High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage
Abstract
1. Introduction
2. Results
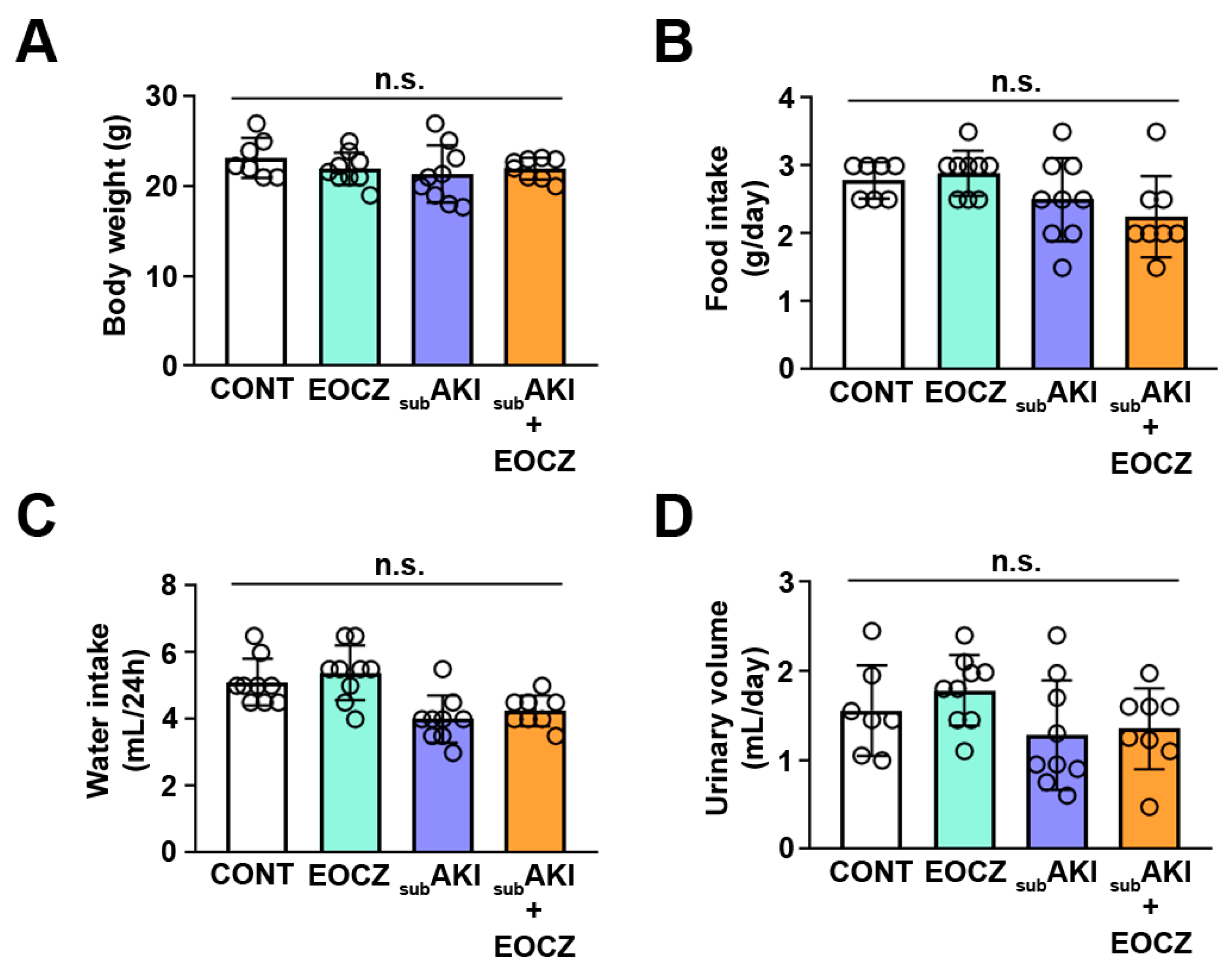
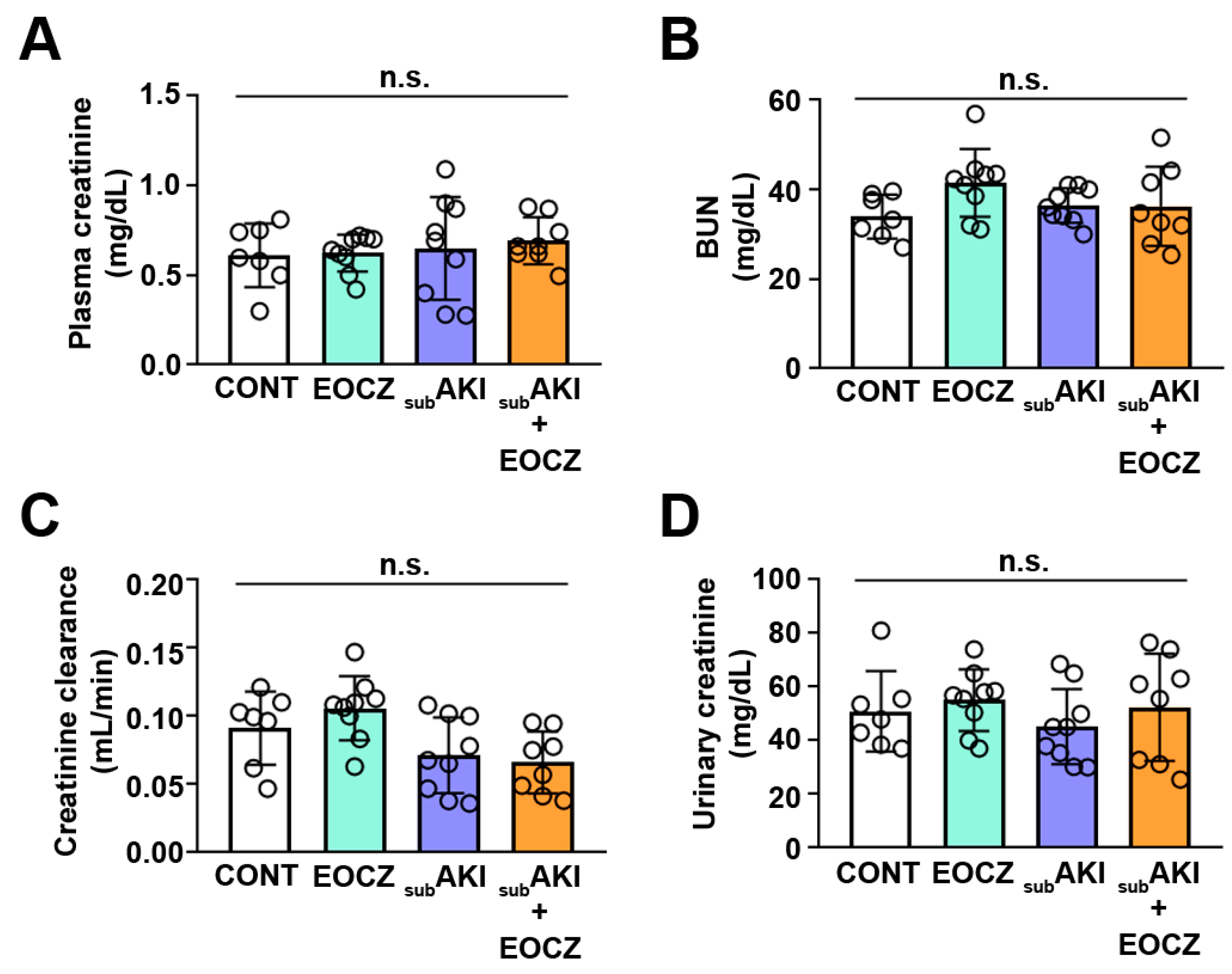
2.1. EOCZ Treatment Induces Changes in Renal Function Parameters
2.2. Urinary Protein Excretion Is Modulated by EOCZ Treatment
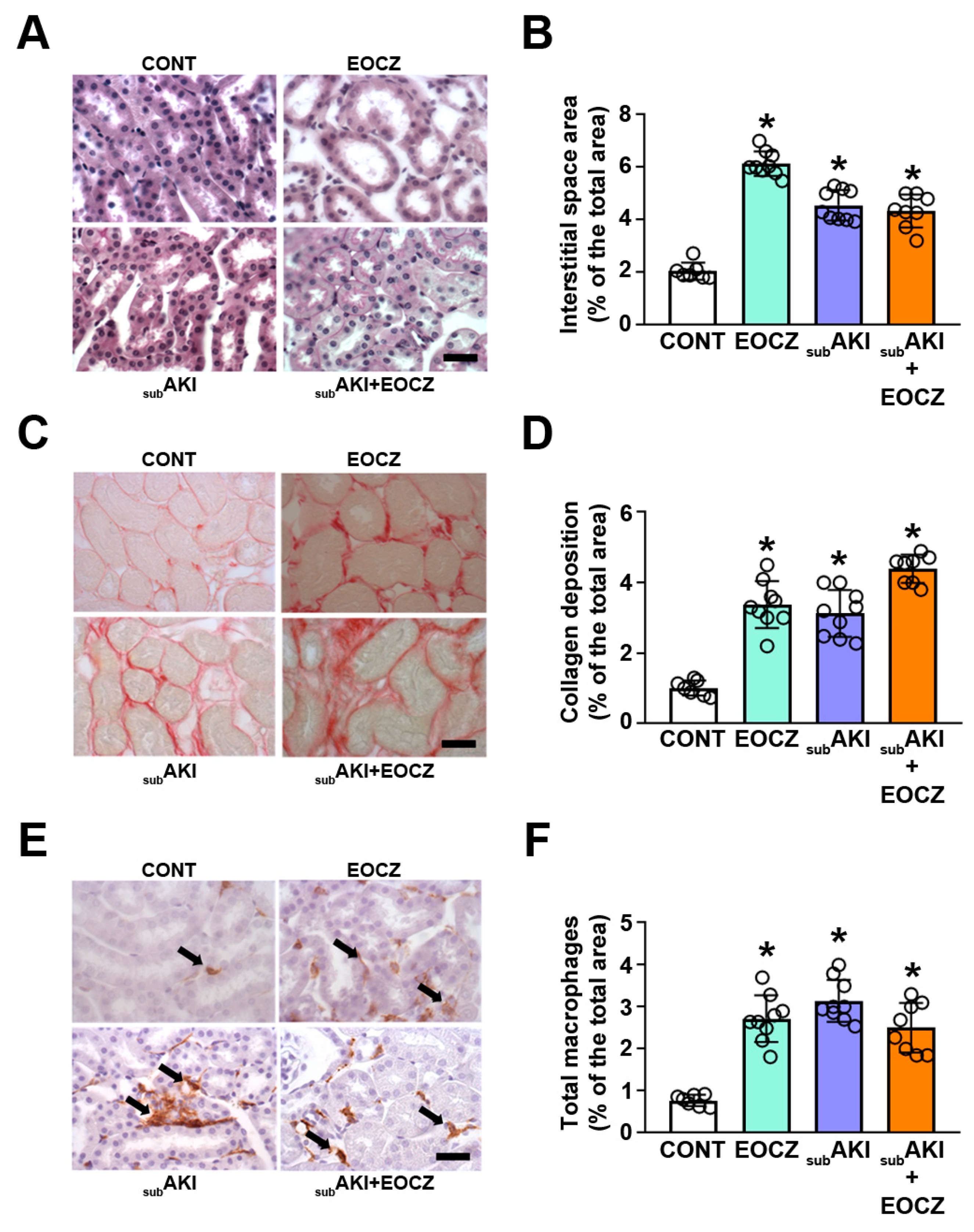
2.3. EOCZ Treatment Promotes Tubulointerstitial Injury
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Chromatographic Analysis of EOCZ
4.3. Animals and Experimental Protocol
4.4. Renal Function Analysis
4.5. Histologic and Immunohistochemical Analyses
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Fang, F.; Hu, X.; Dai, X.; Wang, S.; Bai, Z.; Chen, J.; Pan, J.; Li, X.; Wang, J.; Li, Y. Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children. Crit. Care 2018, 22, 256. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef] [PubMed]
- Theilig, F. Spread of glomerular to tubulointerstitial disease with a focus on proteinuria. Ann. Anat. 2010, 192, 125–132. [Google Scholar] [CrossRef]
- Gorriz, J.L.; Martinez-Castelao, A. Proteinuria: Detection and role in native renal disease progression. Transplant. Rev. (Orlando) 2012, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Portella, V.G.; Silva-Filho, J.L.; Landgraf, S.S.; de Rico, T.B.; Vieira, M.A.; Takiya, C.M.; Souza, M.C.; Henriques, M.G.; Canetti, C.; Pinheiro, A.A.; et al. Sepsis-surviving mice are more susceptible to a secondary kidney insult. Crit. Care Med. 2013, 41, 1056–1068. [Google Scholar] [CrossRef]
- Abreu, T.P.; Silva, L.S.; Takiya, C.M.; Souza, M.C.; Henriques, M.G.; Pinheiro, A.A.; Caruso-Neves, C. Mice rescued from severe malaria are protected against renal injury during a second kidney insult. PLoS ONE 2014, 9, e93634. [Google Scholar] [CrossRef]
