Muscle Protein Hydrolysates and Amino Acid Composition in Fish
Abstract
:1. Introduction
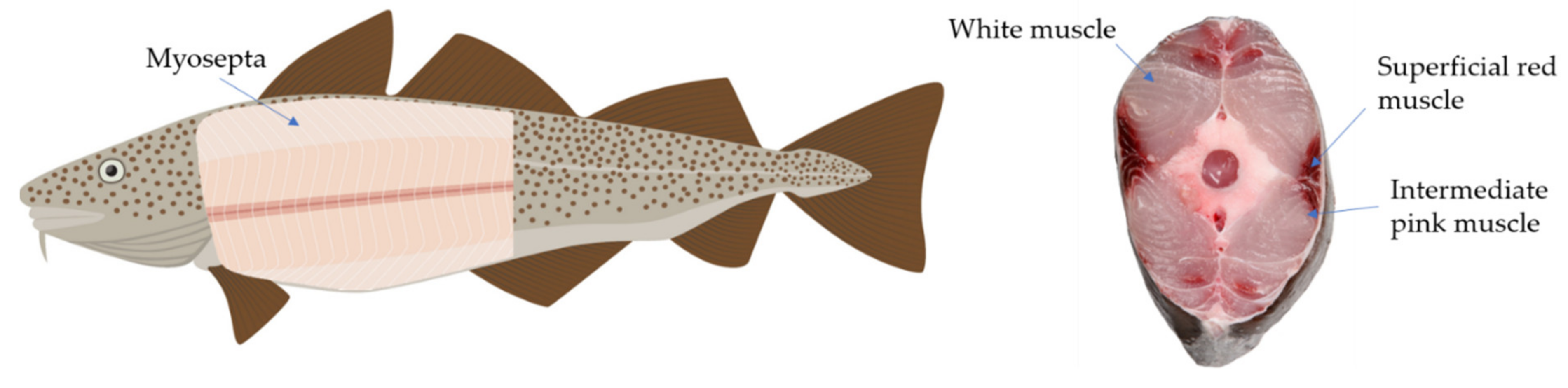
2. Characteristics of Fish Muscle Protein
3. Fish Muscle Protein Hydrolysates
4. Amino Acids in Fish Protein Hydrolysates
5. Amino Acid Composition of Fish Muscle Protein Hydrolysates
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tacon, A.G.J.; Metian, M. Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Wenning, R. The State of World Fisheries and Aquaculture 2020 Report; Food and Agriculture Organization: Hoboken, NJ, USA, 2020. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Food Matters: Fish, Income, and Food Supply—A Comparative Analysis. Rev. Fish. Sci. Aquac. 2017, 26, 15–28. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Longstreth, W.; Lemaitre, R.N.; Manolio, T.A.; Kuller, L.H.; Burke, G.L.; Siscovick, D.S. Fish consumption and stroke risk in elderly individuals: The cardiovascular health study. Arch. Intern. Med. 2005, 165, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012, 15, 725–737. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, Y.J.; Sawada, N.; Mimura, M.; Shikimoto, R.; Nozaki, S.; Hamazaki, K.; Uchitomi, Y.; Tsugane, S. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: A population-based prospective cohort study. Transl. Psychiatry 2017, 7, e1242. [Google Scholar] [CrossRef] [Green Version]
- Rylander, C.; Sandanger, T.M.; Engeset, D.; Lund, E. Consumption of Lean Fish Reduces the Risk of Type 2 Diabetes Mellitus: A Prospective Population Based Cohort Study of Norwegian Women. PLoS ONE 2014, 9, e89845. [Google Scholar] [CrossRef] [Green Version]
- Strasburg, G.M.; Xiong, Y.L. Physiology and chemistry of edible muscle tissues. In Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2017; pp. 955–1015. [Google Scholar]
- Sikorski, Z.E.; Kołakowska, A.; Pan, B.S. The Nutritive Composition of the Major Groups of Marine Food Organisms. In Seafood: Resources, Nutritional Composition, and Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 29–54. [Google Scholar]
- Heffernan, S.; Giblin, L.; O’Brien, N. Assessment of the biological activity of fish muscle protein hydrolysates using in vitro model systems. Food Chem. 2021, 359, 129852. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Montero, P.; Borderias, J. Effect of rigor mortis and ageing on collagen in trout (Salmo irideus) muscle. J. Sci. Food Agric. 1990, 52, 141–146. [Google Scholar] [CrossRef]
- Sato, K.; Ohashi, C.; Ohtsuki, K.; Kawabata, M. Type V collagen in trout (Salmo gairdneri) muscle and its solubility change during chilled storage of muscle. J. Agric. Food Chem. 1991, 39, 1222–1225. [Google Scholar] [CrossRef]
- Espe, M.; Ruohonen, K.; Bjornevik, M.; Froyland, L.; Nortvedt, R.; Kiessling, A. Interactions between ice storage time, collagen composition, gaping and textural properties in fanned salmon muscle harvested at different times of the year. Aquaculture 2004, 240, 489–504. [Google Scholar] [CrossRef]
- Boland, M.; Kaur, L.; Chian, F.M.; Astruc, T. Muscle Proteins. Encycl. Food Chem. 2019, 164–179. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bone, Q. Locomotor Muscle. Fish Physiol. 1978, 7, 361–424. [Google Scholar] [CrossRef]
