A Microencapsulated Mixture of Eugenol and Garlic Tincture Supplementation Mitigates the Effect of Necrotic Enteritis on Intestinal Integrity and Increases Goblet Cells in Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Design and Husbandry
2.3. Dietary Treatments
2.4. Necrotic Enteritis Challenge
2.5. Sampling and FITC-d Inoculation
2.6. Serum FITC-d Measurement
2.7. Histomorphology
2.8. RNA Extraction and cDNA Synthesis
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Extraction of Ileal Bacterial DNA
2.11. Extraction of Cecal Bacterial DNA
2.12. Quantification of Ileal and Caecal Bacterial DNA
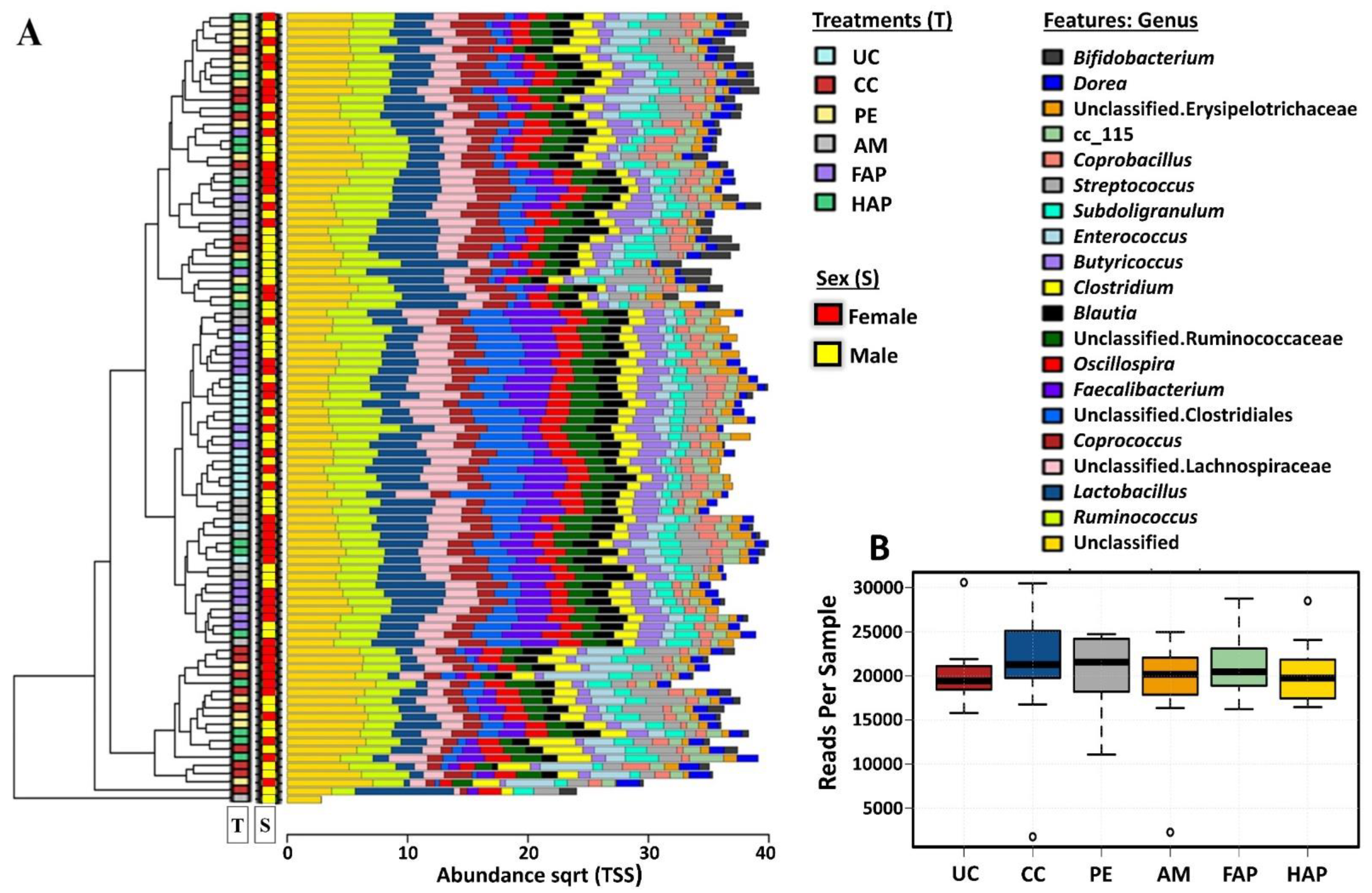
2.13. 16S rRNA Gene Sequencing and Data Analysis
2.14. Data Analysis
3. Results
3.1. Serum FITC-d Concentration
3.2. Histomorphology and Goblet Cell Number
3.3. Jejunal Gene Expression on d 8
3.4. Jejunal Gene Expression on d 16
3.5. Ileal Bacterial Load by qPCR
3.6. Caecal Bacterial Load by qPCR
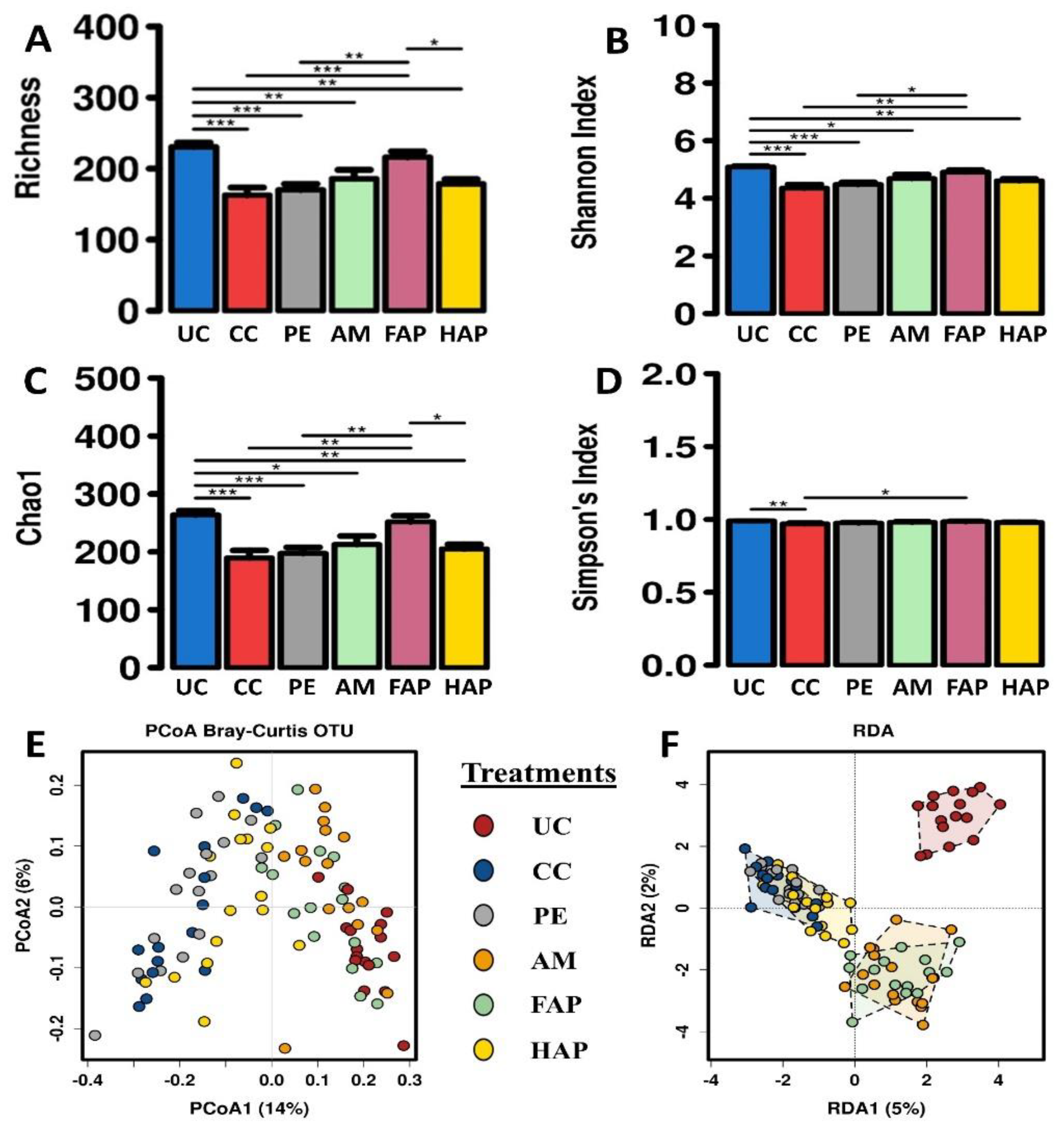
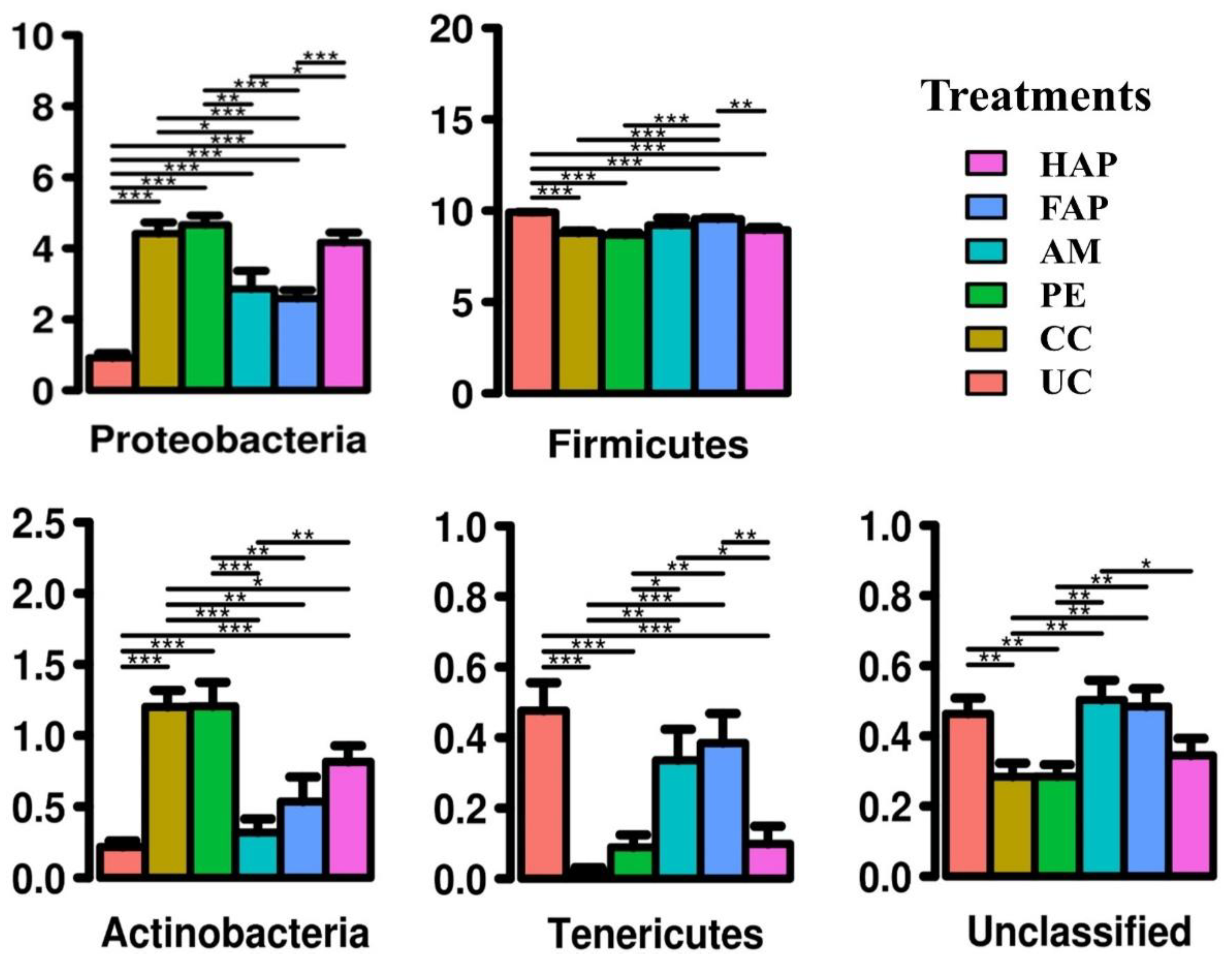
3.7. Caecal Microbiota Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. Poult. World 2015, 31, 16–17. [Google Scholar]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef]
- Annett, C.; Viste, J.; Chirino-Trejo, M.; Classen, H.; Middleton, D.; Simko, E. Necrotic enteritis: Effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002, 31, 598–601. [Google Scholar] [CrossRef]
