Physcomitrium patens Mutants in Auxin Conjugating GH3 Proteins Show Salt Stress Tolerance but Auxin Homeostasis Is Not Involved in Regulation of Oxidative Stress Factors
Abstract
1. Introduction
2. Results
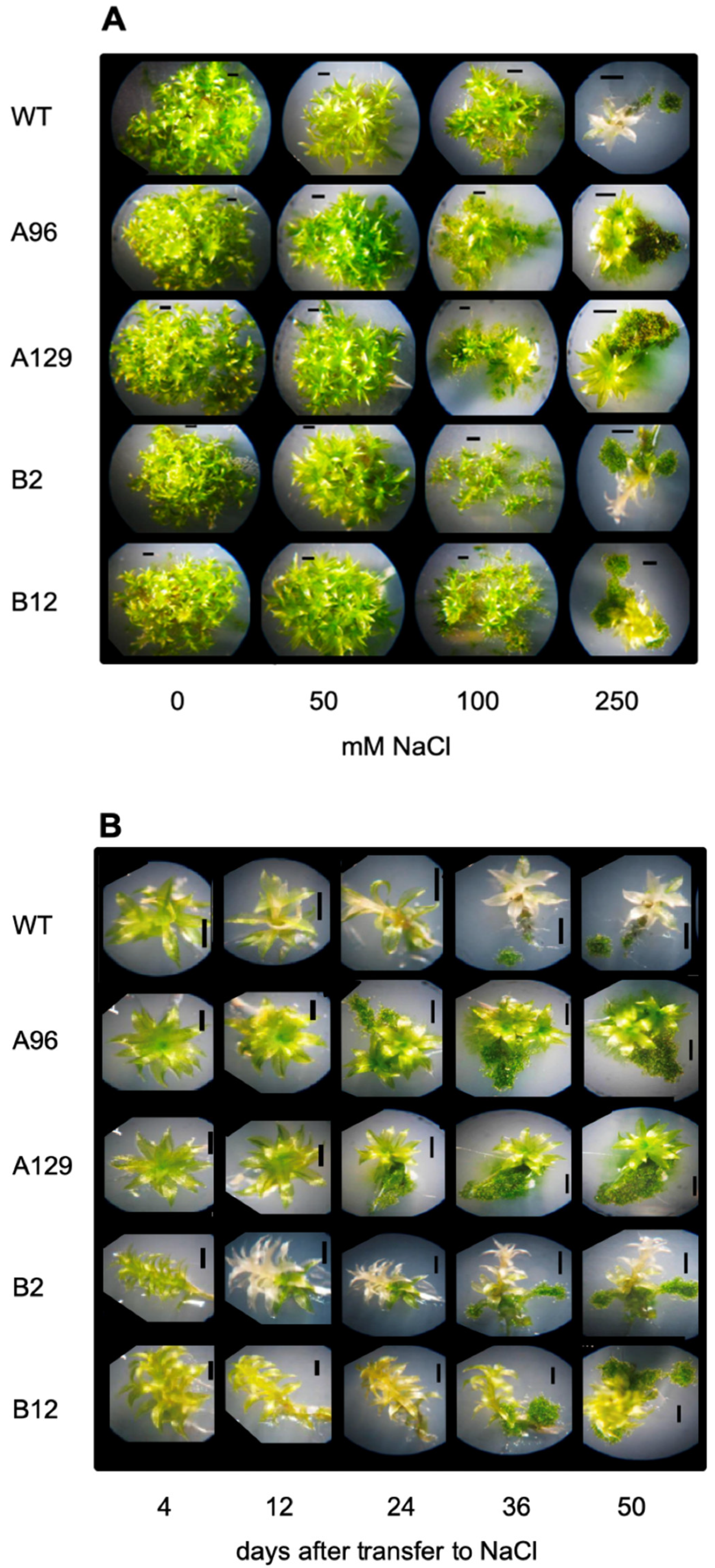
2.1. Growth of Physcomitrium patens at High Salt Concentrations and Revival
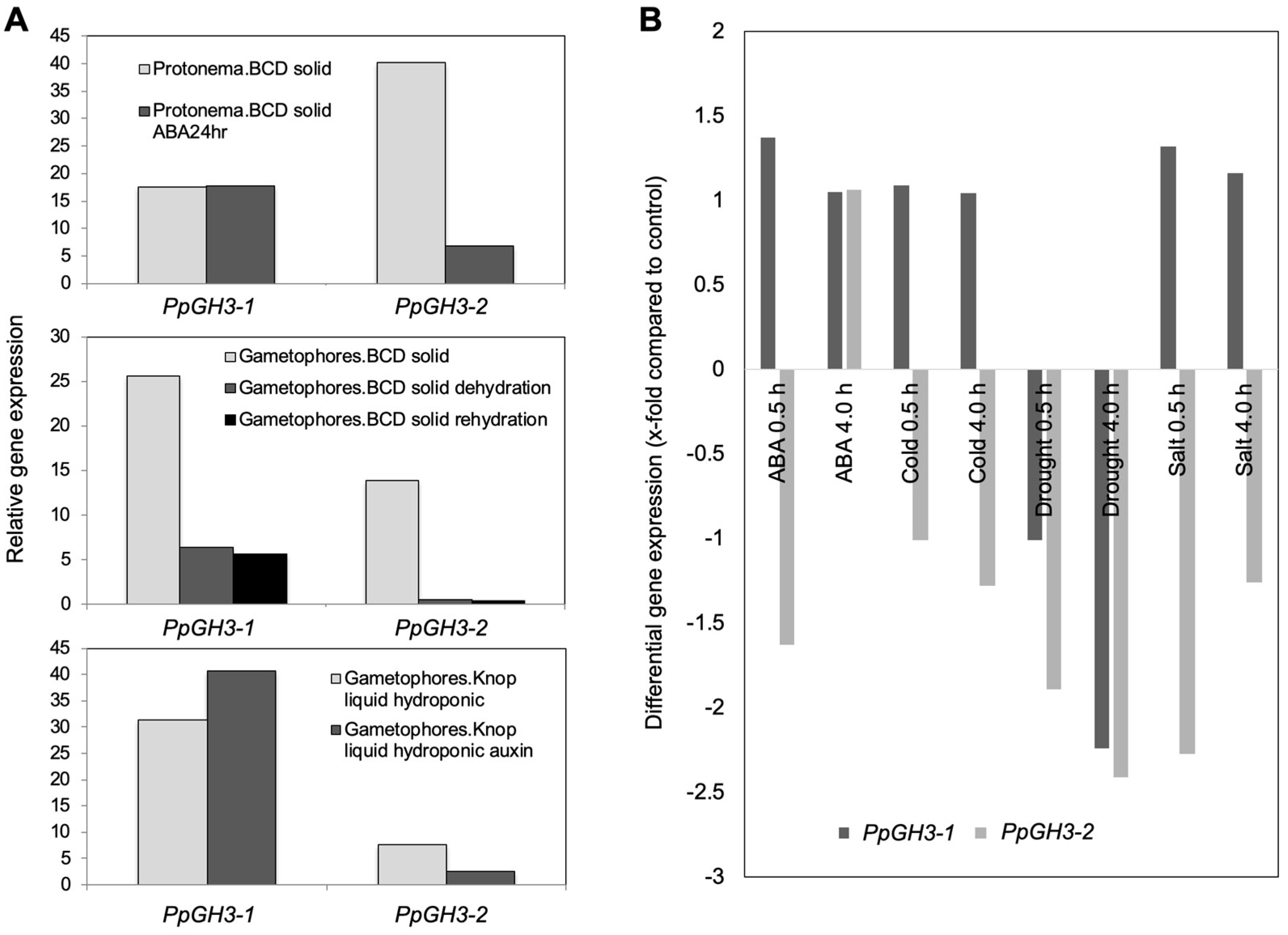
2.2. Digital Expression Analysis Showed That P. patens GH3 Genes Are Differentially Expressed during Abiotic Stress
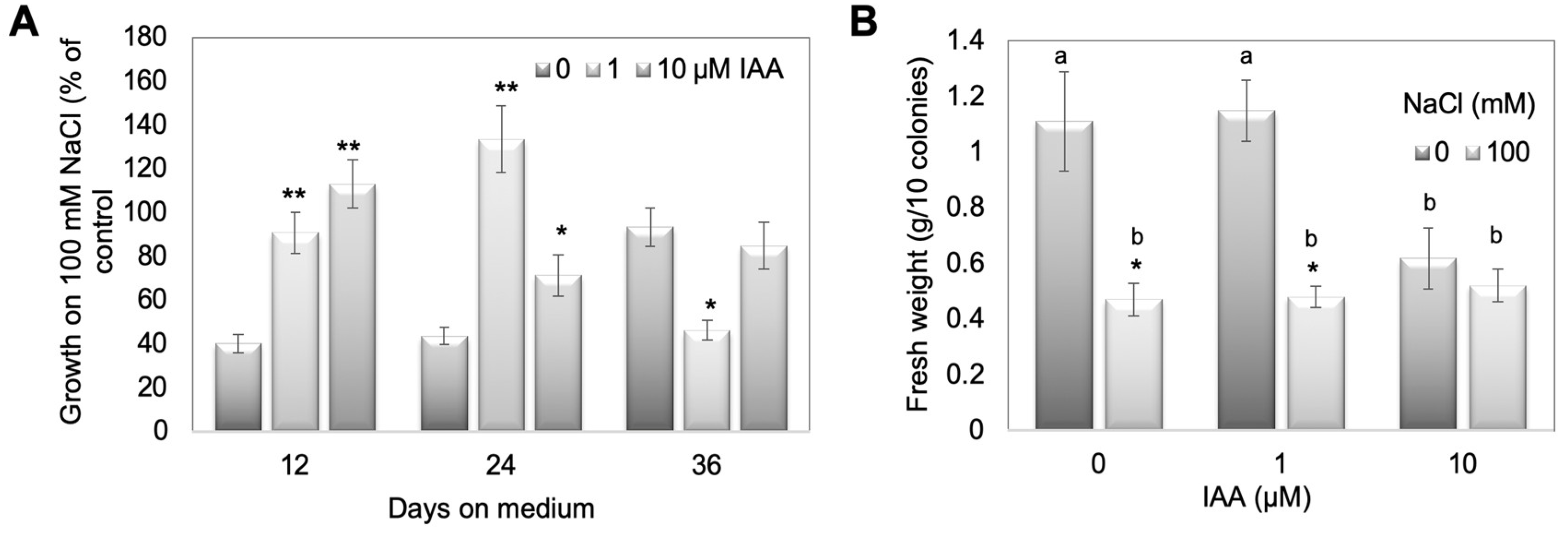
2.3. PpGH3 Double KO Mutants Show More Tolerance to Growth on High Salt
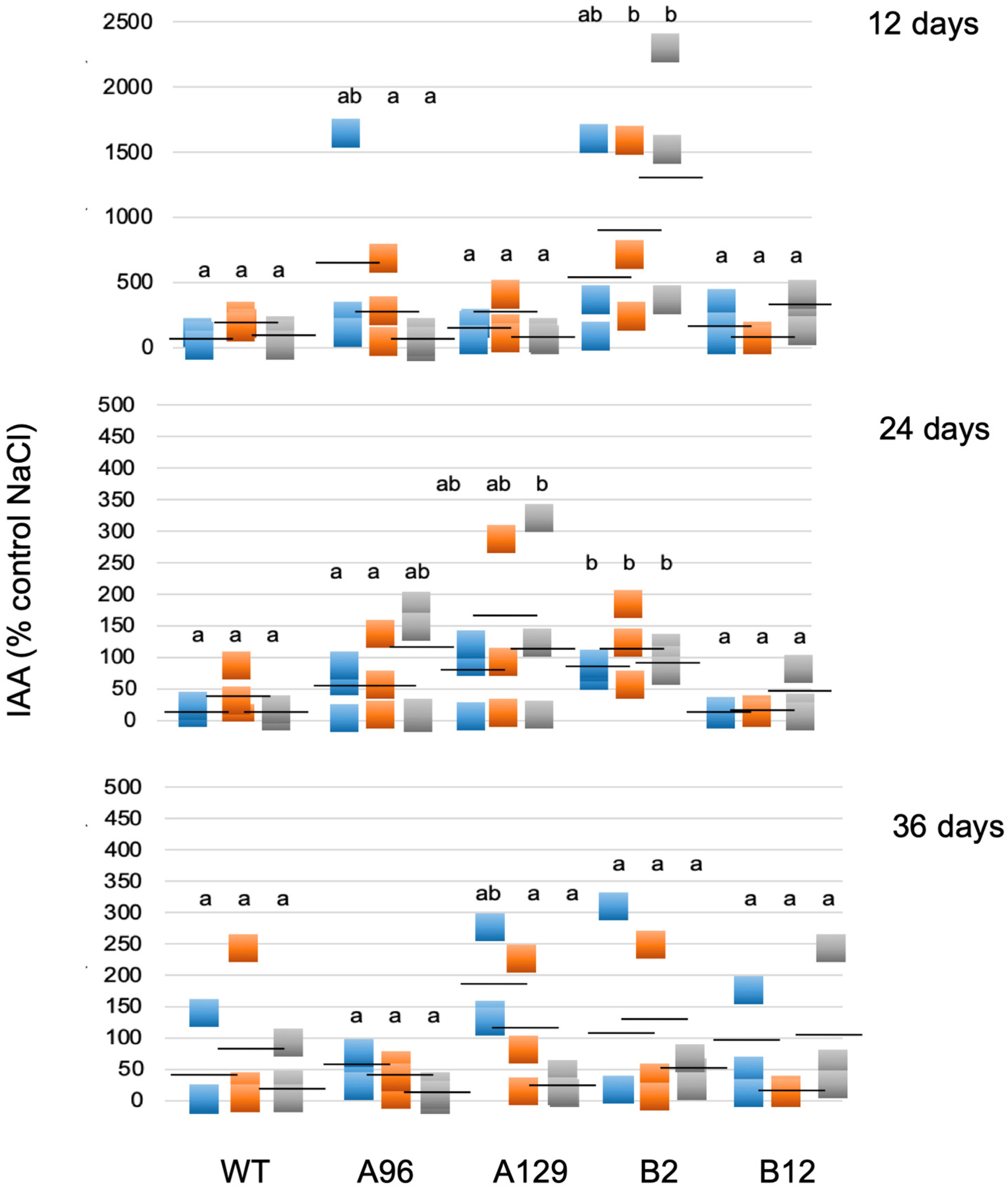
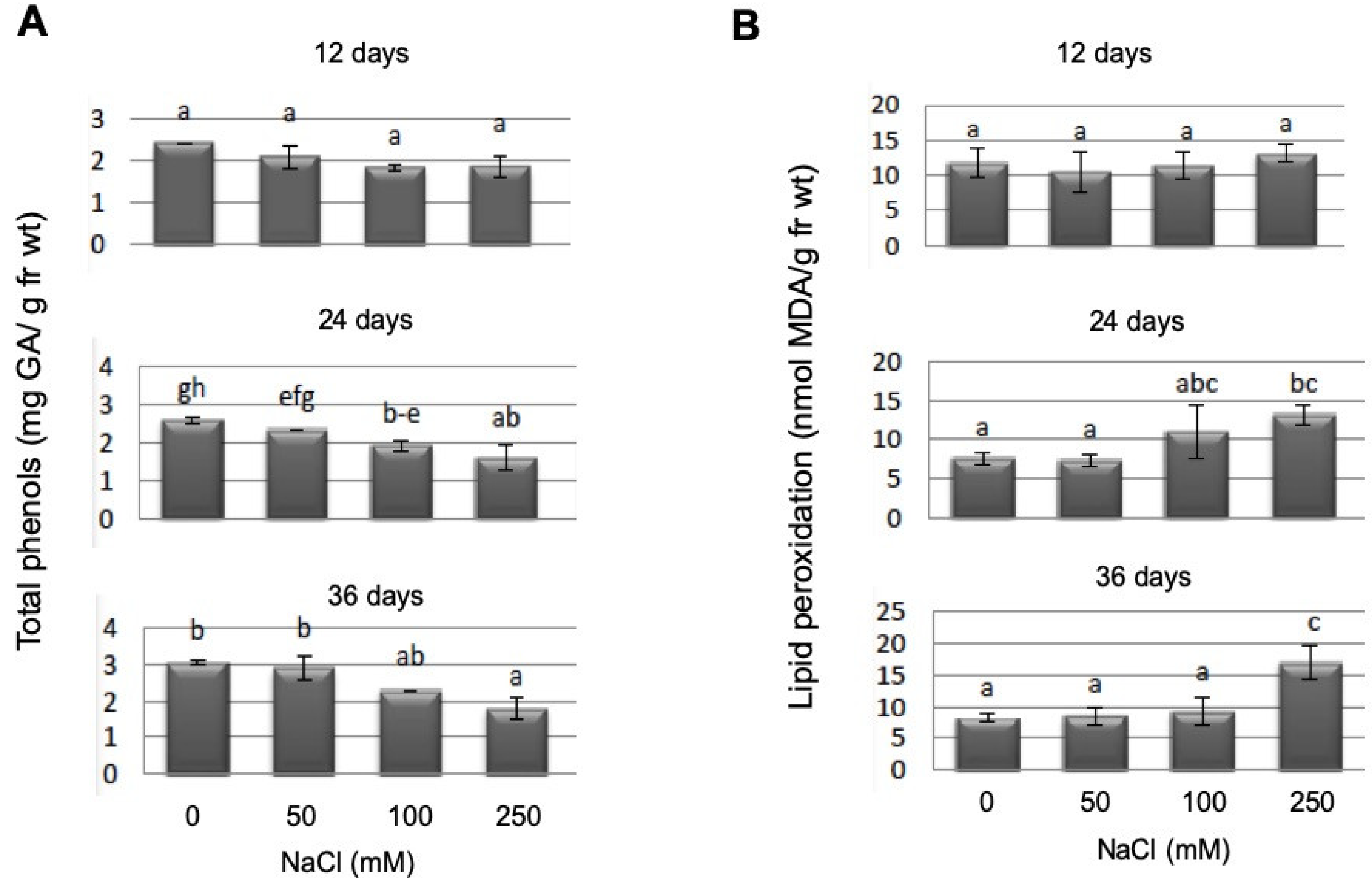
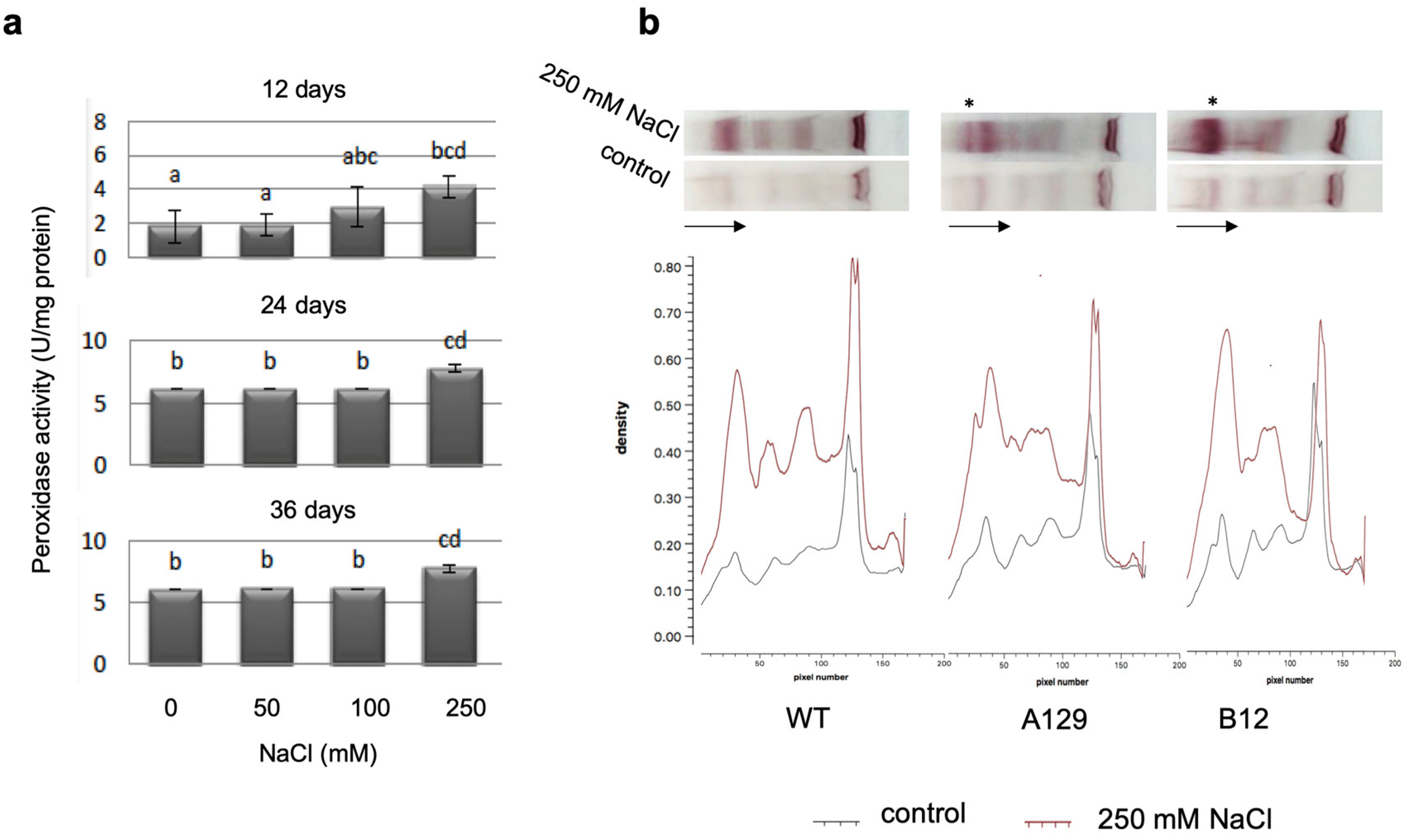
2.4. Salt Stress Upregulates Different Stress Parameters in Gametophores, but PpGH3 Proteins Are Not Involved in Their Upregulation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Free IAA Determination
4.3. Lipid Peroxidation Determination
4.4. Total Phenol Determination
4.5. In Situ Staining of Flavonoids
4.6. Protein Extraction
4.7. Total Protein Determination
4.8. Peroxidase Enzyme Activity
4.9. Native Polyacrylamide Gel Electrophoresis (PAGE)
4.9.1. Peroxidase Isoenzyme Detection
4.9.2. Superoxide Dismutase Isoenzyme Detection
4.9.3. Total Protein Staining with Colloidal Commassie
4.10. Statistical Analysis
4.11. Digital Expression and Promoter Element Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.; Long, W.; Zou, Y.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Goffinet, B.; Meyberg, R.; Wu, S.-Z.; Bezanilla, M. The moss Physcomitrium (Physcomitrella) patens: A model organism for non-seed plants. Plant Cell 2020. [Google Scholar] [CrossRef]