- Landgraf, S.S.; Silva, L.S.; Peruchetti, D.B.; Sirtoli, G.M.; Moraes-Santos, F.; Portella, V.G.; Silva-Filho, J.L.; Pinheiro, C.S.; Abreu, T.P.; Takiya, C.M.; et al. 5-Lypoxygenase products are involved in renal tubulointerstitial injury induced by albumin overload in proximal tubules in mice. PLoS ONE 2014, 9, e107549. [Google Scholar] [CrossRef]
- Peruchetti, D.B.; Silva-Filho, J.L.; Silva-Aguiar, R.P.; Teixeira, D.E.; Takiya, C.M.; Souza, M.C.; Henriques, M.D.G.; Pinheiro, A.A.S.P.; Caruso-Neves, C. IL-4 receptor α chain protects the kidney against tubule-interstitial injury induced by albumin overload. Front. Physiol. 2020, 11, 172. [Google Scholar] [CrossRef]
- Teixeira, D.E.; Peruchetti, D.B.; Silva, L.S.; Silva-Aguiar, R.P.; Oquendo, M.B.; Silva-Filho, J.L.; Takiya, C.M.; Leal-Cardoso, J.H.; Pinheiro, A.A.S.P.; Caruso-Neves, C. Lithium ameliorates tubule-interstitial injury through activation of the mTORC2/protein kinase B pathway. PLoS ONE 2019, 14, e0215871. [Google Scholar] [CrossRef]
- Ishola, D.A., Jr.; van der Giezen, D.M.; Hahnel, B.; Goldschmeding, R.; Kriz, W.; Koomans, H.A.; Joles, J.A. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol. Dial. Transplant. 2006, 21, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Batatinha, M.J.; de Souza-Spinosa, H.; Bernardi, M.M. Croton zehntneri: Possible central nervous system effects of the essential oil in rodents. J. Ethnopharmacol. 1995, 45, 53–57. [Google Scholar] [CrossRef]
- Leal-Cardoso, J.H.; Fonteles, M.C. Pharmacological effects of essential oils of plants of the northeast of Brazil. An. Acad. Bras. Cienc. 1999, 71, 207–213. [Google Scholar] [PubMed]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Essential oil of Croton zehntneri prevents conduction alterations produced by diabetes mellitus on vagus nerve. Plants (Basel) 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, R.O.; Morais, S.M.; Brito, E.H.; Brilhante, R.S.; Cordeiro, R.A.; Nascimento, N.R.; Kerntopf, M.R.; Sidrim, J.J.; Rocha, M.F. Antifungal activity of essential oils of Croton species from the Brazilian Caatinga biome. J. Appl. Microbiol. 