- Moran, E.T. Intestinal events and nutritional dynamics predispose Clostridium perfringens virulence in broilers. Poult. Sci. 2014, 93, 3028–3036. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Schneitz, C.; Hofshagen, M.; Skjerve, E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001, 45, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Morgan, N.; Swick, R.; Choct, M.; Wu, S. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult. Sci. 2019, 98, 6422–6432. [Google Scholar] [CrossRef]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018, 5, 199. [Google Scholar] [CrossRef]
- Keerqin, C.; Rhayat, L.; Zhang, Z.-H.; Gharib-Naseri, K.; Kheravii, S.; Devillard, E.; Crowley, T.; Wu, S.-B. Probiotic Bacillus subtilis 29,784 improved weight gain and enhanced gut health status of broilers under necrotic enteritis condition. Poult. Sci. 2021, 100, 100981. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Benestad, S.L.; Løvland, A. Epidemiologic aspects of necrotic enteritis in broiler chickens–disease occurrence and production performance. Avian Pathol. 2016, 45, 271–274. [Google Scholar] [CrossRef]
- Kocher, A.; Choct, M. Improving Broiler Chicken Performance: The Efficacy of Organic Acids, Prebiotics and Enzymes in Controlling Necrotic Enterities; Australian Government, Rural Industries Research and Development Corporation: Barton, Australia, 2008.
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Bicrobiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Redondo, L.M.; Redondo, E.A.; Dominguez, J.E.; Chacana, A.; Fernandez Miyakawa, M.E. Use of plant extracts as an effective manner to control Clostridium perfringens induced necrotic enteritis in poultry. BioMed. Res. Int. 2016, 2016, 3278359. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Ni, X.; Gong, J.; Yu, H.; Tsao, R.; Han, Y.; Chambers, J. Antimicrobial activity of essential oils and structurally related synthetic food additives towards Clostridium perfringens. J. Appl. Microbiol. 2009, 106, 213–220. [Google Scholar] [CrossRef]