- Frank, W.; Ratnadewi, D.; Reski, R. Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 2005, 220, 384–394. [Google Scholar] [CrossRef]
- Arif, M.A.; Alseekh, S.; Harb, J.; Fernie, A.; Frank, W. Abscisic acid, cold and salt stimulate conserved metabolic regulation in the moss Physcomitrella patens. Plant Biol. 2018, 20, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ruibal, C.; Castro, A.; Carballo, V.; Szabados, L.; Vidal, S. Recovery from heat, salt and osmotic stress in Physcomitrella patens requires a functional small heat shock protein PpHsp16.4. BMC Plant Biol. 2013, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Mittag, J.; Šola, I.; Rusak, G.; Ludwig-Müller, J. Physcomitrella patens auxin conjugate synthetase (GH3) double knockout mutants are more resistant to Pythium infection than wild type. J. Plant Physiol. 2015, 183, 75–83. [Google Scholar] [CrossRef]
- Ponce de León, I.; Oliver, J.; Castro, A.; Gaggero, C.; Bentancor, M.; Vidal, S. Erwinia carotovora elicitors and Botrytis cinerea activate defense responses in Physcomitrella patens. BMC Plant Biol. 2007, 7, 52. [Google Scholar] [CrossRef]
- Richardt, S.; Timmerhaus, G.; Lang, D.; Qudeimat, E.; Corrêa, L.G.G.; Reski, R.; Rensing, S.A.; Frank, W. Microarray analysis of the moss Physcomitrella patens reveals evolutionarily conserved transcriptional regulation of salt stress and abscisic acid signalling. Plant Mol. Biol. 2010, 72, 27–45. [Google Scholar] [CrossRef]