- Hayes, M.; Flower, D. Bioactive peptides from marine processing byproducts. In Bioactive Compounds from Marine Foods: Plant and Animal Sources; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 57–71. [Google Scholar]
- Cerrato, A.; Capriotti, A.; Capuano, F.; Cavaliere, C.; Montone, A.; Montone, C.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Identification and Antimicrobial Activity of Medium-Sized and Short Peptides from Yellowfin Tuna (Thunnus albacares) Simulated Gastrointestinal Digestion. Foods 2020, 9, 1185. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Estrella-Millán, Y.; Betancur-Ancona, D.; Aranda-González, I.; Castellanos-Ruelas, A.; Gallegos-Tintoré, S. Antioxidant, chelating, and angiotensin-converting enzyme inhibitory activities of peptide fractions from red lionfish (Pterois volitans L.) muscle protein hydrolysates. Int. Food Res. J. 2020, 27, 224–233. [Google Scholar]
- Da Rocha, M.; Aleman, A.; Baccan, G.; López-Caballero, M.E.; Gómez-Guillén, C.; Montero, P.; Prentice, C. Anti-Inflammatory, Antioxidant, and Antimicrobial Effects of Underutilized Fish Protein Hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-R.; Byun, H.-G. The Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Rainbow Trout Muscle Hydrolysate. Fish. Aquat. Sci. 2012, 15, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Nazeer, R.; Kumar, N.S.; Ganesh, R.J. In vitro and in vivo studies on the antioxidant activity of fish peptide isolated from the croaker (Otolithes ruber) muscle protein hydrolysate. Peptides 2012, 35, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Thawornchinsombut, S.; Park, J.W. effect of nacl on gelation characteristics of acid- and alkali-treated pacific whiting fish protein isolates. J. Food Biochem. 2007, 31, 427–455. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G. Antioxidative Efficacy of Alkali-Treated Tilapia Protein Hydrolysates: A Comparative Study of Five Enzymes. J. Agric. Food Chem. 2008, 56, 1434–1441. [Google Scholar] [CrossRef]
- Himonides, A.T.; Taylor, A.K.D.; Morris, A.J. A Study of the Enzymatic Hydrolysis of Fish Frames Using Model Systems. Food Nutr. Sci. 2011, 02, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, Y.; Xu, K.; Wu, J.; Dai, Z. Microbiological Changes and Biodiversity of Cultivable Indigenous Bacteria in Sanbao Larger Yellow Croaker (Pseudosciaena crocea), a Chinese Salted and Fermented Seafood. J. Food Sci. 2015, 80, 776. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Lee, K.-H.; Je, J.-Y. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effects of enzymes and molecular weight on bioactivity. Int. J. Food Sci. Technol. 2010, 45, 562–568. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-É. Proteolytic processing of herring (Clupea harengus): Biochemical and nutritional characterisation of hydrolysates. Int. J. Food Sci. Technol. 2009, 44, 2113–2119. [Google Scholar] [CrossRef]
- Dong, Y.-L.; Sheng, G.-Y.; Fu, J.-M.; Wen, K.-W. Chemical characterization and anti-anaemia activity of fish protein hydrolysate fromSaurida elongata. J. Sci. Food Agric. 2005, 85, 2033–2039. [Google Scholar] [CrossRef]
