Mousavi, S.M. Improvement of fish protein film with nanoclay and transglutaminase for food packaging. Food Packag. Shelf Life 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Rawdkuen, S. Effects of plasticizers on the properties of fish myofibrillar protein film. J. Food Sci. Technol. 2018, 55, 3046–3055. [Google Scholar] [CrossRef]
- Nuanmano, S.; Prodpran, T.; Benjakul, S. Potential use of gelatin hydrolysate as plasticizer in fish myofibrillar protein film. Food Hydrocoll. 2015, 47, 61–68. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Prodpran, T.; Benjakul, S.; Phatcharat, S. Effect of phenolic compounds on protein cross-linking and properties of film from fish myofibrillar protein. Int. J. Biol. Macromol. 2012, 51, 774–782. [Google Scholar] [CrossRef]
- Shi, Y.; Li, R.; Tu, Z.; Ma, D.; Wang, H.; Huang, X.; He, N. Effect of γ-irradiation on the physicochemical properties and structure of fish myofibrillar proteins. Radiat. Phys. Chem. 2015, 109, 70–72. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Weng, W.; Wu, F. Water resistance and mechanical property improvement of tilapia (Tilapia zillii) scale gelatin films by dehydrated thermal treatment. J. Food Sci. Technol. 2015, 52, 3358–3366. [Google Scholar] [CrossRef][Green Version]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of collagen derived from tropical freshwater carp fish scale wastes and its amino acid sequence. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Collagen extraction optimization from the skin of the small-spotted catshark (S. canicula) by response surface methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef]
- Ahmad, M.; Nirmal, N.P.; Chuprom, J. Molecular characteristics of collagen extracted from the starry triggerfish skin and its potential in the development of biodegradable packaging film. RSC Adv. 2016, 6, 33868–33879. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Yao, Q.F.; Zhou, D.S.; Yang, J.H.; Huang, W.T. Directly reusing waste fish scales for facile, large-scale and green extraction of fluorescent carbon nanoparticles and their application in sensing of ferric ions. Sustain. Chem. Pharm. 2020, 17, 100305. [Google Scholar] [CrossRef]
- Wu, C.-S. Comparative assessment of the interface between poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and fish scales in composites: Preparation, characterization, and applications. Mater. Sci. Eng. C 2019, 104, 109878. [Google Scholar] [CrossRef]
- Muhammad, N.; Gao, Y.; Iqbal, F.; Ahmad, P.; Ge, R.; Nishan, U.; Rahim, A.; Gonfa, G.; Ullah, Z. Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 2016, 161, 129–135. [Google Scholar] [CrossRef]
- Cavalcante, L.d.A.; Ribeiro, L.S.; Takeno, M.L.; Aum, P.T.P.; Aum, Y.K.P.G.; Andrade, J.C.S. Chlorapatite Derived from Fish Scales. Materials 2020, 13, 1129. [Google Scholar] [CrossRef]
- Nur, R.M. The Utilitation of Fish Scale Waste as A Chitosan. Agrikan J. Agribisnis Perikan 2020, 13, 269–273. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Zhang, W.; Wang, W.; Chang, J.; Kai, J. Fluorescent carbon dots as nanoprobe for determination of lidocaine hydrochloride. Sens. Actuators B Chem. 2018, 262, 928–937. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Yang, X.; Chang, J.; Liu, Z.; Jiang, K. Fish-scale-derived carbon dots as efficient fluorescent nanoprobes for detection of ferric ions. RSC Adv. 2019, 9, 940–949. [Google Scholar] [CrossRef]
- Thammahiwes, S.; Riyajan, S.-A.; Kaewtatip, K. Preparation and properties of wheat gluten based bioplastics with fish scale. J. Cereal Sci. 2017, 75, 186–191. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Sanz, B.; Albillos Sanchez, A.; Tangey, B.; Gilmore, K.; Yue, Z.; Liu, X.; Wallace, G. Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation. Biomedicines 2021, 9, 16. [Google Scholar] [CrossRef]