- Beike, A.K.; Lang, D.; Zimmer, A.D.; Wüst, F.; Trautmann, D.; Wiedemann, G.; Beyer, P.; Decker, E.L.; Reski, R. Insights from the cold transcriptome of Physcomitrella patens: Global specialization pattern of conserved transcriptional regulators and identification of orphan genes involved in cold acclimation. New Phytol. 2015, 205, 869–881. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Yang, P. Proteomic studies of the abiotic stresses response in model moss—Physcomitrella patens. Front. Plant Sci. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Saavedra, L.; Svensson, J.; Carballo, V.; Izmendi, D.; Welin, B.; Vidal, S. A dehydrin gene in Physcomitrella patens is required for salt and osmotic stress tolerance. Plant J. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Bogdanović, M.; Ilić, M.; Živković, S.; Sabovljević, A.; Grubišić, D.; Sabovljević, M. Comparative study on the effects of NaCl on selected moss and fern representatives. Aust. J. Bot. 2011, 59, 734–740. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Jülke, S.; Bierfreund, N.M.; Decker, E.L.; Reski, R. Moss (Physcomitrella patens) GH3 proteins act in auxin homeostasis. New Phytol. 2008, 181, 323–338. [Google Scholar] [CrossRef]
- Mittag, J.; Gabrielyan, A.; Ludwig-Müller, J. Knockout of GH3 genes in the moss Physcomitrella patens leads to increased IAA levels at elevated temperature and in darkness. Plant Physiol. Biochem. 2015, 97, 339–349. [Google Scholar] [CrossRef]
