Parasporin A13-2 of Bacillus thuringiensis Isolates from the Papaloapan Region (Mexico) Induce a Cytotoxic Effect by Late Apoptosis against Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Proteins Isolation
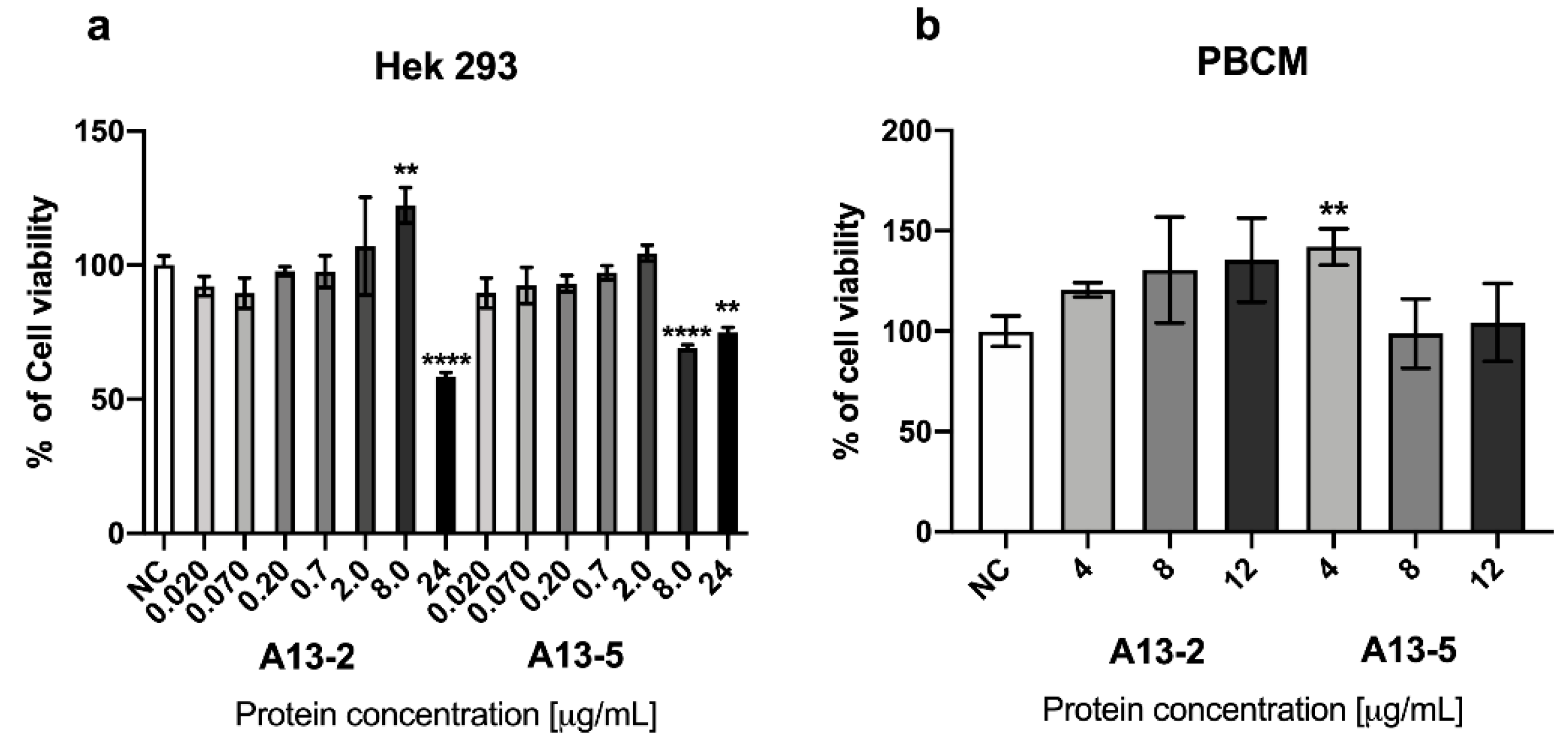
2.2. Cytotoxic Effect of Parasporal Protein A13-2 in Cancer and PBMC Cells
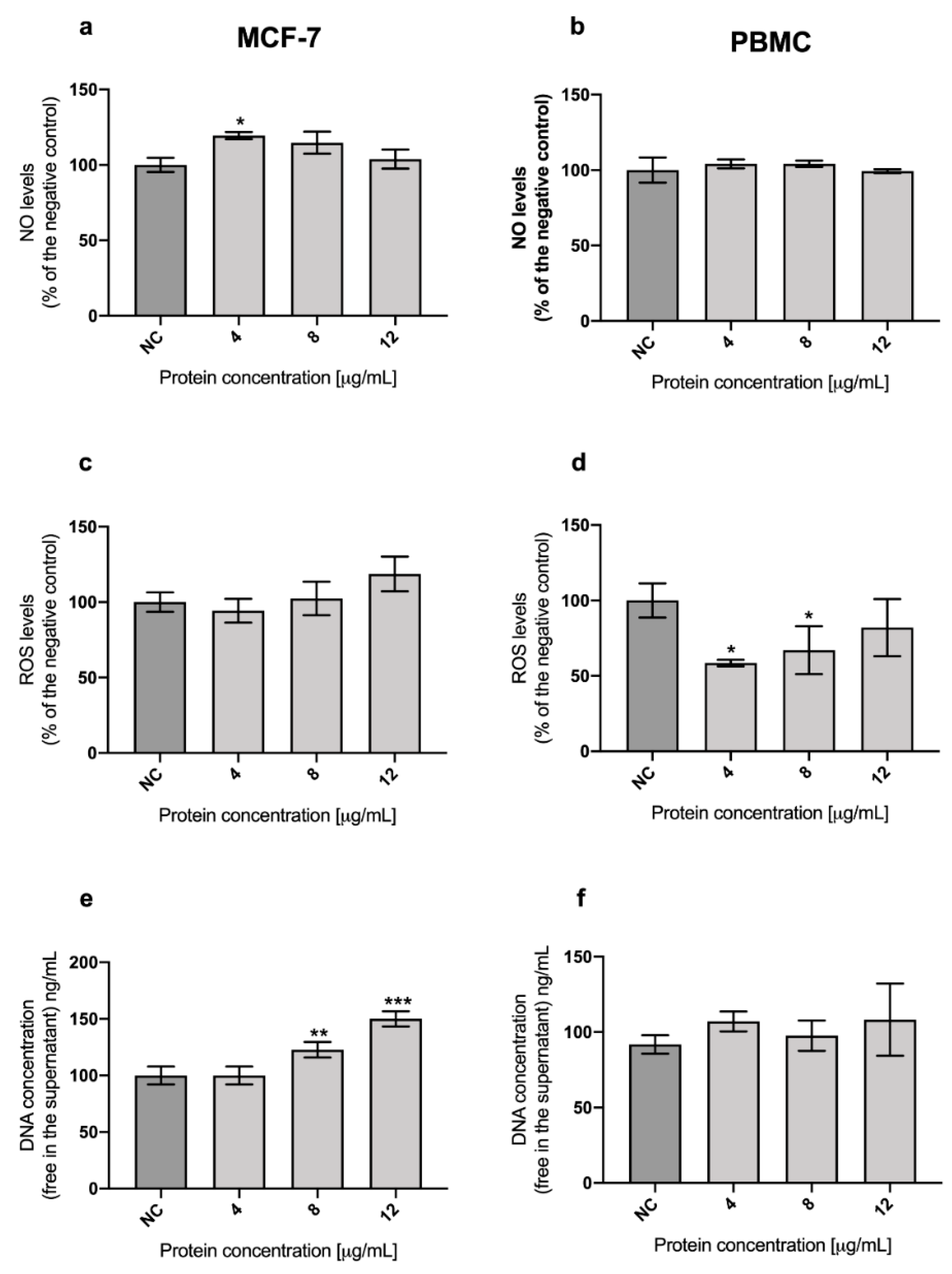
2.3. Role of Oxidative Stress in the Cytotoxicity of A13-2 Protein in MCF-7 and PBMC Cells
2.4. Morphological Changes and Cell Death Mechanism Induced by Parasporal Protein A13-2
3. Discussion
Morphological Changes and Cell Death Mechanism Induced by Parasporal Protein A13-2
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains, Culture Conditions and Parasporal Inclusion Isolation
5.2. Cell Culture Conditions
5.3. Cytotoxicity Assays
5.4. Nitric Oxide Test
5.5. Reactive Oxygen Species Assay
5.6. Genotoxicity Assay
5.7. Apoptosis Detection by Annexin V/PI Assay
5.8. Light Microscopic Observation
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnepf, E.; Crickmore, N.V.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. Am. Soc. Microbiol. 1998, 62, 775–806. [Google Scholar] [CrossRef] [Green Version]