- Cumming, M.H.; Leonard, A.R.; LeCorre-Bordes, D.S.; Hofman, K. Intra-fibrillar citric acid crosslinking of marine collagen electrospun nanofibres. Int. J. Biol. Macromol. 2018, 114, 874–881. [Google Scholar] [CrossRef]
- Luca, S.; Nunzia, G.; Lucia, N.M.; Lorena, C.; Paola, L.; Marta, M.; Stella, B.F.; Angelo, C.; Loredana, C.; Alessandro, S. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 110963. [Google Scholar]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Kozlowska, J.; Prus-Walendziak, W.; Stachowiak, N.; Bajek, A.; Kazmierski, L.; Tylkowski, B. Modification of Collagen/Gelatin/Hydroxyethyl Cellulose-Based Materials by Addition of Herbal Extract-Loaded Microspheres Made from Gellan Gum and Xanthan Gum. Materials 2020, 13, 3507. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A review of bioactive glass/natural polymer composites: State of the art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Process. 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Mohammed, M.A.P. Functional Properties of Tilapia’s Fish Scale Gelatin Film: Effects of Different Type of Plasticizers. Sains Malaysiana 2020, 49, 2221–2229. [Google Scholar]
- Prodpran, T.; Chuaynukul, K.; Nagarajan, M.; Benjakul, S.; Prasarpran, S. Impacts of plasticizer and pre-heating conditions on properties of bovine and fish gelatin films fabricated by thermo-compression molding technique. Ital. J. Food Sci. 2017, 29, 487–504. [Google Scholar] [CrossRef]
- De la Caba, K.; Guerrero, P.; Trung, T.S.; Cruz-Romero, M.; Kerry, J.P.; Fluhr, J.; Maurer, M.; Kruijssen, F.; Albalat, A.; Bunting, S. From seafood waste to active seafood packaging: An emerging opportunity of the circular economy. J. Clean. Prod. 2019, 208, 86–98. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- da Trindade Alfaro, A.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish gelatin: Characteristics, functional properties, applications and future potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Muhammad, K.M.L.; Ariffin, F.; Abd Razak, H.K.B.; Sulaiman, P.D.S. Review of fish gelatin extraction, properties and packaging applications. Food Sci. Nutr. 2016, 56, 47–59. [Google Scholar]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Olden, J.D.; Vitule, J.R.S.; Cucherousset, J.; Kennard, M.J. There’s more to Fish than Just Food: Exploring the Diverse Ways that Fish Contribute to Human Society. Fisheries 2020, 45, 453–464. [Google Scholar] [CrossRef]
- Ottani, V.; Martini, D.; Franchi, M.; Ruggeri, A.; Raspanti, M. Hierarchical structures in fibrillar collagens. Micron 2002, 33, 587–596. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Franco, L.O.; de Campos-Takaki, G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Maia, P.; Alencar, N.d.S.; Farias, L.; Andrade, R.F.S.; Souza, D.; Ribaux, D.R.; Franco, L.d.O.; Campos-Takaki, G.M. Recovery of chitin and chitosan from shrimp waste with microwave technique and versatile application. Arq. Inst. Biol. 2019, 86, 1–7. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Nagel-Hassemer, M.E.; Lapolli, F.R.; Lobo-Recio, M.Á. Potencial dos resíduos do processamento de camarão para remediação de águas contaminadas com drenagem ácida mineral. Polímeros 2016, 26. [Google Scholar] [CrossRef][Green Version]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Insect cuticle-mimetic hydrogels with high mechanical properties achieved via the combination of chitin nanofiber and gelatin. J. Agric. Food Chem. 2019, 67, 5571–5578. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.L.; Vasconcelos, F.P.; Albuquerque, M.F.C. A diversidade no uso e ocupação da zona costeira do brasil: A sustentabilidade como necessidade. Conex. Technol. 2017, 11, 8–16. [Google Scholar] [CrossRef]
- de Souza, F.M.; Ferreira, R.d.M.S.; Cardoso, R. Utilização da Casca de Camarão para Produção de Quitina. Rev. Acad. Cient. 2015, 7, 1–11. [Google Scholar]