- Junghans, U.; Polle, A.; Düchting, P.; Weiler, E.; Kuhlmann, B.; Gruber, F.; Teichmann, T. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ. 2006, 29, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Salopek-Sondi, B.; Samec, D.; Mihaljević, S.; Smolko, A.; Pavlović, I.; Janković, I.; Ludwig-Müller, J. Influence of stress hormones on the auxin homeostasis in Brassica rapa seedlings. Plant Cell Rep. 2013, 32, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenolcontent and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Sztein, A.E.; Cohen, J.D.; Slovin, J.P.; Cooke, T.J. Auxin metabolism in representative land plants. Am. J. Bot. 1995, 82, 1514–1521. [Google Scholar] [CrossRef]
- Sztein, A.E.; Cohen, J.D.; Garcia de la Fuente, I.; Cooke, T.J. Auxin metabolism in mosses and liverworts. Am. J. Bot. 1999, 86, 1544–1555. [Google Scholar] [CrossRef]
- Campanella, J.; Kurdach, S.; Skibitski, R.; Smalley, J.; Desind, S.; Ludwig-Müller, J. Evidence for the early evolutionary loss of the M20D auxin amidohydrolase family from mosses and horizontal gene transfer from soil bacteria of cryptic hydrolase orthologues to Physcomitrella patens. J. Plant Growth Regul. 2019, 38, 1428–1438. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Wang, D.; Ma, H.; Liu, B.; Shi, Z.; Ma, X.; Chen, Y.; Chen, Q. Evolutionary history of the glycoside hydrolase 3 (GH3) family based on the sequenced genomes of 48 plants and identification of jasmonic acid-related GH3 proteins in Solanum tuberosum. Int. J. Mol. Sci. 2018, 19, 1850. [Google Scholar] [CrossRef]
- Campanella, J.J.; Kurdach, S.; Bochis, J.; Smalley, J.V. Evidence for exaptation of the Marchantia polymorpha M20D peptidase MpILR1 into the tracheophyte auxin regulatory pathway. Plant Physiol. 2018, 177, 1595–1604. [Google Scholar] [CrossRef]