- Wasswa, J.; Tang, J.; Gu, X.-H.; Yuan, X.-Q. Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2007, 104, 1698–1704. [Google Scholar] [CrossRef]
- Bucci, L.; Unlu, L. Proteins and amino acid supplements in exercise and sport. In Energy-Yielding Macronutrients Energy Metabolism in Sports Nutrition; CRC Press: Boca Raton, FL, USA, 2000; pp. 191–212. [Google Scholar]
- Jemil, I.; Jridi, M.; Nasri, R.; Ktari, N.; Salem, R.B.S.-B.; Mehiri, M.; Hajji, M.; Nasri, M. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process. Biochem. 2014, 49, 963–972. [Google Scholar] [CrossRef]
- Fujita, H.; Yamagami, T.; Ohshima, K. Effects of an ace-inhibitory agent, katsuobushi oligopeptide, in the spontaneously hypertensive rat and in borderline and mildly hypertensive subjects. Nutr. Res. 2001, 21, 1149–1158. [Google Scholar] [CrossRef]
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigón, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, W.; Yang, F.; Nie, X. Physical and chemical changes of silver carp sausages during fermentation with Pediococcus pentosaceus. Food Chem. 2010, 122, 633–637. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Braun, S. State of the Art Manufacturing of Protein Hydrolysates. Protein Hydrolysates Biotechnol. 2008, 11–32. [Google Scholar] [CrossRef]
- Dos Santos, S.D.; Martins, V.; Salas-Mellado, M.; Prentice, C. Evaluation of Functional Properties in Protein Hydrolysates from Bluewing Searobin (Prionotus punctatus) Obtained with Different Microbial Enzymes. Food Bioprocess Technol. 2009, 4, 1399–1406. [Google Scholar] [CrossRef]
- Srichanun, M.; Tantikitti, C.; Kortner, T.M.; Krogdahl, Å.; Chotikachinda, R. Effects of different protein hydrolysate products and levels on growth, survival rate and digestive capacity in Asian seabass (Lates calcarifer Bloch) larvae. Aquaculture 2014, 428-429, 195–202. [Google Scholar] [CrossRef]
- Ha, N.C.; Hien, D.M.; Thuy, N.T.; Nguyen, L.T.; Devkota, L. Enzymatic Hydrolysis of Catfish (Pangasius hypophthalmus) By-Product: Kinetic Analysis of Key Process Parameters and Characteristics of the Hydrolysates Obtained. J. Aquat. Food Prod. Technol. 2017, 26, 1070–1082. [Google Scholar] [CrossRef]
- Shen, Q.; Guo, R.; Dai, Z.; Zhang, Y. Investigation of Enzymatic Hydrolysis Conditions on the Properties of Protein Hydrolysate from Fish Muscle (Collichthys niveatus) and Evaluation of Its Functional Properties. J. Agric. Food Chem. 2012, 60, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Silva, C.; Silva, G.; Prentice, C. Enzymatic hydrolysis of cobia (Rachycentron canadum) meat and wastes using different microbial enzymes. J. Int. Food Res. J. 2016, 23, 152. [Google Scholar]
- Khantaphant, S.; Benjakul, S.; Kishimura, H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process. Biochem. 2011, 46, 318–327. [Google Scholar] [CrossRef]
- Liu, J.; Lyu, F.; Zhou, X.; Wang, B.; Wang, X.; Ding, Y. Preparation of skipjack tuna (Katsuwonus pelamis) protein hydrolysate using combined controlled enzymatic hydrolysis and glycation for improved solubility and emulsifying properties. J. Food Nutr. Res. 2015, 3, 471–477. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Phillips, M.; Kailasapathy, K. Isolation and characterization of nutraceutically potential ACE-Inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT 2017, 80, 430–436. [Google Scholar] [CrossRef]