- Ru, G.; Wu, S.; Yan, X.; Liu, B.; Gong, P.; Wang, L.; Feng, J. Inverse solubility of chitin/chitosan in aqueous alkali solvents at low temperature. Carbohydr. Polym. 2019, 206, 487–492. [Google Scholar] [CrossRef]
- Henry García, Y.; Troncoso-Rojas, R.; Tiznado-Hernández, M.E.; Báez-Flores, M.E.; Carvajal-Millan, E.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Martínez-Robinson, K.G. Enzymatic treatments as alternative to produce chitin fragments of low molecular weight from Alternaria alternata. J. Appl. Polym. Sci. 2019, 136, 47339. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Sugiyanti, D.; Darmadji, P.; Anggrahini, S.; Anwar, C.; Santoso, U. Preparation and characterization of chitosan from Indonesian Tambak Lorok shrimp shell waste and crab shell waste. Pak. J. Nutr. 2018, 17, 446–453. [Google Scholar] [CrossRef]
- Samrot, A.V.; Burman, U.; Philip, S.A.; Shobana, N.; Chandrasekaran, K. Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inform. Med. Unlocked 2018, 10, 159–182. [Google Scholar] [CrossRef]
- Ali, M.; Shakeel, M.; Mehmood, K. Extraction and characterization of high purity chitosan by rapid and simple techniques from mud crabs taken from Abbottabad. Pak. J. Pharm. Sci 2019, 32, 171–175. [Google Scholar] [PubMed]
- Tanabtabzadeh, M.S.; Javanbakht, V.; Golshirazi, A.H. Extraction of betacyanin and betaxanthin pigments from red beetroots by chitosan extracted from shrimp wastes. Waste Biomass Valorization 2019, 10, 641–653. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Yang, C.-L.; Palmer, J.; Brigham, C.J. Chitin extraction from lobster shell waste using microbial culture-based methods. Appl. Food Biotechnol. 2018, 5, 141–154. [Google Scholar]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018, 172, 4140–4151. [Google Scholar] [CrossRef]
- Marzieh, M.-N.; Zahra, F.; Tahereh, E.; Sara, K.-N. Comparison of the physicochemical and structural characteristics of enzymatic produced chitin and commercial chitin. Int. J. Biol. Macromol. 2019, 139, 270–276. [Google Scholar] [CrossRef]
- Castro, R.; Guerrero-Legarreta, I.; Bórquez, R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018, 20, e00287. [Google Scholar] [CrossRef]
- Zaku, S.G.; Aguzue, S.A.E.O.C.; Thomas, S.A. Extraction and characterization of chitin; a functional biopolymer obtained from scales of common carp fish (Cyprinus carpio L.): A lesser known source. Afr. J. Food Sci. 2011, 5, 478–483. [Google Scholar]
- Kumari, S.; Rath, P.K. Extraction and characterization of chitin and chitosan from (Labeo rohit) fish scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Arrouze, F.; Desbrieres, J.; Rhazi, M.; Essahli, M.; Tolaimate, A. Valorization of chitins extracted from North Morocco shrimps: Comparison of chitin reactivity and characteristics. J. Appl. Polym. Sci. 2019, 136, 47804. [Google Scholar] [CrossRef]
- Younes, I.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Cytotoxicity of chitosans with different acetylation degrees and molecular weights on bladder carcinoma cells. Int. J. Biol. Macromol. 2016, 84, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, A.; García-Payo, C.; Khayet, M. Structural, mechanical, and transport properties of electron beam-irradiated chitosan membranes at different doses. Polymers 2018, 10, 117. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Goyal, N.; Rastogi, D.; Jassal, M.; Agrawal, A.K. Chitosan as a potential stabilizing agent for titania nanoparticle dispersions for preparation of multifunctional cotton fabric. Carbohydr. Polym. 2016, 154, 167–175. [Google Scholar] [CrossRef]