- Drábková, L.Z.; Dobrev, P.I.; Motyka, V. Phytohormone profiling across the bryophytes. PLoS ONE 2015, 10, e0125411. [Google Scholar] [CrossRef]
- Landberg, K.; Simura, J.; Ljung, K.; Sundberg, E.; Thelander, M. Studies of moss reproductive development indicate that auxin biosynthesis in apical stem cells may constitute an ancestral function for focal growth control. New Phytol. 2021, 229, 845–860. [Google Scholar] [CrossRef]
- Thelander, M.; Landberg, K.; Sundberg, E. Auxin-mediated developmental control in the moss Physcomitrella patens. J. Exp. Bot. 2018, 69, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Rubery, P.H.; Bopp, M. The mechanism of auxin uptake and accumulation in moss protonemata. Physiol. Plant. 1983, 58, 52–56. [Google Scholar] [CrossRef]
- Viaene, T.; Landberg, K.; Thelander, M.; Medvecka, E.; Pederson, E.; Feraru, E.; Cooper, E.D.; Karimi, M.; Delwiche, C.F.; Ljung, K.; et al. Directional auxin transport mechanisms in early diverging land plants. Current Biol. 2014, 24, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Mohanta, N. Genome wide identification of auxin efflux carrier gene family in Physcomitrella patens. J. Biotechnol. Sci. 2013, 1, 54–64. Available online: http://drabbas.org/jbs/ (accessed on 7 July 2021).
- Porco, S.; Pěnčík, A.; Rashed, A.; Voß, U.; Casanova-Sáez, R.; Bishopp, A.; Golebiowska, A.; Bhosale, R.; Swarup, R.; Swarup, K.; et al. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 11016–11021. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, J.E.; Harris, C.; Campos Mastrotti Pereira, F.; Wu, F.; Blakeslee, J.J.; Peer, W.A. DAO1 catalyzes temporal and tissue-specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 11010–11015. [Google Scholar] [CrossRef] [PubMed]