- Applegate, T.; Klose, V.; Steiner, T.; Ganner, A.; Schatzmayr, G. Probiotics and phytogenics for poultry: Myth or reality? J. Appl. Poult. Res. 2010, 19, 194–210. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Gaafar, K.M.; Elbahy, N.; Helal, M.A.; Mahboub, H.D.; Sameh, G. Bioactive effect of dietary supplementation with essential oils blend of oregano, thyme and garlic oils on performance of broilers infected with Eimeria species. Glob. Vet. 2014, 13, 977–985. [Google Scholar]
- Kirubakaran, A.; Moorthy, M.; Chitra, R.; Prabakar, G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet. World 2016, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, K.J.; Omar, J.A. Effect of garlic powder on performance and lipid profile of broilers. Open J. Anim. Sci. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.; Spais, A. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, E.; Skoufos, I.; Marugan-Hernandez, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Blake, D.; Tzora, A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, faecal oocyst output and intestinal microbiota in vivo. Front. Vet. Sci. 2020, 7, 420. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Rose, S.; Catherine, I.; Blanchard, A. Phytogenic feed additives can alleviate the negative impact of necrotic enteritis in broilers. In Proceedings of the 6th International Conference on Poultry Intestinal Health, Rome, Italy, 3–5 April 2019. [Google Scholar]
- NHMRC. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, Australia, 2013.