- Demitri, C.; Moscatello, A.; Giuri, A.; Raucci, M.G.; Esposito Corcione, C. Preparation and characterization of eg-chitosan nanocomposites via direct exfoliation: A green methodology. Polymers 2015, 7, 2584–2594. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Giuri, A.; Masi, S.; Colella, S.; Listorti, A.; Rizzo, A.; Liscio, A.; Treossi, E.; Palermo, V.; Gigli, G.; Mele, C.; et al. GO/PEDOT: PSS nanocomposites: Effect of different dispersing agents on rheological, thermal, wettability and electrochemical properties. Nanotechnology 2017, 28, 174001. [Google Scholar] [CrossRef]
- Catalano, M.; Taurino, A.; Zhu, J.; Crozier, P.A.; Dal Zilio, S.; Amati, M.; Gregoratti, L.; Bozzini, B.; Mele, C. Dy-and Tb-doped CeO 2-Ni cermets for solid oxide fuel cell anodes: Electrochemical fabrication, structural characterization, and electrocatalytic performance. J. Solid State Electrochem. 2018, 22, 3761–3773. [Google Scholar] [CrossRef]
- Castel-Molieres, M.; Conzatti, G.; Torrisani, J.; Rouilly, A.; Cavalie, S.; Carrere, N.; Tourrette, A. Influence of homogenization technique and blend ratio on chitosan/alginate polyelectrolyte complex properties. J. Med. Biol. Eng. 2018, 38, 10–21. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Montembault, A.; Sudre, G.; Alcouffe, P.; Marcon, L.; Gehan, H.; Lux, F.; Albespy, K.; Centis, V.; Campos, D. Self-crosslinked fibrous collagen/chitosan blends: Processing, properties evaluation and monitoring of degradation by bi-fluorescence imaging. Int. J. Biol. Macromol. 2019, 131, 353–367. [Google Scholar] [CrossRef]
- Kasai, D.; Chougale, R.; Masti, S.; Chalannavar, R.; Malabadi, R.B.; Gani, R.; Gouripur, G. An investigation into the influence of filler Piper nigrum leaves extract on physicochemical and antimicrobial properties of chitosan/poly (vinyl alcohol) blend films. J. Polym. Environ. 2019, 27, 472–488. [Google Scholar] [CrossRef]
- Gopi, S.; Pius, A.; Kargl, R.; Kleinschek, K.S.; Thomas, S. Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym. Bull. 2019, 76, 1557–1571. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Heras Caballero, A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; De Benedictis, V.M.; Madaghiele, M.; Corcione, C.E.; Maffezzoli, A. Nanostructured active chitosan-based films for food packaging applications: Effect of graphene stacks on mechanical properties. Measurement 2016, 90, 418–423. [Google Scholar] [CrossRef]
- Leceta, I.; Peñalba, M.; Arana, P.; Guerrero, P.; De La Caba, K. Ageing of chitosan films: Effect of storage time on structure and optical, barrier and mechanical properties. Eur. Polym. J. 2015, 66, 170–179. [Google Scholar] [CrossRef]
- Jasour, M.S.; Ehsani, A.; Mehryar, L.; Naghibi, S.S. Chitosan coating incorporated with the lactoperoxidase system: An active edible coating for fish preservation. J. Sci. Food Agric. 2015, 95, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.; Hanjabam, M.D.; Gudipati, V.; Kannuchamy, N. Protective Effect of Fish Gelatin-Based Natural Antimicrobial Coatings on Quality of I ndian S almon Fillets during Refrigerated Storage. J. Food Process Eng. 2017, 40, e12270. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Wang, H. Scale-up preparation and characterization of collagen/sodium alginate blend films. J. Food Qual. 2017, 2017, 4954259. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Nazarewicz, S.; Góral, D.; Krawczuk, A.; Domin, M. Lyophilized Protein Structures as an Alternative Biodegradable Material for Food Packaging. Sustainability 2019, 11, 7002. [Google Scholar] [CrossRef]
- Gokoglu, N. Innovations in Seafood Packaging Technologies: A Review. Food Rev. Int. 2020, 36, 340–366. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Ruiz-Salmón, I.; Margallo, M.; Laso, J.; Villanueva-Rey, P.; Mariño, D.; Quinteiro, P.; Dias, A.C.; Nunes, M.L.; Marques, A.; Feijoo, G. Addressing challenges and opportunities of the European seafood sector under a circular economy framework. Curr. Opin. Environ. Sci. Health 2020, 13, 101–106. [Google Scholar] [CrossRef]