- Mizuki, E.; Ohba, M.; Akao, T.; Yamashita, S.; Saitoh, H.; Park, Y.S. Unique activity associated with non-insecticidal Bacillus thuringiensis parasporal inclusions: In vitro cell-killing action on human cancer cells. J. Appl. Microbiol. 1999, 86, 477–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Georghiou, S.B.; Kelleher, A.J.; Aroian, R.V. Bacillus thuringiensis Cry5B Protein Is Highly Efficacious as a Single-Dose Therapy against an Intestinal Roundworm Infection in Mice. PLoS Negl. Trop. Dis. 2010, 4, e614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, S.; Ohba, M.; Mizuki, E.; Crickmore, N.; Côté, J.C.; Nagamatsu, Y.; Kitada, S.; Sakai, H.; Harata, K.; Shin, T. Parasporin Nomenclature. 2011. Available online: http//parasporin.fitc.pref.fukuoka.jp./intro.html (accessed on 21 April 2021).
- Ito, A.; Sasaguri, Y.; Kitada, S.; Kusaka, Y.; Kuwano, K.; Masutomi, K.; Mizuki, E.; Akao, T.; Ohba, M. A Bacillus thuringiensis crystal protein with selective cytocidal action to human cells. J. Biol. Chem. 2004, 279, 21282–21286. [Google Scholar] [CrossRef] [Green Version]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Wasano, N.; Yamashita, S.; Kusumoto, K.I.; Akao, T.; Mizuki, E.; Ohba, M.; Inouye, K. Identification of a novel cytotoxic protein, Cry45Aa, from Bacillus thuringiensis A1470 and its selective cytotoxic activity against various mammalian cell lines. J. Agric. Food Chem. 2005, 53, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Cardona, L.F.V.; Torres, D.S.R.; Salamanca, J.C. Toxinas de Bacillus thuringiensis con actividad anticancerígena: Parasporinas. Rev. Colomb. Biotecnol. 