2008, 104, 1383–1390. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Leal-Cardoso, J.H.; Santos, C.F.; Morais, S.M.; Coelho-de-Souza, S.M. Antinociceptive effects of the essential oil of Croton zehntneri in mice. Braz. J. Med. Biol. Res. 2011, 34, 1471–1474. [Google Scholar] [CrossRef]
- Ponte, E.L.; Sousa, P.L.; Rocha, M.V.; Soares, P.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.; Assreuy, A.M. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol. Rep. 2012, 264, 984–990. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Lahlou, S.; Barreto, J.E.; Yum, M.E.; Oliveira, A.C.; Oliveira, H.D.; Celedônio, N.R.; Feitosa, R.G.; Duarte, G.P.; Santos, C.F.; et al. Essential oil of Croton zehntneri and its major constituent anethole display gastroprotective effect by increasing the surface mucous layer. Fundam. Clin. Pharmacol. 2013, 27, 288–298. [Google Scholar] [CrossRef]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Albuquerque, A.A.; do Vale, O.C.; Leal-Cardoso, J.H. Essential oil of Croton zehntneri and its main constituent anethole block excitability of rat peripheral nerve. Planta Med. 2015, 81, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, J.M.; Leal-Cardoso, J.H.; Diniz, L.R.; Portella, V.G.; Costa, C.O.; Linard, C.F.; Alves, K.; Rocha, M.V.; Lima, C.C.; Cecatto, V.M.; et al. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J. Ethnopharmacol. 2012, 144, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.W.; Ellenhorn, M.J.; Hulbert, T.V.; McCarron, M. Clove oil ingestion in an infant. Hum. Exp. Toxicol. 1991, 10, 291–294. [Google Scholar] [CrossRef]