- Qamar, S.A.; Asgher, M.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 109625. [Google Scholar]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Thermoplastic properties of fish myofibrillar proteins: Application to biopackaging fabrication. Polymer 1997, 38, 4071–4078. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Rungraeng, N.; Osako, K.; Rawdkuen, S. Properties of fish myofibrillar protein film incorporated with catechin-Kradon extract. Food Packag. Shelf Life 2017, 13, 56–65. [Google Scholar] [CrossRef]
- Romani, V.P.; Machado, A.V.; Olsen, B.D.; Martins, V.G. Effects of pH modification in proteins from fish (Whitemouth croaker) and their application in food packaging films. Food Hydrocoll. 2018, 74, 307–314. [Google Scholar] [CrossRef]
- Garcıa, F.T.; Sobral, P.J.D.A. Effect of the thermal treatment of the filmogenic solution on the mechanical properties, color and opacity of films based on muscle proteins of two varieties of Tilapia. LWT Food Sci. Technol. 2005, 38, 289–296. [Google Scholar] [CrossRef]
- Leerahawong, A.; Tanaka, M.; Okazaki, E.; Osako, K. Stability of the physical properties of plasticized edible films from squid (Todarodes pacificus) mantle muscle during storage. J. Food Sci. 2012, 77, E159–E165. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.S.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of blend film based on cuttlefish (Sepia pharaonis) skin gelatin and mungbean protein isolate. Int. J. Biol. Macromol. 2011, 49, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of film based on protein isolate from red tilapia muscle with negligible yellow discoloration. Int. J. Biol. Macromol. 2011, 48, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Neves, E.M.P.X.; Pereira, R.R.; da Silva Pereira, G.V.; da Silva Pereira, G.V.; Vieira, L.L.; de Fátima Henriques Lourenço, L. Effect of polymer mixture on bioplastic development from fish waste. Boletim Instituto Pesca 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Ishak, N.S.; Thng, C.S.; Marsilla, K.I.K. The effect of crosslinking agent on protein-based bioplastic from fish waste. AIP Conf. Proc. 2020, 2267, 20030. [Google Scholar] [CrossRef]
- Sommer, A.; Dederko-Kantowicz, P.; Staroszczyk, H.; Sommer, S.; Michalec, M. Enzymatic and Chemical Cross-Linking of Bacterial Cellulose/Fish Collagen Composites—A Comparative Study. Int. J. Mol. Sci. 2021, 22, 3346. [Google Scholar] [CrossRef]
- Ahmad, M.; Nirmal, N.P.; Danish, M.; Chuprom, J.; Jafarzedeh, S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016, 6, 82191–82204. [Google Scholar] [CrossRef]
- Ben Slimane, E.; Sadok, S. Collagen from cartilaginous fish by-products for a potential application in bioactive film composite. Mar. Drugs 2018, 16, 211. [Google Scholar] [CrossRef]
- Valdés, A.; Garcia-Serna, E.; Martínez-Abad, A.; Vilaplana, F.; Jimenez, A.; Garrigós, M.C. Gelatin-Based Antimicrobial Films Incorporating Pomegranate (Punica granatum L.) Seed Juice by-Product. Molecules 2020, 25, 166. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, S.; Zhou, Z.; Lei, T.; Dong, B.; Xu, P. The Effect of Shear Deformation on Permeability of 2.5 D Woven Preform. Materials 2019, 12, 3594. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Preparation and characterization of chitosan nanoparticles-loaded fish gelatin-based edible films. J. Food Process Eng. 2016, 39, 521–530. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Three-layer sheets based on gelatin and poly (lactic acid), part 1: Preparation and properties. J. Appl. Polym. Sci. 2010, 118, 3102–3110. [Google Scholar] [CrossRef]
