Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study
Abstract
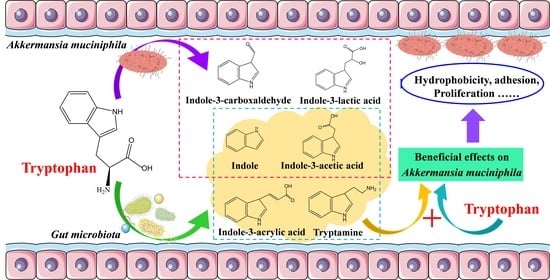
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Bacterial Strains and Culture Conditions
2.3. Auto-Aggregation and Hydrophobicity Analysis
2.4. Scanning Electron Microscopy (SEM)
2.5. Epithelial Cell Lines
2.6. Bacterial Adhesion to HT-29 Cells
2.7. UPLC/Q-TRAP MS Method for Tryptophan Metabolomics of A. muciniphila
2.8. Statistical Analysis
3. Results
3.1. The Effect of Tryptophan on the Growth of A. muciniphila
3.2. The Effect of Tryptophan on the Auto-Aggregation, Hydrophobicity, and the Surface Morphology of A. muciniphila
3.3. The Effect of Tryptophan on the Adhesion Ability of A. muciniphila to HT-29 Cells
3.4. The Tryptophan Metabolites of A. muciniphila
3.5. The Effects of Tryptophan Metabolites Produced by Akkermansia on the Growth of A. muciniphila BAA-835T
3.6. The Effects of Tryptophan Metabolites Produced by Other Gut Microbes on the Growth of A. muciniphila BAA-835T
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov. sp. nov. a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 5, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Lugt, B.; van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.P.; Brandt, R.M.C.; de Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (-/Delta7) mice. Immun. Ageing 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 2020. [Google Scholar] [CrossRef] [Green Version]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Yang, F.; DeLuca, J.A.A.; Menon, R.; Garcia-Vilarato, E.; Callaway, E.; Landrock, K.K.; Lee, K.; Safe, S.H.; Chapkin, R.S.; Allred, C.D.; et al. Effect of diet and intestinal AhR expression on fecal microbiome and metabolomic profiles. Microb. Cell Fact. 2020, 19, 219. [Google Scholar] [CrossRef]
- Shi, Z.; Lei, H.; Chen, G.; Yuan, P.; Cao, Z.; Ser, H.L.; Zhu, X.; Wu, F.; Liu, C.; Dong, M.; et al. Impaired intestinal Akkermansia muciniphila and aryl hydrocarbon receptor ligands contribute to nonalcoholic fatty liver disease in mice. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the gut microbiota of children with autism spectrum disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef]
- Song, J.; Ma, W.; Gu, X.; Zhao, L.; Jiang, J.; Xu, Y.; Zhang, L.; Zhou, M.; Yang, L. Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J. Transl. Med. 2019, 17, 224. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, D.N.; Swimm, A.; Sonowal, R.; Bretin, A.; Gewirtz, A.T.; Jones, R.M.; Kalman, D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc. Natl. Acad. Sci. USA 2020, 117, 21519–21526. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Zhang, B.; Yu, Z.; Hu, Y.; Lv, H.; Ji, X.; Wang, J.; Peng, B.; Wang, S. Ameliorative effect of dietary tryptophan on neurodegeneration and inflammation in d-galactose-induced aging mice with the potential mechanism relying on AMPK/SIRT1/PGC-1alpha pathway and gut microbiota. J. Agric. Food Chem. 2021, 69, 4732–4744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Y.; Liu, S.; Lv, H.; Hu, Y.; Wang, Y.; Li, Z.; Wang, J.; Ji, X.; Ma, H.; et al. Dietary supplementation of foxtail millet ameliorates colitis-associated colorectal cancer in mice via activation of gut receptors and suppression of the STAT3 pathway. Nutrients 2020, 12, 2367. [Google Scholar] [CrossRef]
- Hsu, S.A.; Chou, J.Y. Yeasts in fermented food and kefir: In vitro characterization of probiotic traits. J. Anim. Plant Sci. JAPS 2021, 31, 567–582. [Google Scholar]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.D.F.; de Souza, K.M.S.; Albuquerque, W.W.C.; Teixeira, J.A.C.; Cavalcanti, M.T.H.; Porto, A.L.F. Saccharomyces cerevisiae from Brazilian kefir-fermented milk: An in vitro evaluation of probiotic properties. Microb. Pathog. 2017, 110, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, B.; Champomier-Verges, M.C.; Anglade, P.; Baraige, F.; Reyes-Gavilan, C.G.D.; Margolles, A.; Zagorec, M. Proteomic analysis of global changes in protein expression during bile salt exposure of Bifidobacterium longum NCIMB 8809. J. Bacteriol. 2005, 187, 5799–5808. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Douillard, F.P.; Wang, G.; Zhai, Z.; Yang, J.; Song, S.; Cui, J.; Ren, F.; Luo, Y.; Zhang, B.; et al. Integrated transcriptomic and proteomic analysis of the bile stress response in a centenarian-originated probiotic Bifidobacterium longum BBMN68. Mol. Cell. Proteom. 2014, 13, 2558–2572. [Google Scholar] [CrossRef] [Green Version]