- Kumar, A.; Sharma, N.K.; Kheravii, S.K.; Keerqin, C.; Ionescu, C.; Blanchard, A.; Wu, S.-B. Potential of a microencapsulated mixture of eugenol and garlic tincture to improve performance and intestinal health in broilers under necrotic enteritis challenge. Anim. Nutr. 2021. under review. [Google Scholar] [CrossRef]
- England, A.; Kheravii, S.; Musigwa, S.; Kumar, A.; Daneshmand, A.; Sharma, N.; Gharib-Naseri, K.; Wu, S. Sexing chickens (Gallus gallus domesticus) with high resolution melting analysis using feather crude DNA. Poult. Sci. 2020, 100, 100924. [Google Scholar] [CrossRef]
- Cobb500. Cobb Broiler Management Guide; Cobb-Vantress: Siloam Springs, AR, USA, 2018. [Google Scholar]
- Cobb500. Broiler Performance and Nutrition Supplement; Cobb-Vantress, Inc.: Siloam Springs, AR, USA, 2018. [Google Scholar]
- Rodgers, N.J.; Swick, R.A.; Geier, M.S.; Moore, R.J.; Choct, M.; Wu, S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015, 59, 38–45. [Google Scholar] [CrossRef]
- Golder, H.; Geier, M.; Forder, R.; Hynd, P.; Hughes, R. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br. Poult. Sci. 2011, 52, 500–506. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hirose, H.; Onizuka, A.; Hayashi, M.; Futamura, N.; Kawamura, Y.; Ezaki, T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000, 94, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Roberts, J.; Wu, S.-B. Downregulation of ALAS1 by nicarbazin treatment underlies the reduced synthesis of protoporphyrin IX in shell gland of laying hens. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Hellemans, J.; Mortier, G.; de Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Swick, R.A.; Choct, M.; Wu, S.-B. Differential expression of intestinal genes in necrotic enteritis challenged broiler chickens with two different Clostridium perfringens strains. Poult. Sci. 2021, 100, 100886. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kheravii, S.K.; Li, L.; Wu, S.-B. Monoglycerides blend reduces mortality, improves nutrient digestibility and intestinal health in broilers subjected to clinical necrotic enteritis challenge. Animals 2021, 11, 1432. [Google Scholar] [CrossRef]
- Fan, X.; Liu, S.; Liu, G.; Zhao, J.; Jiao, H.; Wang, X.; Song, Z.; Lin, H. Vitamin a deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS ONE 2015, 10, e0139131. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.P.; Bang, D.D.; Handberg, K.J.; Jorgensen, P.H.; Zhang, M.F. Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus. Vet. Microbiol. 2005, 110, 155–165. [Google Scholar] [CrossRef]
- Bagés, S.; Estany, J.; Tor, M.; Pena, R. Investigating reference genes for quantitative real-time PCR analysis across four chicken tissues. Gene 2015, 561, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchipudi, S.V.; Tellabati, M.; Nelli, R.K.; White, G.A.; Perez, B.B.; Sebastian, S.; Slomka, M.J.; Brookes, S.M.; Brown, I.H.; Dunham, S.P. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol. J. 2012, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kheravii, S.; Swick, R.A.; Choct, M.; Wu, S.-B. Dietary sugarcane bagasse and coarse particle size of corn are beneficial to performance and gizzard development in broilers fed normal and high sodium diets. Poult. Sci. 2017, 96, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S.-B. Organic acid blends improve intestinal integrity, modulate short-chain fatty acids profiles and alter microbiota of broilers under necrotic enteritis challenge. Anim. Nutr. 2021, in press. [Google Scholar]