- Wijesekara, I.; Qian, Z.-J.; Ryu, B.; Ngo, D.-H.; Kim, S.-K. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res. Int. 2011, 44, 703–707. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Deng, Y.-Y.; Wang, Y.-M.; Deng, S.-G.; Ma, J.-Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Mosquera, M.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Incorporation of liposomes containing squid tunic ACE-inhibitory peptides into fish gelatin. J. Sci. Food Agric. 2016, 96, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.-Y.; Kang, N.; Lee, J.-H.; Kim, J.-S.; Kim, W.-S.; Park, S.-J.; Kim, Y.-T.; Jeon, Y.-J. Angiotensin I-converting enzyme inhibitory peptides from an enzymatic hydrolysate of flounder fish (Paralichthys olivaceus) muscle as a potent anti-hypertensive agent. Process. Biochem. 2016, 51, 535–541. [Google Scholar] [CrossRef]
- Pacheco-Aguilar, R.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 2008, 109, 782–789. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011, 124, 1354–1362. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef]
- Darewicz, M.; Borawska, J.; Vegarud, G.E.; Minkiewicz, P.; Iwaniak, A. Angiotensin I-converting enzyme (ACE) inhibitory activity and ACE inhibitory peptides of salmon (Salmo salar) protein hydrolysates obtained by human and porcine gastrointestinal enzymes. Int. J. Mol. Sci. 2014, 15, 14077–14101. [Google Scholar] [CrossRef] [Green Version]
- Jemil, I.; Abdelhedi, O.; Nasri, R.; Mora, L.; Jridi, M.; Aristoy, M.-C.; Toldrá, F.; Nasri, M. Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res. Int. 2017, 100, 121–133. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Barkia, A.; Aristoy, M.-C.; Nasri, M.; Toldrá, F. Angiotensin I-converting enzyme inhibitory peptides FQPSF and LKYPI identified in Bacillus subtilis A26 hydrolysate of thornback ray muscle. Int. J. Food Sci. Technol. 2016, 51, 1604–1609. [Google Scholar] [CrossRef]
- Gbogouri, G.; Linder, M.; Fanni, J.; Parmentier, M. Influence of Hydrolysis Degree on the Functional Properties of Salmon Byproducts Hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Neklyudov, A.D.; Ivankin, A.N.; Berdutina, A.V. Production and purification of protein hydrolysates (review). Appl. Biochem. Microbiol. 2000, 36, 317–324. [Google Scholar] [CrossRef]
- UG, Y.; Bhat, I.; Karunasagar, I.; BS, M. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2018, 59, 2363–2374. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Kumar, B.D.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Lima, M.M.; Vanier, N.L.; Dias, A.R.G.; Zavareze, E.; Prentice, C.; Moreira, A.D. Whitemouth croaker (Micropogonias furnieri) protein hydrolysates: Chemical composition, molecular mass distribution, antioxidant activity and amino acid profile. Int Food Res. J. 2019, 26, 247–254. [Google Scholar]