- Chen, X.; Serag, E.S.; Sneed, K.B.; Zhou, S. Herbal bioactivation, molecular targets and the toxicity relevance. Chem. Biol. Interact. 2011, 192, 161–176. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Boersma, M.G.; van der Woude, H.; Jeurissen, S.M.; Schutte, M.E.; Alink, G.M. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. 2005, 574, 124–138. [Google Scholar] [CrossRef]
- BIBRA. Toxicity Profile for Eucalyptus Oil; BIBRA Toxicology International: London, UK, 1991. [Google Scholar]
- Kristiansen, E.; Madsen, C. Induction of protein droplet (alpha 2 mu-globulin) nephropathy in male rats after short-term dosage with 1,8-cineole and l-limonene. Toxicol. Lett. 1995, 80, 147–152. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Silano, M.; De Vincenzi, A.; Maialetti, F.; Scazzocchio, B. Constituents of aromatic plants: Eucalyptol. Fitoterapia 2002, 73, 269–275. [Google Scholar] [CrossRef]
- Gekle, M. Renal tubule albumin transport. Annu. Rev. Physiol. 2005, 67, 573–594. [Google Scholar] [CrossRef]
- Landgraf, S.S.; Wengert, M.; Silva, J.S.; Zapata-Sudo, G.; Sudo, R.T.; Takiya, C.M.; Pinheiro, A.A.; Caruso-Neves, C. Changes in angiotensin receptors expression play a pivotal role in the renal damage observed in spontaneously hypertensive rats. Am. J. Physiol. Renal Physiol. 2011, 300, F499–F510. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.H.; de Morais Campos, R.; Fonteles, M.C.; Santos, C.F.; Leal-Cardoso, J.H.; do Nascimento, N.R. Effects of the essential oil of Croton zehntneri and its major components, anethole and estragole, on the rat corpora cavernosa. Life Sci. 2014, 112, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.S.; Moreira Gomes, M.D.; Cavalcante, F.S.Á.; Leal-Cardoso, J.H. Essential oil of Croton zehntneri attenuates lung injury in the OVA-induced asthma model. J. Asthma 2019, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I. Chinese herbal medicines, aristolochic acid and Balkan endemic nephropathy. Occup. Environ. Med. 2011, 68, 237. [Google Scholar] [CrossRef]