- Wise, M.G.; Siragusa, G.R. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poult. Sci. 2017, 96, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Requena, T.; Burton, J.; Matsuki, T.; Munro, K.; Simon, M.A.; Tanaka, R.; Watanabe, K.; Tannock, G.W. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 2002, 68, 2420–2427. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.; McKay, L.; Williams, D.; Garrett, V.; Gentry, R.; Sayler, G. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 2006, 72, 4214–4224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, D.; Yu, B.; He, J.; Yu, J.; Mao, X.; Wang, J.; Luo, J.; Huang, Z.; Cheng, G. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J. Anim. Sci. 2015, 93, 2967–2976. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef] [Green Version]
- Wise, M.G.; Siragusa, G.R. Quantitative detection of Clostridium perfringens in the broiler fowl gastrointestinal tract by real-time PCR. Appl. Environ. Microbiol. 2005, 71, 3911–3916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-H.; Zo, Y.-G.; Kim, S.-J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics, Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High resolution sample inference from amplicon data. bioRxiv 2015, 024034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformation 2017, 33, 782–783. [Google Scholar] [CrossRef] [Green Version]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. Ther. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Wu, S.-B.; Rodgers, N.; Swick, R.A.; Moore, R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS ONE 2014, 9, e104739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-B.; Rodgers, N.J.; Cui, G.; Sun, Y.; Choct, M. Dynamics of intestinal metabolites and morphology in response to necrotic enteritis challenge in broiler chickens. Avian Pathol. 2016, 45, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Eeckhaut, V.; Van Driessche, K.; Onrust, L.; Haesebrouck, F.; Ducatelle, R.; Moore, R.J.; Van Immerseel, F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016, 45, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [Green Version]
- Phong, S.; Shanmugavelu, S.; Thayalini, K.; Noraini, S.; Wong, H. Detection of Lactobacillus, Bacteroides and Clostridium perfringens in the gastrointestinal contents of chicken fed different diets by real-time PCR. J. Trop. Agric. Food Sci. 2010, 38, 81–87. [Google Scholar]
- Kleessen, B.; Kroesen, A.; Buhr, H.; Blaut, M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand. J. Gastroenterol. 2002, 37, 1034–1041. [Google Scholar] [CrossRef]

| Treatments 1 | Additives | Inclusion Level; Starter (d 0 to 9), Grower (d 9 to 21) and Finisher (d 21 to 35) Phases, ppm | Necrotic Enteritis Challenge 2 |
|---|---|---|---|
| UC | - | - | Unchallenged |
| CC | - | - | Challenged |
| PE | Plant extract | 100 | Challenged |
| AM | Antimicrobial | 50 of narasin and nicarbasin | Challenged |
| FAP | AM full dose + PE | 50 + 100 | Challenged |