- Chyun, J.-H.; Griminger, P. Improvement of nitrogen retention by arginine and glycine supplementation and its relation to collagen synthesis in traumatized mature and aged rats. J. Nutr. 1984, 114, 1697–1704. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yamamoto, K.; Sato, Y.; Inoue, S.; Morinaga, T.; Hirano, E. Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed. Res. 2016, 37, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Ngo, D.-H.; Qian, Z.-J.; Ryu, B.; Park, J.W.; Kim, S.-K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.-P.; Ben Amar, R. Antioxidant properties of peptide fractions from tuna dark muscle protein by-product hydrolysate produced by membrane fractionation process. Food Res. Int. 2014, 65, 329–336. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.-Q.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Cândido, L.M.B.; Sgarbieri, V.C. Enzymatic hydrolysis of Nile tilapia (Oreochromus niloticus) myofibrillar proteins: Effects on nutritional and hydrophilic properties. J. Sci. Food Agric. 2003, 83, 937–944. [Google Scholar] [CrossRef]
- Abdul-Hamid, A.; Bakar, J.; Bee, G.H. Nutritional quality of spray dried protein hydrolysate from Black Tilapia (Oreochromis mossambicus). Food Chem. 2002, 78, 69–74. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem. 2007, 103, 1385–1394. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K. Amino Acid Composition and Antioxidative Peptides from Protein Hydrolysates of Yellow Stripe Trevally (Selaroides leptolepis). J. Food Sci. 2009, 74, C126–C133. [Google Scholar] [CrossRef]
- Nakajima, K.; Yoshie-Stark, Y.; Ogushi, M. Comparison of ACE inhibitory and DPPH radical scavenging activities of fish muscle hydrolysates. Food Chem. 2009, 114, 844–851. [Google Scholar] [CrossRef]
- Jung, W.-K.; Mendis, E.; Je, J.-Y.; Park, P.-J.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
| Source | Ref. | Protease | Enzymolysis Condition | DH | Functional and Biological Properties |
|---|---|---|---|---|---|
| Monkfish | [47] | Trypsin | Enzymolysis time 4 h, E/P 2%, Temp. 40 °C and pH 8.0 | 19.83% ± 0.82% | Hydroxyl radical scavenger |
| Red lionfish | [20] | Alcalase | Enzymolysis time 30, 60, and 90 min, E/P 0.3 AU/g protein, Temp. 50 °C and pH 8 | 30.78% ± 1.57%, 27.14% ± 1.20%, and 30.08% ± 0.25% (30, 60, and 90 min) | Antioxidant, chelator, angiotensin-converting enzyme inhibitor |
| Cobia (Rachycentron canadum) | [42] | Alcalase, Flavorase, Protamex | Alcalase (pH 8.0, 50 °C, E/P 99.75 U/g), Flavourzyme (pH 7.0, 50 °C, E/P 2.07 U/g) and Protamex (pH 7.0, 40 °C, E/P 8.41 U/g), Enzymolysis time 560 min | 12.5%, 11.7%, 33.1% | - |
| Catfish (Pangasius hypothalamus) | [40] | Neutrase, Papain, Bromelain | Neutrase (50 °C, pH 6.5), Papain (55 °C, pH 7.5), Bromelain (55 °C, pH 6.5), Enzymolysis time 180 min | 13.30%, 31.16%, 29.36% | - |
| Leatherjacket fish (Meuchenia sp.) | [45] | Bromelain, Papain, Flavourzyme | Bromelain, Papain (0.5% for water soluble protein and 1.5% for insoluble protein), Flavourzyme (0.25 % for water-soluble protein and 0.75% for insoluble protein), 50 °C, without any pH adjustment, 2 to 10 h | - | ACE inhibitor |
| Skipjack tuna (Katsuwonus pelamis) | [44] | Alcalase, Protamex, Flavorase, Neutrase | Alcalase (55 °C, pH 8), Protamex (50 °C, pH 7), Flavorase (55 °C, pH 7), Neutrase (50 °C, pH 7), Enzymolysis time 5 h, solid/liquid ratio 1:2 | 2.43%, 78.33%, 33.80%, and 56.72% | Solubilizer, emulsifier |