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef]
- De Jonge, H.; Vanrenterghem, Y. Aristolochic acid: The common culprit of Chinese herbs nephropathy and Balkan endemic nephropathy. Nephrol. Dial. Transplant. 2008, 23, 39–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Schmeiser, H.H.; Stiborova, M.; Arlt, V.M. Chemical and molecular basis of the carcinogenicity of Aristolochia plants. Curr. Opin. Drug Discov. Devel. 2009, 12, 141–148. [Google Scholar]
- Aprotosoaie, A.C.; Costache, I.; Miron, A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [CrossRef]
- Aydemir, D.; Öztaşcı, B.; Barlas, N.; Ulusu, N.N. Effects of butylparaben on antioxidant enzyme activities and histopathological changes in rat tissues. Arh. Hig. Rada Toksikol. 2019, 70, 315–324. [Google Scholar] [CrossRef]
- Luft, F.C. Biomarkers and predicting acute kidney injury. Acta Physiol. (Oxf). 2021, 231, e13479. [Google Scholar] [CrossRef]
- Murugan, R.; Kellum, J.A. Acute kidney injury: What’s the prognosis? Nat. Rev. Nephrol. 2011, 7, 209–217. [Google Scholar] [CrossRef]
- Wang, K.; Kestenbaum, B. Proximal Tubular Secretory Clearance: A Neglected Partner of Kidney Function. Clin. J. Am. Soc. Nephrol. 2018, 13, 1291–1296. [Google Scholar] [CrossRef]
- Palmer, L.G.; Schnermann, J. Integrated control of Na transport along the nephron. Clin. J. Am. Soc. Nephrol. 2015, 10, 676–687. [Google Scholar] [CrossRef]
- Vallon, V. Tubular transport in acute kidney injury: Relevance for diagnosis, prognosis and intervention. Nephron 2016, 134, 160–166. [Google Scholar] [CrossRef]