2018, 20, 89–100. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Okamura, S.; Saitou, H.; Akao, T.; Mizuki, E. Three Cry toxins in two types from Bacillus thuringiensis strain M019 preferentially kill human hepatocyte cancer and uterus cervix cancer cells. Biosci. Biotechnol. Biochem. 2010, 74, 494–498. [Google Scholar] [CrossRef]
- Espino-Vázquez, A.; Gómez-Treviño, A.; Galán-Wong, L.; Pereyra-Alférez, B. Isolation of Bacillus thuringiensis strains with cytotoxic activity against MOLT-4, a leukemia cell line. In Microbes in Applied Research: Current Advances and Challenges; World Scientific Publishing Co.: Singapore, 2012; pp. 147–151. [Google Scholar]
- Bravo-D, H.R.; Cruz-Nolasco, A.; Gutiérrez-Lucas, L.R.; Navarro-Mtz, A.K. Bioinformatics Analysis of NprR-NprX Quorum-Sensing System of Bacillus thuringiensis Isolates from the Papaloapan Region, Oaxaca-Mexico. Adv. Biol. Chem. 2015, 5, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Aboul-Soud, M.A.M.; Al-Amri1, M.Z.; Kumar, A.; Al-Sheikh, Y.A.; Ashour, A.E.; El-Kersh, T.A. Specific cytotoxic effects of parasporal Crystal proteins isolated from native Saudi Arabian Bacillus thuringiensis strains against cervical cancer cells. Molecules 2019, 24, 506. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Cancer. 2020. Available online: https://www.who.int (accessed on 21 April 2021).