| HAP | AM half dose + PE | 25 + 100 | Challenged |
| Item | Sequence | Size (pb) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| TJP1 | F-GGATGTTTATTTGGGCGGC R-GTCACCGTGTGTTGTTCCCAT | 187 | 60 | Gharib-Naseri et al. [41] |
| OCLN | F-ACGGCAGCACCTACCTCAA R-GGGCGAAGAAGCAGATGAG | 123 | 60 | Du et al. [42] |
| CLDN1 | F-CTTCATCATTGCAGGTCTGTCAG R-AAATCTGGTGTTAACGGGTGTG | 103 | 60 | Gharib-Naseri et al. [41] |
| CLDN5 | F-GCAGGTCGCCAGAGATACAG R-CCACGAAGCCTCTCATAGCC | 162 | 61 | Kumar et al. [43] |
| JAM2 | F-AGACAGGAACAGGCAGTGCTAG R-ATCCAATCCCATTTGAGGCTAC | 135 | 60 | Kumar et al. [43] |
| MUC2 | F-CCCTGGAAGTAGAGGTGACTG R-TGACAAGCCATTGAAGGACA | 143 | 60 | Fan et al. [44] |
| Reference Genes on d 8 | ||||
| TBP | F-TAGCCCGATGATGCCGTAT R-GTTCCCTGTGTCGCTTGC | 66 | 61 | Li et al. [45] |
| YWHAZ | F-TTGCTGCTGGAGATGACAAG R-CTTCTTGATACGCCTGTTG | 61 | 60 | Bagés et al. [46] |
| Reference Genes on d 16 | ||||
| GAPDH | F: GAAGCTTACTGGAATGGCTTTCC R: CGGCAGGTCAGGTCAACAA | 66 | 61 | Kuchipudi et al. [47] |
| TBP | F-TAGCCCGATGATGCCGTAT R-GTTCCCTGTGTCGCTTGC | 147 | 62 | Li et al. [45] |
| Bacteria Group | Primer Sequence (5′→3′) | Annealing Temperature (°C) | Reference |
|---|---|---|---|
| Lactobacillus spp. | F-CAC CGC TAC ACA TGG AG R-AGC AGT AGG GAA TCT TCC A | 63 | Rinttilä et al. [52] |
| Bifidobacterium spp. | F-GCG TCC GCT GTG GGC R-CTT CTC CGG CAT GGT GTT G | 63 | Requena et al. [53] |
| Bacteroides spp. | F-GAG AGG AAG GTC CCC CAC R-CGC TAC TTG GCT GGT TCA G | 63 | Layton et al. [54] |
| Bacillus spp. | F-GCA ACG AGC GCA ACC CTT GA R-TCA TCC CCA CCT TCC TCC GGT | 63 | Zhang et al. [55] |
| Ruminococcus spp. | F-GGC GGC YTR CTG GGC TTT R-CCA GGT GGA TWA CTT ATT GTG TTA A | 63 | Ramirez-Farias et al. [56] |
| Enterobacteriaceae | F-CAT TGA CGT TAC CCG CAG AAG AAG C R-CTC TAC GAG ACT CAA GCT TGC | 63 | Bartosch et al. [57] |
| Clostridium perfringens | F-ATG CAA GTC GAG CGA KG R-TAT GCG GTA TTA ATC TYC CTT T TaqMan Probe-5′-FAM-TCA TCA TTC AAC CAA AGG AGC AAT CC-TAMRA-3′ | 60 | Rinttilä et al. [52]; Wise and Siragusa [58] |
| Total Bacteria | F-CGG YCC AGA CTC CTA CGG G R-TTA CCG CGG CTG CTG GCA C | 63 | Lee et al. [59] |
| Treatment 2 | Serum FITC-d |
|---|---|
| Experimental treatment | |
| UC | 0.221 d |
| CC | 0.647 a |
| PE | 0.558 b |
| AM | 0.458 c |
| FAP | 0.428 c |
| HAP | 0.548 b |
| SEM | 0.018 |
| Sex | |
| Male | 0.462 |
| Female | 0.491 |
| SEM | 0.011 |
| p-value | |
| Experimental treatment | <0.001 |
| Sex | 0.064 |
| Experimental treatment × Sex | 0.502 |
| Item | UC | NE Challenged 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CC | PE | AM | FAP | HAP | ||||
| d 8 (before NE challenge) | ||||||||
| VH, μm | 1471 | 1460 | 1514 | 1480 | 1523 | 1495 | 32 | 0.715 |
| CD, μm | 219 | 218 | 209 | 222 | 217 | 210 | 8 | 0.789 |
| VW, μm | 149 | 146 | 149 | 148 | 149 | 147 | 2 | 0.788 |
| VH:CD | 6.75 | 6.76 | 7.31 | 6.72 | 7.03 | 7.17 | 0.20 | 0.198 |
| VSA, mm 2 | 0.686 | 0.669 | 0.707 | 0.686 | 0.714 | 0.687 | 0.015 | 0.323 |
| Goblet cell number/villus | 89.7 c | 90.3 b,c | 93.5 a–c | 92.7 a–c | 96.3 a | 94.9 a,b | 1.6 | 0.041 |
| d 16 (after NE challenge) | ||||||||
| VH, μm | 1685 a | 1057 d | 1153 c,d | 1306 b | 1316 b | 1290 b,c | 35 | <0.001 |