- Queiroz-Madeira, E.P.; Lara, L.S.; Wengert, M.; Landgraf, S.S.; Líbano-Soares, J.D.; Zapata-Sudo, G.; Sudo, R.T.; Takiya, C.M.; Gomes-Quintana, E.; Lopes, A.G.; et al. Na(+)-ATPase in spontaneous hypertensive rats: Possible AT(1) receptor target in the development of hypertension. Biochim. Biophys. Acta 2010, 1798, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Chen, J.; Yang, W.; Appel, L.J.; Kusek, J.W.; Alper, A.; Delafontaine, P.; Keane, M.G.; Mohler, E.; Ojo, A.; et al. Chronic Renal Insufficiency Cohort (CRIC) study investigators, Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 2016, 315, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Peruchetti, D.B.; Freitas, A.C.; Pereira, V.C.; Lopes, J.V.; Takiya, C.M.; Nascimento, N.R.F.; Pinheiro, A.A.S.P.; Caruso-Neves, C. PKB is a central molecule in the modulation of Na+-ATPase activity by albumin in renal proximal tubule cells. Arch. Biochem. Biophys. 2019, 674, 108115. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.F.; Peruchetti, D.B.; Sirtoli, G.M.; Takiya, C.M.; Pinheiro, A.A.S.; Leal-Cardoso, J.H.; Caruso-Neves, C. High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage. Plants 2021, 10, 1400. https://doi.org/10.3390/plants10071400
Silva KF, Peruchetti DB, Sirtoli GM, Takiya CM, Pinheiro AAS, Leal-Cardoso JH, Caruso-Neves C. High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage. Plants. 2021; 10(7):1400. https://doi.org/10.3390/plants10071400
Chicago/Turabian StyleSilva, Katarine F., Diogo B. Peruchetti, Gabriela M. Sirtoli, Christina M. Takiya, Ana Acacia S. Pinheiro, José Henrique Leal-Cardoso, and Celso Caruso-Neves. 2021. "High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage" Plants 10, no. 7: 1400. https://doi.org/10.3390/plants10071400
APA StyleSilva, K. F., Peruchetti, D. B., Sirtoli, G. M., Takiya, C. M., Pinheiro, A. A. S., Leal-Cardoso, J. H., & Caruso-Neves, C. (2021). High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage. Plants, 10(7), 1400. https://doi.org/10.3390/plants10071400