| Bighead croaker (Collichthys niveatus) | [41] | Alcalase, Neutrase | Alcalase (pH 8.0, 60 °C), Neutrase (pH 7.0, 50 °C), Enzymolysis time 4 h | 17.03%, 15.04% | - |
| Pacific whiting (Merluccius productus) | [50] | Alcalase | pH 8.0, 50 °C, up to 2 h | 10%, 15% and 20% (pH 4.0, 7.0 and 10, respectively) | Solubilizer, emulsifier, foaming capacity, foam stability |
| Ornate threadfin bream (Nemipterus hexodon) | [51] | Pepsin from skipjack tuna | pH 8.0, 50 °C, E/P 0.11 to 0.52 %, 1 h of hydrolysis | 10%, 20% and 30% by adding skipjack tuna pepsin (140–650 μL) | ABTS and DPPH radical scavenger, chelator |
| Brownstripe red snapper (Lutjanus vitta) | [43] | Alcalase or Flavourzyme, as the first step and Pyloric caeca protease (PCP) | Alcalase (50 °C, pH 8.0), Flavourzyme (50 °C, pH 7.0), PCP (60 °C, pH 8.5), Enzymolysis time 10 min | 40% | Antioxidant and ACE inhibitor |
| Cuttle fish (Sepia officinalis) | [52] | A21 proteases, Cuttlefish proteases | A21 proteases (pH 10.0; 50 °C), Cuttlefish hepatopancreas (pH 8.0; 50 °C), E/P 3:1 U/mg | 16%, 8% | ACE inhibitor |
| Salmon (Salmon salar) | [53] | Human and Porcine gastrointestinal enzymes | - | - | ACE inhibitor |
| Sardinelle (Sardina pilchardus) | [54] | A26 proteases | pH 8.0; 45 °C, E/P 3:1 U/mg, Enzymolysis time 300 min | 10% | Antibacterial, antioxidant and antihypertensive |
| Thornback ray fish (Raja clavate) | [55] | A26 proteases | pH 8.0; 40 °C, E/P 3:1 U/mg, Enzymolysis time 390 min | 18% | ACE inhibitor |
| Pipefish (Syngnathus schlegeli) | [46] | Papain, Alcalase, Neutrase, Pronase, Pepsin and Trypsin | E/P 1%, Enzymolysis time 8 h | - | ACE inhibitor |
| Flounder (Daralichthys olivaceus) | [49] | Pepsin, Papain, Trypsin, Kojizyme | E/P 1:500 | - | ACE inhibitor |
| Amino Acid | Studies | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuna Dark Muscle (g/100 g) [68] | Red Lionfish Muscle (g/100 g) [20] | Capelin % [69] | Nile Tilapia (g/kg) [70] | ||||||||||||||
| Enzyme | Alcalase | Alcalase | Protein | Alcalase | Protein | Flavourzime | |||||||||||
| Size | TPH | 1–4 kDa | F 5–3 (3–5 kDa) | F < 1 (<1kDa) | - | - | - | - | |||||||||
| Protein content | 72 ± 1.61 (g·L−1) | 76 ± 1.59 (g·L−1) | - | - | 13.9 ± 0.21 | 72.4 ± 0.70 | 823 g kg−1 | 899 g kg−1 | |||||||||
| Aspartic acid | 5.4 ± 0.1 | 7.5 ± 0.2 | 8.44 ± 0.80 | 8.80 ± 0.42 | 8.88 ± 0.15 | 9.89 ± 0.53 | - | - | |||||||||
| Threonine | 3 ± 0.1 | 4 ± 0.1 | 3.57 ± 0.02 | 4.58 ± 0.15 | 4.82 ± 0.05 | 4.56 ± 0.03 | 49 | 52 | |||||||||
| Serine | 2.3 ± 0.1 | 3 ± 0.32 | 2.97 ± 0.07 | 4.09 ± 0.12 | 4.18 ± 0.05 | 4.24 ± 0.10 | - | - | |||||||||
| Glutamic acid | 8.5 ± 0.2 | 12.1 ± 0.1 | 13.24 ± 0.54 | 11.00 ± 0.41 | 13.2 ± 0.03 | 13.4 ± 0.03 | - | - | |||||||||
| Proline | 2.2 ± 0.0 | 3 ± 0.2 | 14.52 ± 0.37 | 5.71 ± 0.57 | 3.70 ± 0.15 | 3.67 ± 0.03 | - | - | |||||||||
| Glycine | 3.6 ± 0. | 4.8 ± 0.1 | 4.24 ± 0.19 | 4.48 ± 0.16 | 5.32 ± 0.04 | 5.14 ± 0.01 | - | - | |||||||||
| Alanine | 4.6 ± 0.2 | 6.1 ± 0.3 | 2.43 ± 0.04 | 2.21 ± 0.19 | 5.57 ± 0.04 | 6.00 ± 0.01 | - | - | |||||||||
| Valine | 3.2 ± 0.1 | 3.9 ± 0.1 | 14.86 ± 0.20 | 13.27 ± 0.02 | 5.71 ± 0.12 | 5.77 ± 0.01 | 51 | 53 | |||||||||