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Viñas, G.; Puig, T.; Porta, R. Estréss oxidativo en pacientes con cáncer: Dos caras de una misma moneda. Med. Clin. (Barc.) 2012, 139, 171–175. [Google Scholar] [CrossRef]
- Nair, K.; Iskandarani, A.; Al-Thani, R.; Mohammad, R.; Jaoua, S. The replacement of five consecutive amino acids in the cyt1a protein of Bacillus thuringiensis enhances its cytotoxic activity against lung epithelial cancer cells. Toxins 2018, 10, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasseur, K.; Auger, P.; Asselin, E.; Parent, S.; Côté, J.-C.; Sirois, M. Parasporin-2 from a new Bacillus thuringiensis 4R2 strain induces caspases activation and apoptosis in human cancer cells. PLoS ONE 2015, 10, e0135106. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.P. A versatile stain for pollen fungi, yeast and bacteria. Stain Technol. 1980, 55, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Obeidat, M. In vitro selective cytotoxicity of activated parasporal proteins produced by Bacillus thuringiensis serovars kumamotoensis and tohokuensis against human cancer cell lines. Afr. J. Biotechnol. 2017, 16, 2181–2188. [Google Scholar]
- Krishnan, K.; Ker, J.E.A.; Mohammed, S.M.; Nadarajah, V.D. Identification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a binding protein for a 68-kDa Bacillus thuringiensis parasporal protein cytotoxic against leukaemic cells. J. Biomed. Sci. 2010, 17, 86. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S.Y.; Mohamed, S.M.; Nadarajah, V.D.; Tengku, I.A.T. Characterisation of the binding properties of Bacillus thuringiensis 18 toxin on leukaemic cells. J. Exp. Clin. Cancer Res. 2010, 29, 86. [Google Scholar] [CrossRef] [Green Version]
- Chubicka, T.; Girija, D.; Deepa, K.; Salini, S.; Meera, N.; Raghavamenon, A.C.; Divya, M.K.; Babu, T.D. A parasporin from Bacillus thuringiensis native to Peninsular India induces apoptosis in cancer cells through intrinsic pathway. J. Biosci. 2018, 43, 407–416. [Google Scholar] [CrossRef]
- Kitada, S.; Abe, Y.; Shimada, H.; Kusaka, Y.; Matsuo, Y.; Katayama, H.; Okumura, S.; Akao, T.; Mizuki, E.; Kuge, O.; et al. Cytocidal actions of parasporin-2, an anti-tumor crystal toxin from Bacillus thuringiensis. J. Biol. Chem. 2006, 281, 26350–26360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazamian, E.; Bahador, N.; Azarpira, N.; Rasouli, M. Anti-cancer parasporin toxins of new Bacillus thuringiensis against human colon (HCT-116) and blood (CCRF-CEM) cancer cell lines. Curr. Microbiol. 2018, 75, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K. Nitric oxide: Role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother. Pharmacol. 2011, 67, 1211–1224. [Google Scholar] [CrossRef]
- Basudhar, D.; Miranda, K.M.; Wink, D.A.; Ridnour, L.A. Advances in breast cancer therapy using Nitric Oxide and Nitroxyl donor agents. In Redox-Active Therapeutics. Oxidative Stress in Applied Basic Research and Clinical Practice; Batinić-Haberle, I., Rebouças, J., Spasojević, I., Eds.; Springer: Cham, Switzerland, 2016; pp. 377–403. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–23794. [Google Scholar] [CrossRef]