- Keilig, K.; Ludwig-Müller, J. Effect of Flavonoids on Heavy Metal Tolerance in Arabidopsis thaliana Seedlings. Botan. Stud. 2009, 50, 311–318. Available online: http://ejournal.sinica.edu.tw/bbas/content/2009/3/Bot503-05.pdf (accessed on 7 July 2021).
- Emiliani, J.; Grotewold, E.; Ferreyra, M.L.F.; Casati, P. Flavonols protect arabidopsis plants against UV-B deleterious effects. Mol. Plant 2013, 6, 1376–1379. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef]
- Wolf, L.; Rizzini, L.; Stracke, R.; Ulm, R.; Rensing, S.A. The molecular and physiological responses of Physcomitrella patens to Ultraviolet-B radiation. Plant Physiol. 2010, 153, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Pinhero, R.G.; Rao, M.V.; Paliyath, C.; Murr, D.P.; Fletcher, R.A. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol induced chilling tolerance of maize seedlings. Plant Physiol. 1997, 114, 695–704. [Google Scholar] [CrossRef]
- Lunde, C.; Drew, D.P.; Jacobs, A.K.; Tester, M. Exclusion of Na+ via Sodium ATPase (PpENA1) ensures normal growth of Physcomitrella patens under moderate salt stress. Plant Physiol. 2007, 144, 1786–1796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- JGI Phytozome 12—The Plant Genomic Resource. Available online: https://phytozome.jgi.doe.gov/pz/portal.html#!gene?search=1&detail=1&method=5013&searchText=transcriptid:32910187 (accessed on 13 August 2019).