- Bae, H.J.; Darby, D.O.; Kimmel, R.M.; Park, H.J.; Whiteside, W.S. Effects of transglutaminase-induced cross-linking on properties of fish gelatin–nanoclay composite film. Food Chem. 2009, 114, 180–189. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; de la Caba, K.; Focarete, M.L. Electrospinning of fish gelatin solution containing citric acid: An environmentally friendly approach to prepare crosslinked gelatin fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Tabibiazar, M.; Mohammadi, M.; Pezeshki, A. Advanced properties of gelatin film by incorporating modified kappa-carrageenan and zein nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 183, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Farahnaky, A.; Dadfar, S.M.M.; Shahbazi, M. Physical and mechanical properties of gelatin-clay nanocomposite. J. Food Eng. 2014, 122, 78–83. [Google Scholar] [CrossRef]
- Qiang, X.; Zhou, S.; Zhang, Z.; Quan, Q.; Huang, D. Synergistic Effect of Halloysite Nanotubes and Glycerol on the Physical Properties of Fish Gelatin Films. Polymers 2018, 10, 1258. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Bilatto, S.; Paschoalin, R.T.; Soares, A.C.; Moreira, F.K.V.; Oliveira, O.N., Jr.; Mattoso, L.H.C.; Bras, J. Eco-friendly gelatin films with rosin-grafted cellulose nanocrystals for antimicrobial packaging. Int. J. Biol. Macromol. 2020, 165, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Kreith, F.; Manglik, R.; Bohn, M. Principles of Heat Transfer; Cengage Learning: Boston, MA, USA, 2010; ISBN 1133714854. [Google Scholar]
- Shabanpour, B.; Kazemi, M.; Ojagh, S.M.; Pourashouri, P. Bacterial cellulose nanofibers as reinforce in edible fish myofibrillar protein nanocomposite films. Int. J. Biol. Macromol. 2018, 117, 742–751. [Google Scholar] [CrossRef]
- Pan, L.; Li, P.; Tao, Y. Preparation and Properties of Microcrystalline Cellulose/Fish Gelatin Composite Film. Materials 2020, 13, 4370. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Beroual, M.; Trache, D.; Mehelli, O.; Boumaza, L.; Tarchoun, A.F.; Derradji, M.; Khimeche, K. Effect of the delignification process on the physicochemical properties and thermal stability of microcrystalline cellulose extracted from date palm fronds. Waste Biomass Valorization 2021, 12, 2779–2793. [Google Scholar] [CrossRef]
- Rouhi, J.; Mahmud, S.; Naderi, N.; Ooi, C.H.R.; Mahmood, M.R. Physical properties of fish gelatin-based bio-nanocomposite films incorporated with ZnO nanorods. Nanoscale Res. Lett. 2013, 8, 364. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties and characteristics of nanocomposite films from tilapia skin gelatin incorporated with ethanolic extract from coconut husk. J. Food Sci. Technol. 2015, 52, 7669–7682. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements-a review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar]
- Afshar, S.; Baniasadi, H. Investigation the effect of graphene oxide and gelatin/starch weight ratio on the properties of starch/gelatin/GO nanocomposite films: The RSM study. Int. J. Biol. Macromol. 2018, 109, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Adilah, A.N.; Hean, C.G.; Hanani, Z.A.N. Incorporation of graphene oxide to enhance fish gelatin as bio-packaging material. Food Packag. Shelf Life 2021, 28, 100679. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef] [PubMed]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Reungsang, A.; Rachtanapun, P. Antioxidant Films from Cassava Starch/Gelatin Biocomposite Fortified with Quercetin and TBHQ and Their Applications in Food Models. Polymers 2021, 13, 1117. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Vioque, B.; Rubio-Senent, F.; Fernández-Bolaños, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3,4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Kusznierewicz, B.; Malinowska-Pańczyk, E.; Sinkiewicz, I.; Gottfried, K.; Kołodziejska, I. Fish gelatin films containing aqueous extracts from phenolic-rich fruit pomace. LWT 2020, 117, 108613. [Google Scholar] [CrossRef]