- Rubbo, H.; Radi, R. Antioxidant properties of nitric oxide. In Handbook of Antioxidants; Cadenas, E., Packer, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 689–706. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., II. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatla, S.; Woodhead, V.; Foreman, J.C.; Chain, B.M. The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic. Biol. Med. 1999, 26, 14–24. [Google Scholar] [CrossRef]
- Baran, C.P.; Zeigler, M.M.; Tridandapani, S.; Marsh, C.B. The role of ROS and RNS in regulating life and death of blood monocytes. Curr. Pharm. Des. 2004, 10, 855–866. [Google Scholar] [CrossRef]
- Wierzbicki, P.M.; Adrych, K.; Kartanowicz, D.; Dobrowolski, S.; Stanislawowski, M.; Chybicki, J.; Godlewski, J.; Korybalski, B.; Smoczynski, M.; Kmiec, Z. Fragile histidine triad (FHIT) gene is overexpressed in colorectal cancer. J. Physiol. Pharmacol. 2009, 60, 63–70. [Google Scholar]
- Yu, G.R.; Qin, W.W.; Li, J.P.; Hua, W.; Meng, Y.L.; Chen, R.; Yan, B.; Wang, L.; Zhang, X.; Jia, L.T.; et al. HIV-TAT-fused FHIT protein functions as a potential pro-apoptotic molecule in hepatocellular carcinoma cells. Biosci. Rep. 2012, 32, 271–279. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Papapostolou, I.; Grintzalis, K. Protocol for the quantitative assessment of DNA concentration and damage (fragmentation and nicks). Nat. Protoc. 2009, 4, 125. [Google Scholar] [CrossRef]
- Duprez, L.; Wirawan, E.; Berghe TVanden Vandenabeele, P. Major cell death pathways at a glance. Microbes Infect. 2009, 11, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Akiba, T.; Abe, Y.; Kitada, S.; Kusaka, Y.; Ito, A.; Ichimatsu, T.; Katayama, H.; Akao, T.; Higuchi, K.; Mizuki, E.; et al. Crystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cells. J. Mol. Biol. 2009, 386, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.C.; Naylor, C.E.; Smedley, I.I.I.J.G.; Lukoyanova, N.; Robertson, S.; Moss, D.S.; McClane, B.A.; Basak, A.K. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J. Mol. Biol. 2011, 413, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Diep, D.B.; Nelson, K.L.; Raja, S.M.; Pleshak, E.N.; Buckley, J.T. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J. Biol. Chem. 1998, 273, 2355–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, A.I.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. Characterization of PicoGreen interaction with dsDNA and the origin of its fluorescence enhancement upon binding. Biophys. J. 2010, 99, 3010–3019. [Google Scholar] [CrossRef] [Green Version]
- Ohba, M.; Mizuki, E.; Uemori, A. Parasporin, a new anticancer protein group from Bacillus thuringiensis. Anticancer Res. 2009, 29, 427–433. [Google Scholar]
- Abe, Y.; Shimada, H.; Kitada, S. Raft-targeting and oligomerization of Parasporin-2, a Bacillus thuringiensis crystal protein with anti-tumour activity. J. Biochem. 2007, 143, 269–275. [Google Scholar] [CrossRef]
- Yamashita, S.; Katayama, H.; Saitoh, H.; Akao, T.; Park, Y.S.; Mizuki, E.; Ohba, M.; Ito, A. Typical three-domain Cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J. Biochem. 2005, 138, 663–672. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.-C.; Yu, Z.; Sun, M. Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef] [Green Version]