- Schaechter, M.; Maaloe, O.; Kjeldgaard, N.O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J. Gen. Microbiol. 1958, 19, 592–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Bai, Y.; Jiang, M.; Tokuyasu, T.A.; Huang, X.; Zhong, F.; Wu, Y.; Fu, X.; Kleckner, N.; Hwa, T.; et al. General quantitative relations linking cell growth and the cell cycle in Escherichia coli. Nat. Microbiol. 2020, 5, 995–1001. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepika, G.; Rastall, R.A.; Charalampopoulos, D. Effect of food models and low-temperature storage on the adhesion of Lactobacillus rhamnosus GG to Caco-2 cells. J. Agric. Food Chem. 2011, 59, 8661–8666. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 2020, 61, 102138. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Rohrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Van Beek, A.A.; Hugenholtz, F.; Meijer, B.; Sovran, B.; Perdijk, O.; Vermeij, W.P.; Brandt, R.M.; Barnhoorn, S.; Hoeijmakers, J.H.; de Vos, P.; et al. Frontline Science: Tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1(-/Delta7) mice. J. Leukoc. Biol. 2017, 101, 811–821. [Google Scholar] [CrossRef]
- Sonowal, R.; Swimm, A.; Sahoo, A.; Luo, L.; Matsunaga, Y.; Wu, Z.; Bhingarde, J.A.; Ejzak, E.A.; Ranawade, A.; Qadota, H.; et al. Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA 2017, 114, E7506–E7515. [Google Scholar] [CrossRef] [Green Version]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Inaba, T.; Obana, N.; Habe, H.; Nomura, N. Biofilm formation by Streptococcus mutans is enhanced by indole via the quorum sensing pathway. Microbes Environ. 2020, 35, ME19164. [Google Scholar] [CrossRef]
- Rattanaphan, P.; Mittraparp-Arthorn, P.; Srinoun, K.; Vuddhakul, V.; Tansila, N. Indole signaling decreases biofilm formation and related virulence of Listeria monocytogenes. FEMS Microbiol. Lett. 2020, 367. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Mol. Cell Proteom. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, W.; Liang, Y.; Sun, L.; Yin, Y.; Zhang, W. Anti-inflammatory and anti-oxidative activity of indole-3-acetic acid involves induction of HO-1 and neutralization of free radicals in RAW264.7 Cells. Int. J. Mol. Sci. 2020, 21, 1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghi, M.; Pariano, M.; Solito, V.; Puccetti, M.; Bellet, M.M.; Stincardini, C.; Renga, G.; Vacca, C.; Sellitto, F.; Mosci, P.; et al. Targeting the aryl hydrocarbon receptor with indole-3-aldehyde protects from vulvovaginal candidiasis via the il-22-il-18 cross-talk. Front. Immunol. 2019, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cho, K.Y.; Meng, D.; Walker, W.A. The impact of indole-3-lactic acid on immature intestinal innate immunity and development: A transcriptomic analysis. Sci. Rep. 2021, 11, 8088. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, W.; Zhang, F.; Zhong, S.; Sun, Y.; Huo, J.; Zhu, J.; Wu, C. The gut microbiota-produced indole-3-propionic acid confers the antihyperlipidemic effect of mulberry-derived 1-deoxynojirimycin. mSystems 2020, 5. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Song, Y.; Hu, Y.; Wang, Y.; Zhang, B.; Wang, J.; Ji, X.; Wang, S. Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study. Microorganisms 2021, 9, 1511. https://doi.org/10.3390/microorganisms9071511
Yin J, Song Y, Hu Y, Wang Y, Zhang B, Wang J, Ji X, Wang S. Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study. Microorganisms. 2021; 9(7):1511. https://doi.org/10.3390/microorganisms9071511
Chicago/Turabian StyleYin, Jia, Yujie Song, Yaozhong Hu, Yuanyifei Wang, Bowei Zhang, Jin Wang, Xuemeng Ji, and Shuo Wang. 2021. "Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study" Microorganisms 9, no. 7: 1511. https://doi.org/10.3390/microorganisms9071511
APA StyleYin, J., Song, Y., Hu, Y., Wang, Y., Zhang, B., Wang, J., Ji, X., & Wang, S. (2021). Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study. Microorganisms, 9(7), 1511. https://doi.org/10.3390/microorganisms9071511