- Perroud, P.; Haas, F.B.; Hiss, M.; Ullrich, K.K.; Alboresi, A.; Amirebrahimi, M.; Barry, K.; Bassi, R.; Bonhomme, S.; Chen, H.; et al. The Physcomitrella patens gene atlas project: Large-scale RNA-seq based expression data. Plant J. 2018, 95, 168–182. [Google Scholar] [CrossRef] [PubMed]
- BAR—The Bio-Analytic Resource for Plant Biology. Available online: http://bar.utoronto.ca/efp_physcomitrella/cgi-bin/efpWeb.cgi (accessed on 13 August 2019).
- Ortiz-Ramírez, C.; Hernandez-Coronado, M.; Thamm, A.; Catarino, B.; Wang, M.; Dolan, L.; Feijó, J.A.A.; Becker, J.D. A Transcriptome Atlas of Physcomitrella patens provides insights into the evolution and development of land plants. Mol. Plant 2016, 9, 205–220. [Google Scholar] [CrossRef]
- Oldenhof, H.; Wolkers, W.F.; Bowman, J.L.; Tablin, F.; Crowe, J.H. Freezing and desiccation tolerance in the moss Physcomitrella patens: An in situ Fourier transform infrared spectroscopic study. Biochim. Biophys. Acta 2006, 1760, 1226–1234. [Google Scholar] [CrossRef]
- Koster, K.L.; Balsamo, R.A.; Espinoza, C.; Oliver, M.J. Desiccation sensitivity and tolerance in the moss Physcomitrella patens: Assessing limits and damage. Plant Growth Regul. 2010, 62, 293–302. [Google Scholar] [CrossRef]
- Cuming, A.C.; Cho, S.H.; Kamisugi, Y.; Graham, H.; Quatrano, R.S. Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol. 2007, 176, 275–287. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef]
- Li, D.-D.; Ni, R.; Wang, P.-P.; Zhang, X.-S.; Wang, P.-Y.; Zhu, T.-T.; Sun, C.-J.; Liu, C.-J.; Lou, H.-X.; Cheng, A.-X. Molecular basis for chemical evolution of flavones to flavonols and anthocyanins in land plants. Plant Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Jahns, O.; Keck, M.; Tohge, T.; Niehaus, K.; Fernie, A.R.; Weisshaar, B. Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytol. 2010, 188, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Rusak, G.; Cerni, S.; Stupin Polancec, D.; Ludwig-Müller, J. The Responsiveness of the IAA2 Promoter to IAA and IBA is Differentially Affected in Arabidopsis Roots and Shoots by Flavonoids. Biol. Plant. 2010, 54, 403–414. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Thermann, P.; Pieper, K.; Hilgenberg, W. Peroxidase and chitinase isoenzyme activiti.es during root infection of Chinese cabbage with Plasmodiophora brassicae. Physiol. Plant. 1994, 90, 661–670. [Google Scholar] [CrossRef]
- Pitzschke, A.; Fraundorfer, A.; Guggemos, M.; Fuchs, N. Antioxidative responses during germination in quinoa grown in Vitamin B-rich medium. Food Sci. Nutr. 2015, 3, 242–251. [Google Scholar] [CrossRef]
- Bertrand, R.L.; Eze, M.O. Modifying polyacrylamide background color for the nitroblue tetrazolium-based superoxide dismutase staining assay. Adv. Enz. Res. 2014, 2, 77–81. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Ghanem, M.E.; Martinez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Martinez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Prakash, L.; Prathapasenan, G. NaCl-and gibberellic acid-induced changes in the content of auxin and the activities of cellulase and pectin lyase during leaf growth in rice (Oryza sativa). Ann. Bot. 1990, 65, 251–257. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Popko, J.; Hänsch, R.; Mendel, R.R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Pěnčík, A.; Novák, O.; Vujcic, V.; Brkanac, S.R.; Lepedus, H.; Strnad, M.; Salopek-Sondi, B. Short-term salt stress in Brassica rapa seedlings causes alterations in auxin metabolism. Plant Physiol. Biochem. 2018, 125, 74–84. [Google Scholar] [CrossRef]
- Teichmann, T.; Bolu-Arianto, W.H.; Olbrich, A.; Langenfeld-Heyser, R.; Göbel, C.; Grzeganek, P.; Feussner, I.; Hänsch, R.; Polle, A. GH3::GUS reflects cell-specific developmental patterns and stress-induced changes in wood anatomy in the poplar stem. Tree Physiol. 2008, 28, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.G.; Zrÿd, J.-P. The moss Physcomitrella patens, now and then. Plant Physiol. 2001, 127, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Cho, S.H.; Marella, H.; Sakata, Y.; Perroud, P.F.; Pan, A.; Quatrano, R.S. Role of ABA and ABI3 in desiccation tolerance. Science 2010, 327, 546. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.L.; Frank, W.; Sarnighausen, E.; Reski, R. Moss systems biology en route: Phytohormones in Physcomitrella development. Plant Biol. 2006, 8, 397–406. [Google Scholar] [CrossRef]
- Cove, D.; Bezanilla, M.; Harries, P.; Quatrano, R. Mosses as model systems for the study of metabolism and development. Annu. Rev. Plant. Biol. 2006, 57, 497–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prigge, M.J.; Lavy, M.; Ashton, N.W.; Estelle, M. Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr. Biol. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Thelander, M.; Olsson, T.; Ronne, H. Effect of the energy supply on filamentous growth and development in Physcomitrella patens. J. Exp. Bot. 2005, 412, 653–662. [Google Scholar] [CrossRef]
- Glime, J.M. Ecophysiology of Development: Protonemata. In Bryophyte Ecology (Digital Commons@Michigan Tech); Glime, J.M., Ed.; Physiological Ecology, 2013; Volume 1, Chapter 5-3; pp. 1–18. Available online: www.bryoecol.mtu.edu (accessed on 14 January 2021).