- Farajzadeh, F.; Motamedzadegan, A.; Shahidi, S.-A.; Hamzeh, S. The effect of chitosan-gelatin coating on the quality of shrimp (Litopenaeus vannamei) under refrigerated condition. Food Control 2016, 67, 163–170. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Hirano, S.; Itakura, C.; Seino, H.; Akiyama, Y.; Nonaka, I.; Kanbara, N.; Kawakami, T. Chitosan as an ingredient for domestic animal feeds. J. Agric. Food Chem. 1990, 38, 1214–1217. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and properties of chitosan. Appl. Chitin Chitosan 2020, 3–29. [Google Scholar]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Butler, B.L.; Vergano, P.J.; Testin, R.F.; Bunn, J.M.; Wiles, J.L. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 1996, 61, 953–956. [Google Scholar] [CrossRef]
- Nadarajah, K.; Prinyawiwatkul, W.; No, H.K.; Sathivel, S.; Xu, Z. Sorption behavior of crawfish chitosan films as affected by chitosan extraction processes and solvent types. J. Food Sci. 2006, 71, E33–E39. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Sustainable production of chitosan. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems; Springer: Cham, Switzerland, 2020; pp. 45–60. [Google Scholar]
- Zakaria, S.; Chia, C.H.; Ahmad, W.H.W.; Kaco, H.; Chook, S.W.; Chan, C.H. Mechanical and antibacterial properties of paper coated with chitosan. Sains Malays. 2015, 44, 905–911. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio-and fossil-based polymeric blends and nanocomposites for packaging: Structure-property relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Martino, S.; Torre, L. Nanocomposites Based on Biodegradable Polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Striani, R.; Ferrari, F.; Visconti, P.; Rizzo, D.; Greco, A. An Innovative Method for the Recycling of Waste Carbohydrate-Based Flours. Polymers 2020, 12, 1414. [Google Scholar] [CrossRef]
- Stasi, E.; Giuri, A.; Ferrari, F.; Armenise, V.; Colella, S.; Listorti, A.; Rizzo, A.; Ferraris, E.; Corcione, C.E. Biodegradable Carbon-based Ashes/Maize Starch Composite Films for Agricultural Applications. Polymers 2020, 12, 524. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: II. Application in bio-based plastics for active packaging. Carbohydr. Polym. 2013, 96, 586–592. [Google Scholar] [CrossRef]
- Alix, S.; Mahieu, A.; Terrie, C.; Soulestin, J.; Gerault, E.; Feuilloley, M.G.J.; Gattin, R.; Edon, V.; Ait-Younes, T.; Leblanc, N. Active pseudo-multilayered films from polycaprolactone and starch based matrix for food-packaging applications. Eur. Polym. J. 2013, 49, 1234–1242. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, J.; Liu, X.; Hu, Q.; Huang, J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J. Mater. Chem. B 2013, 1, 3477–3485. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Khwaldia, K.; Basta, A.H.; Aloui, H.; El-Saied, H. Chitosan–caseinate bilayer coatings for paper packaging materials. Carbohydr. Polym. 2014, 99, 508–516. [Google Scholar] [CrossRef]
- Yuceer, M.; Caner, C. Antimicrobial lysozyme–chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014, 94, 153–162. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Salara, A.R.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, S.-H.; Eun, C.-J.; Yoo, J.; Seo, Y.-S. Dispersion of chitosan nanoparticles stable over a wide pH range by adsorption of polyglycerol monostearate. Nanomater. Nanotechnol. 2020, 10, 1847980420917260. [Google Scholar] [CrossRef]
- Chiarathanakrit, C.; Riyajan, S.-A.; Kaewtatip, K. Transforming fish scale waste into an efficient filler for starch foam. Carbohydr. Polym. 2018, 188, 48–53. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Ashori, A.; Tabrizi, A.K. Characterization and biodegradability of polypropylene composites using agricultural residues and waste fish. Compos. Part B Eng. 2014, 56, 279–283. [Google Scholar] [CrossRef]
- Erik de Laurens. Available online: https://www.erikdelaurens.com (accessed on 9 November 2020).
- MarinaTex. Available online: https://www.marinatex.co.uk/ (accessed on 7 February 2021).