- Ekino, K.; Okumura, S.; Ishikawa, T.; Kitada, S.; Saitoh, H.; Akao, T.; Oka, T.; Nomura, Y.; Ohba, M.; Shin, T.; et al. Cloning and characterization of a unique cytotoxic protein parasporin-5 produced by Bacillus thuringiensis A1100 strain. Toxins 2014, 6, 1882–1895. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of Structural Proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Brüne, B.; von Knethen, A.; Sandau, K.B. Nitric oxide and its role in apoptosis. Eur. J. Pharmacol. 1998, 351, 261–272. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Porter, A.G. Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J. Biol. Chem. 2004, 279, 20096–20107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.-S.; Shin, P.-G.; Lee, J.-H.; Kim, G.-D. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264. 7 macrophage cells. Cell. Immunol. 2012, 280, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Hwang, D.; Lee, E.S.; Hyun, J.W.; Yi, P.H.; Kim, G.S.; Lee, S.E.; Pang, C.; Park, Y.J.; Chung, K.H.; et al. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J. Ethnopharmacol. 2015, 163, 106–112. [Google Scholar] [CrossRef]
- Degli Esposti, M. Measuring mitochondrial reactive oxygen species. Methods 2002, 26, 335–340. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.T.; Huy, N.T.; Murao, L.A.; Lan, N.T.; Thuy, T.T.; Tuan, H.M.; Nga, C.T.; Tuong, V.V.; Dat, T.V.; Kikuchi, M.; et al. Elevated levels of cell-free circulating DNA in patients with acute dengue virus infection. PLoS ONE 2011, 6, e25969. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borin, D.B.; Castrejón-Arroyo, K.; Cruz-Nolasco, A.; Peña-Rico, M.; Sagrillo, M.R.; Santos, R.C.V.; Baco, L.S.d.; Pérez-Picaso, L.; Camacho, L.; Navarro-Mtz, A.K. Parasporin A13-2 of Bacillus thuringiensis Isolates from the Papaloapan Region (Mexico) Induce a Cytotoxic Effect by Late Apoptosis against Breast Cancer Cells. Toxins 2021, 13, 476. https://doi.org/10.3390/toxins13070476
Borin DB, Castrejón-Arroyo K, Cruz-Nolasco A, Peña-Rico M, Sagrillo MR, Santos RCV, Baco LSd, Pérez-Picaso L, Camacho L, Navarro-Mtz AK. Parasporin A13-2 of Bacillus thuringiensis Isolates from the Papaloapan Region (Mexico) Induce a Cytotoxic Effect by Late Apoptosis against Breast Cancer Cells. Toxins. 2021; 13(7):476. https://doi.org/10.3390/toxins13070476
Chicago/Turabian StyleBorin, Diego Becker, Karen Castrejón-Arroyo, Alain Cruz-Nolasco, Miguel Peña-Rico, Michele Rorato Sagrillo, Roberto C. V. Santos, Lucas Silva de Baco, Lemuel Pérez-Picaso, Luz Camacho, and A. Karin Navarro-Mtz. 2021. "Parasporin A13-2 of Bacillus thuringiensis Isolates from the Papaloapan Region (Mexico) Induce a Cytotoxic Effect by Late Apoptosis against Breast Cancer Cells" Toxins 13, no. 7: 476. https://doi.org/10.3390/toxins13070476
APA StyleBorin, D. B., Castrejón-Arroyo, K., Cruz-Nolasco, A., Peña-Rico, M., Sagrillo, M. R., Santos, R. C. V., Baco, L. S. d., Pérez-Picaso, L., Camacho, L., & Navarro-Mtz, A. K. (2021). Parasporin A13-2 of Bacillus thuringiensis Isolates from the Papaloapan Region (Mexico) Induce a Cytotoxic Effect by Late Apoptosis against Breast Cancer Cells. Toxins, 13(7), 476. https://doi.org/10.3390/toxins13070476