| CD, μm | 236 c | 365 a,b | 349 b | 391 a | 396 a | 372 a,b | 14 | <0.001 |
| VW, μm | 172 d | 212 c | 215 b,c | 230 a,b | 245 a | 222 b,c | 6 | <0.001 |
| VH:CD | 7.23 a | 2.94 c | 3.36 b,c | 3.34 b,c | 3.33 b,c | 3.48 b | 0.17 | <0.001 |
| VSA, mm 2 | 0.909 a,b | 0.703 c | 0.780 b,c | 0.942 a | 1.015 a | 0.897 a,b | 0.032 | <0.001 |
| Goblet cell number/villus | 92.4 a | 59.7 d | 68.1 c | 74.8 b,c | 77.8 b | 75.7 b,c | 2.8 | <0.001 |
| Treatment | CLDN1 | MUC2 | OCLN | TJP1 |
|---|---|---|---|---|
| Experimental treatment | ||||
| UC | 1.025 | 1.187 | 1.122 | 1.142 |
| CC | 1.023 | 1.140 | 1.117 | 1.057 |
| PE | 1.028 | 0.965 | 1.031 | 1.143 |
| AM | 1.057 | 1.145 | 0.989 | 1.027 |
| FAP | 1.075 | 1.063 | 0.976 | 1.091 |
| HAP | 1.133 | 1.096 | 1.187 | 1.074 |
| SEM | 0.094 | 0.111 | 0.096 | 0.109 |
| Sex | ||||
| Male | 1.015 | 1.121 | 1.155 a | 0.988 b |
| Female | 1.099 | 1.077 | 0.985 b | 1.190 a |
| SEM | 0.055 | 0.064 | 0.055 | 0.063 |
| p-value | ||||
| Experimental treatment | 0.959 | 0.777 | 0.568 | 0.968 |
| Sex | 0.278 | 0.633 | 0.033 | 0.026 |
| Experimental treatment × Sex | 0.996 | 0.563 | 0.569 | 0.381 |
| Treatment | CLDN1 | TJP1 | JAM2 |
|---|---|---|---|
| Experimental treatment | |||
| UC | 0.540 b | 1.148 | 0.818 d |
| CC | 1.429 a | 0.986 | 1.198 a |
| PE | 1.312 a | 1.143 | 1.120 a,b |
| AM | 1.181 a | 1.163 | 0.893 c,d |
| FAP | 1.151 a | 1.154 | 1.008 b,c |
| HAP | 1.295 a | 1.157 | 1.096 a,b |
| SEM | 0.128 | 0.090 | 0.060 |
| Sex | |||
| Male | 1.153 | 1.226 a | 1.050 |
| Female | 1.150 | 1.024 b | 0.989 |
| SEM | 0.074 | 0.052 | 0.035 |
| p-value | |||
| Experimental treatment | <0.001 | 0.721 | <0.001 |
| Sex | 0.965 | 0.008 | 0.214 |
| Experimental treatment × Sex | 0.151 | 0.723 | 0.085 |
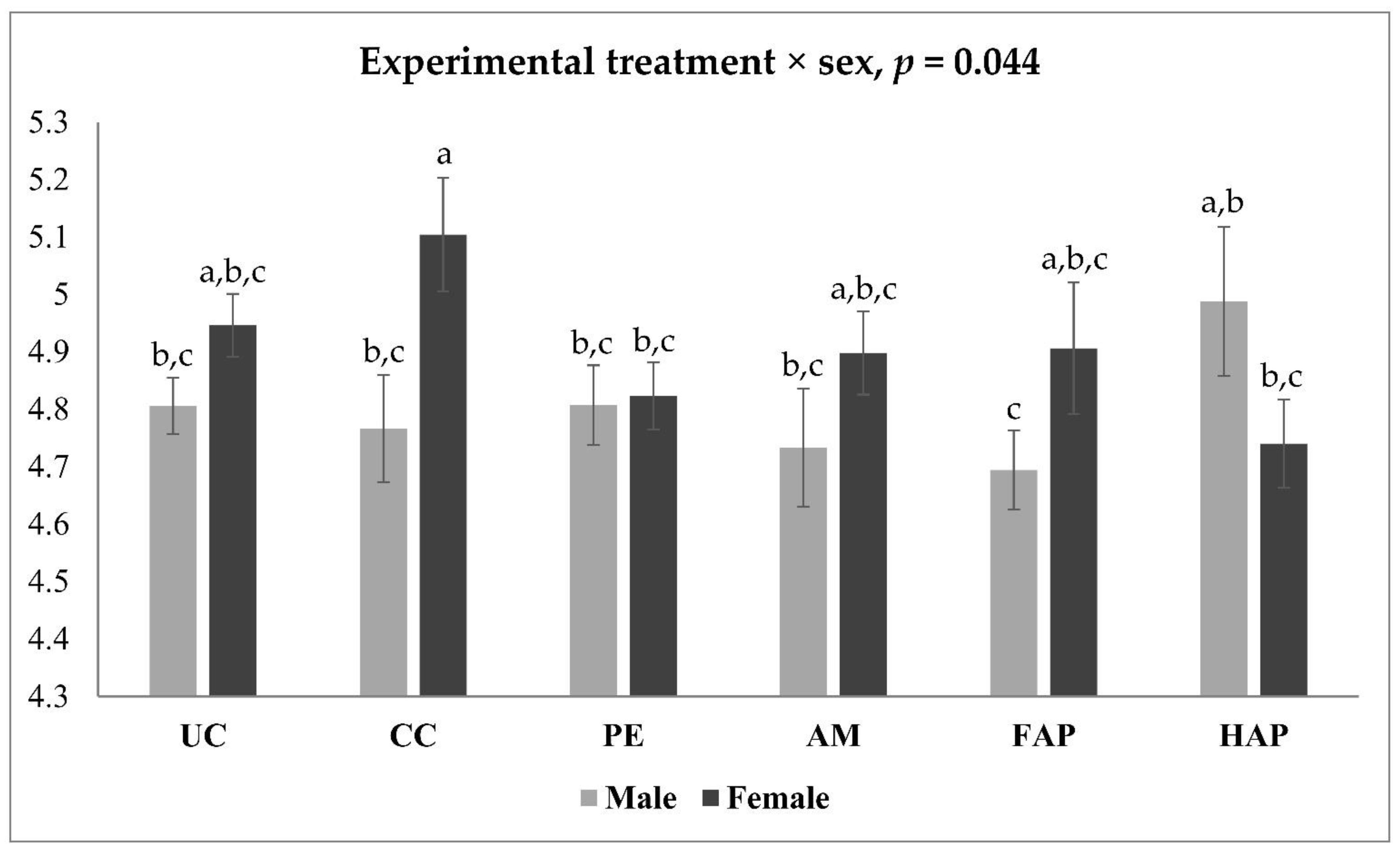
| Sex | Treatment | CLDN5 | OCLN | MUC2 |
|---|---|---|---|---|
| Male | UC | 1.221 a,b | 2.191 a | 2.041 a |
| CC | 1.363 a | 1.111 b–d | 0.707 d | |
| PE | 0.984 b–d | 1.270 b | 0.956 b–d | |
| AM | 0.831 d | 1.160 b,c | 1.005 b–d | |
| FAP | 0.878 c,d | 1.217 b,c | 1.186 b,c | |
| HAP | 1.039 a–d | 1.102 b–d | 1.248 b,c | |
| Female | UC | 0.816 d | 0.999 b–e | 1.329 b |
| CC | 0.990 b–d | 0.767 e | 0.870 c,d | |
| PE | 1.245 a,b | 0.677 e | 0.874 c,d | |
| AM | 0.944 b–d | 0.809 d,e | 1.080 b–d | |
| FAP | 1.280 a,b | 0.822 d,e | 1.224 b,c | |
| HAP | 1.175 a–c | 0.907 c–e | 1.018 b–d | |
| p-value | ||||
| Experimental treatment | 0.243 | <0.001 | <0.001 | |
| Sex | 0.750 | <0.001 | 0.108 | |
| Experimental treatment × Sex | 0.004 | <0.001 | 0.029 | |
| Treatment | Lactobacillus | Bifidobacteria | Bacillus | Ruminococcus | Enterobacteriaceae | C. perfringens | Total Bacteria |
|---|---|---|---|---|---|---|---|
| Experimental treatment | |||||||
| UC | 7.26 d | 5.96 e | 6.07 d | 5.69 | 5.30 c | 3.94 c | 8.82 d |
| CC | 8.20 a | 6.86 a | 6.81 a,b | 6.08 | 8.21 a | 9.16 a | 9.92 a |
| PE | 8.06 a,b | 6.74 a,b | 6.95 a | 5.68 | 8.11 a | 8.94 a,b | 9.75 a,b |
| AM | 7.57 c,d | 6.42 c,d | 6.63 b,c | 5.75 | 7.18 b | 4.99 c | 9.40 c |
| FAP | 7.51 c,d | 6.20 d,e | 6.58 c | 5.61 | 7.08 b | 5.19 c | 9.41 c |
| HAP | 7.72 b,c | 6.53 b,c | 6.81 a,b | 5.59 | 7.24 b | 7.73 b | 9.57 b,c |
| SEM | 0.10 | 0.09 | 0.07 | 0.16 | 0.21 | 0.46 | 0.10 |
| Sex | |||||||