- Jang, G.; Dolan, L. Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1 and PpRSL2 in Physcomitrella patens. New Phytol. 2011, 192, 319–327. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-Glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef]
- Dunlap, J.R.; Binzel, M.L. NaCl reduces indole-3-acetic acid levels in the roots of tomato plants independent of stress-induced abscisic acid. Plant Physiol. 1996, 112, 379–384. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Decker, E.L.; Reski, R. Dead end for auxin conjugates in Physcomitrella? Plant Signal. Behav. 2009, 4, 116–118. [Google Scholar] [CrossRef][Green Version]
- Smolko, A.; Ludwig-Müller, J.; Salopek-Sondi, B. Auxin amidohydrolases—From structure to function: Revisited. Croat. Chem. Acta 2018, 91, 233–239. [Google Scholar] [CrossRef]
- Reginato, M.A.; Castagna, A.; Furlán, A.; Castro, S.; Ranieri, A.; Luna, V. Physiological responses of a halophytic shrub to salt stress by Na2SO4 and NaCl: Oxidative damage and the role of polyphenols in antioxidant protection. AoB Plants 2014, 6, plu042. [Google Scholar] [CrossRef] [PubMed]
- Sekmena, A.H.; Türkan, I.; Takio, S. Differential Responses of Antioxidative Enzymes and Lipid Peroxidation to Salt Stress in Salt-Tolerant Plantago maritime and Salt-Sensitive Plantago media. Physiol. Plant. 2007, 131, 399–411. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Ciarkowska, A.; Jakubowska, A. The auxin conjugate indole-3-acetyl-aspartate affects responses to cadmium and salt stress in Pisum sativum L. J. Plant Physiol. 2016, 191, 63–72. [Google Scholar] [CrossRef]
- Higashi, Y.; Takechi, K.; Takano, H.; Takio, S. Maintenance of normal stress tolerance in the moss Physcomitrella patens lacking chloroplastic CuZn-superoxide dismutase. Am. J. Plant Sci. 2015, 6, 591–601. [Google Scholar] [CrossRef]
- Migowska, N.; Stepnowski, P.; Paszkiewicz, M.; Golebiowski, M.; Kumirska, J. Trimethylsilyldiazomethane (TMSD) as a new derivatization reagent for trace analysis of selected non-steroidal anti-inflammatory drugs (NSAIDs) by gas chromatography methods. Anal. Bioanal. Chem. 2010, 397, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Campanella, J.J.; Ludwig-Müller, J.; Bakllamaja, V.; Sharma, V.; Cartier, A. ILR1 and sILR1 IAA amidohydrolase homologs differ in expression pattern and substrate specificity. Plant Growth Regul. 2003, 41, 215–223. [Google Scholar] [CrossRef]
- Cohen, J.D.; Baldi, B.G.; Slovin, J.P. 13C6-[benzene ring]—indole-3-acetic acid. Plant Physiol. 1986, 80, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi Jr., J. A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Qureshi, M.I.; Abdin, M.Z.; Ahmad, J.; Iqbal, M. Effect of long-term salinity on cellular antioxidants, compatible solute and fatty acid profile of Sweet Annie (Artemisia annua L.). Phytochemistry 2013, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Bortz, J.; Schuster, C. Statistik Für Human-und Sozialwissenschaftler; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9783642127700. [Google Scholar]
- Horn, M.; Vollandt, R. Multiple Test- und Auswahlverfahren; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 9783437205101. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koochak, H.; Ludwig-Müller, J. Physcomitrium patens Mutants in Auxin Conjugating GH3 Proteins Show Salt Stress Tolerance but Auxin Homeostasis Is Not Involved in Regulation of Oxidative Stress Factors. Plants 2021, 10, 1398. https://doi.org/10.3390/plants10071398
Koochak H, Ludwig-Müller J. Physcomitrium patens Mutants in Auxin Conjugating GH3 Proteins Show Salt Stress Tolerance but Auxin Homeostasis Is Not Involved in Regulation of Oxidative Stress Factors. Plants. 2021; 10(7):1398. https://doi.org/10.3390/plants10071398
Chicago/Turabian StyleKoochak, Haniyeh, and Jutta Ludwig-Müller. 2021. "Physcomitrium patens Mutants in Auxin Conjugating GH3 Proteins Show Salt Stress Tolerance but Auxin Homeostasis Is Not Involved in Regulation of Oxidative Stress Factors" Plants 10, no. 7: 1398. https://doi.org/10.3390/plants10071398
APA StyleKoochak, H., & Ludwig-Müller, J. (2021). Physcomitrium patens Mutants in Auxin Conjugating GH3 Proteins Show Salt Stress Tolerance but Auxin Homeostasis Is Not Involved in Regulation of Oxidative Stress Factors. Plants, 10(7), 1398. https://doi.org/10.3390/plants10071398