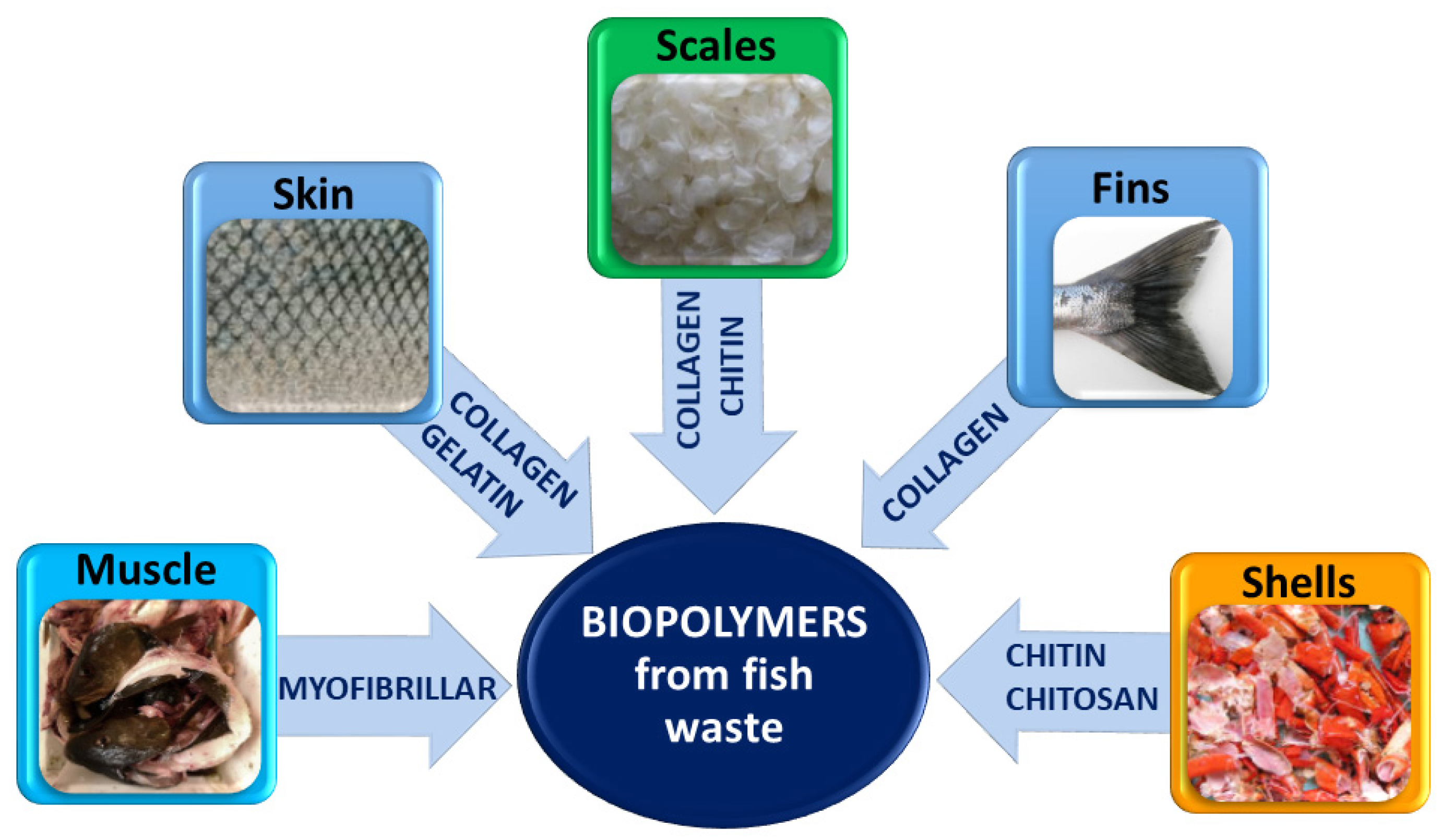

| Biopolymer from Fish Waste | Application | Advantages | Disadvantages | Problem Resolution |
|---|---|---|---|---|
| Myofibrillar protein | edible films | Functional properties with antioxidants, vitamins and coloring agents | Low mechanical properties | Gelatin addition Plasticizer addition |
| Marine Collagen | edible films and coatings | Low cost | Low thermal stability Poor mechanical properties | Blending with biopolymers |
| Fish gelatin | edible films and coatings | Good film-forming properties Low cost Biocompatibility | High hygroscopicity Low barrier properties Low mechanical strength | Cross-linking Nanofillers Blending with biopolymers |
| Chitosan | edible films | Biocompatibility Low cost Antimicrobial properties | Low barrier properties Low mechanical properties | Cross-linking Nanofillers Blending with biopolymers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionetto, F.; Esposito Corcione, C. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers 2021, 13, 2337. https://doi.org/10.3390/polym13142337
Lionetto F, Esposito Corcione C. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers. 2021; 13(14):2337. https://doi.org/10.3390/polym13142337
Chicago/Turabian StyleLionetto, Francesca, and Carola Esposito Corcione. 2021. "Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging" Polymers 13, no. 14: 2337. https://doi.org/10.3390/polym13142337
APA StyleLionetto, F., & Esposito Corcione, C. (2021). Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers, 13(14), 2337. https://doi.org/10.3390/polym13142337