| Male | 7.61 b | 6.52 | 6.58 b | 5.35 b | 7.09 | 6.50 | 9.46 |
| Female | 7.83 a | 6.38 | 6.70 a | 6.11 a | 7.28 | 6.81 | 9.50 |
| SEM | 0.06 | 0.05 | 0.04 | 0.09 | 0.12 | 0.27 | 0.06 |
| p-value | |||||||
| Experimental treatment | <0.001 | <0.001 | <0.001 | 0.244 | <0.001 | <0.001 | <0.001 |
| Sex | 0.008 | 0.070 | 0.039 | <0.001 | 0.271 | 0.412 | 0.608 |
| Experimental treatment × Sex | 0.460 | 0.193 | 0.160 | 0.109 | 0.436 | 0.557 | 0.255 |
| Treatment | Lactobacillus | Bifidobacteria | Bacteroides | Bacillus | Ruminococcus | Enterobacteriaceae | C. perfringens | Total Bacteria |
|---|---|---|---|---|---|---|---|---|
| Experimental treatment | ||||||||
| UC | 8.64 c | 8.46 bc | 5.85 | 7.64 a | 9.57 a | 8.48 d | 0.27 c | 10.79 b |
| CC | 9.41 a | 8.65 a | 6.01 | 6.84 c | 9.04 b | 10.62 a | 9.62 a | 11.05 a |
| PE | 9.09 b | 8.54 a,b | 5.88 | 7.02 b,c | 9.05 b | 10.23 a,b | 9.29 a | 10.85 a,b |
| AM | 8.41 c | 8.40 b,c | 5.78 | 7.20 b | 9.14 b | 9.28 c | 2.89 b | 10.64 b |
| FAP | 8.43 c | 8.36 c | 5.80 | 7.36 a,b | 9.21 b | 9.43 c | 3.87 b | 10.68 b |
| HAP | 8.64 c | 8.50 a–c | 5.85 | 7.11 b,c | 9.08 b | 9.79 b,c | 8.80 a | 10.83 a,b |
| SEM | 0.11 | 0.06 | 0.07 | 0.13 | 0.06 | 0.15 | 0.58 | 0.09 |
| Sex | ||||||||
| Male | 8.27 b | 8.31 b | 5.85 | 7.17 | 9.24 a | 9.59 | 5.44 | 10.71 b |
| Female | 9.27 a | 8.66 a | 5.87 | 7.22 | 9.13 b | 9.69 | 6.14 | 10.90 a |
| SEM | 0.06 | 0.03 | 0.04 | 0.07 | 0.04 | 0.09 | 0.33 | 0.05 |
| p-value | ||||||||
| Experimental treatment | <0.001 | 0.007 | 0.242 | <0.001 | <0.001 | <0.001 | <0.001 | 0.018 |
| Sex | <0.001 | <0.001 | 0.742 | 0.655 | 0.031 | 0.457 | 0.136 | 0.007 |
| Experimental treatment × Sex | 0.670 | 0.100 | 0.553 | 0.761 | 0.665 | 0.538 | 0.538 | 0.592 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Kheravii, S.K.; Ionescu, C.; Blanchard, A.; Barekatain, R.; Bajagai, Y.S.; Wu, S.-B. A Microencapsulated Mixture of Eugenol and Garlic Tincture Supplementation Mitigates the Effect of Necrotic Enteritis on Intestinal Integrity and Increases Goblet Cells in Broilers. Microorganisms 2021, 9, 1451. https://doi.org/10.3390/microorganisms9071451
Kumar A, Kheravii SK, Ionescu C, Blanchard A, Barekatain R, Bajagai YS, Wu S-B. A Microencapsulated Mixture of Eugenol and Garlic Tincture Supplementation Mitigates the Effect of Necrotic Enteritis on Intestinal Integrity and Increases Goblet Cells in Broilers. Microorganisms. 2021; 9(7):1451. https://doi.org/10.3390/microorganisms9071451
Chicago/Turabian StyleKumar, Alip, Sarbast K. Kheravii, Catherine Ionescu, Alexandra Blanchard, Reza Barekatain, Yadav S. Bajagai, and Shu-Biao Wu. 2021. "A Microencapsulated Mixture of Eugenol and Garlic Tincture Supplementation Mitigates the Effect of Necrotic Enteritis on Intestinal Integrity and Increases Goblet Cells in Broilers" Microorganisms 9, no. 7: 1451. https://doi.org/10.3390/microorganisms9071451
APA StyleKumar, A., Kheravii, S. K., Ionescu, C., Blanchard, A., Barekatain, R., Bajagai, Y. S., & Wu, S.-B. (2021). A Microencapsulated Mixture of Eugenol and Garlic Tincture Supplementation Mitigates the Effect of Necrotic Enteritis on Intestinal Integrity and Increases Goblet Cells in Broilers. Microorganisms, 9(7), 1451. https://doi.org/10.3390/microorganisms9071451