| Methionine | 1.7 ± 0.1 | 2.2 ± 0.2 | - | 3.12 ± 0.34 | 3.09 ± 0.02 | 2.05 ± 0.01 | 21 | 24 | |||||||||
| Isoleucine | 4.1 ± 0.6 | 4.0 ± 0.1 | 7.04 ± 0.22 | 8.68 ± 0.11 | 4.72 ± 0.08 | 4.25 ± 0.04 | 49 | 49 | |||||||||
| Leucine | 4.9 ± 0.2 | 6 ± 0.2 | 2.77 ± 0.11 | 2.64 ± 0.04 | 8.15 ± 0.05 | 7.60 ± 0.00 | 86 | 84 | |||||||||
| Tyrosine | 1.8 ± 0.1 | 2.2 ± 0.2 | 2.57 ± 0.05 | 3.42 ± 0.05 | 3.34 ± 0.01 | 2.47 ± 0.06 | 35 | 35 | |||||||||
| Phenylalanine | 2.4 ± 0.1 | 3 ± 0.2 | 3.11 ± 0.20 | 4.19 ± 0.09 | 3.80 ± 0.01 | 3.19 ± 0.00 | 43 | 44 | |||||||||
| Histidine | 2.4 ± 0.1 | 3 ± 0.2 | 1.23 ± 0.15 | 1.64 ± 0.12 | 2.43 ± 0.00 | 2.09 ± 0.02 | 24 | 23 | |||||||||
| Lysine | 4.7 ± 0.1 | 6.1 ± 0.1 | 7.74 ± 0.18 | 6.43 ± 0.09 | 8.47 ± 0.09 | 8.49 ± 0.06 | 105 | 92 | |||||||||
| Arginine | 2.4 ± 0.2 | 4 ± 0.21 | 11.13 ± 0.12 | 15.23 ± 0.40 | 5.99 ± 0.10 | 5.70 ± 0.02 | - | - | |||||||||
| Tryptophan | 0.3 ± 0.0 | 0.5 ± 0.0 | - | 0.51 ± 0.06 | 1.07 ± 0.01 | 0.43 ± 0.01 | 11 | 14 | |||||||||
| Cystine | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.13 ± 0.18 | - | 1.33 ± 0.10 | 1.34 ± 0.00 | 6 | 8 | |||||||||
| HAA | 25.3 | 30.8 | 47.4 | 43.24 | 39.41 | 36.34 | |||||||||||
| AAA | 4.5 | 5.7 | 5.68 | 8.12 | 8.21 | 6.09 | 89 | 93 | |||||||||
| NCAA | 13.9 | 19.6 | 21.68 | 19.8 | 22.08 | 23.29 | - | - | |||||||||
| PCAA | 9.5 | 13.1 | 20.1 | 23.3 | 16.89 | 16.28 | - | - | |||||||||
| EAA | 26.4 | 32.2 | 40.29 | 44.55 | 41.19 | 38 | 428 | 421 | |||||||||
| Argentine Croaker (mg/g) [21] | Black Tilapia (mg/g) [71] | Round Scad (%) [72] | Yellow Stripe Trevally (%) [73] | Bighead Croaker (%) [41] | |||||||||||||
| Enzyme | Alcalase | Protamex | AC | Alcalase | Flavourzyme | Protein | Alcalase | Flavourzyme | Neutrase | ||||||||
| Size | 1083 Da | 1350 Da | - | - | - | - | <7 kDa | 7–8 kDa | - | ||||||||
| Protein content | - | - | - | 49.6 ± 0.19 | 69.0 ± 3.57 | - | - | - | - | ||||||||
| Aspartic acid | 136.93 ± 0.38 | 136.73 ± 0.60 | 45.4 ± 0.16 | 67.2 ± 5.30 | 2.04 | 10.01 | 9.55 | 9.40 | 1.91 | ||||||||
| Threonine | 45.38 ± 0.62 | 44.54 ± 0.15 | 20.1 ± 6.42 | 30.2 ± 6.89 | 5.09 | 2.92 | 5.35 | 5.40 | 10.92 | ||||||||
| Serine | 46.04 ± 0.40 | 49.08 ± 0.41 | 21.5 ± 0.78 | 24.0 ± 0.10 | 8.16 | 5.46 | 5.21 | 5.15 | 3.36 | ||||||||
| Glutamic acid | 188.60 ± 1.37 | 196.19 ±0.33 | 141 ± 6.48 | 151 ± 7.05 | 3.47 | 9.88 | 13.77 | 13.89 | 10.85 | ||||||||
| Proline | 33.37 ± 0.46 | 31.71 ± 0.45 | 17.5 ± 2.42 | 22.6 ± 2.98 | 0.51 | 3.33 | 3.81 | 3.84 | 0.72 | ||||||||
| Glycine | 35.87 ± 0.28 | 36.25 ± 0.12 | 31.8 ± 3.16 | 40.9 ± 1.66 | 1.49 | 16.48 | 8.87 | 8.65 | 2.01 | ||||||||
| Alanine | 58.40 ± 0.04 | 62.31 ± 0.35 | 32.4 ± 0.99 | 37.7 ± 2.01 | 5.31 | 9.64 | 9.49 | 9.46 | 5.21 | ||||||||
| Valine | 35.87 ± 0.02 | 34.02 ± 0.21 | 23.0 ± 0.25 | 31.9 ± 0.56 | 6.77 | 2.77 | 3.61 | 3.38 | 5.12 | ||||||||
| Methionine | 41.68 ± 0.48 | 39.33 ± 0.21 | 19.9 ± 2.09 | 27.6 ± 3.01 | 4.51 | 1.76 | 2.58 | 1.87 | 4.19 | ||||||||
| Isoleucine | 31.00 ± 0.09 | 26.24 ± 0.09 | 24.4 ± 3.15 | 33.6 ± 1.26 | 3.15 | 4.31 | 4.14 | 4.49 | 4.24 | ||||||||
| Leucine | 83.96 ±0.19 | 81.28 ± 0.30 | 56.0 ± 0.78 | 68.9 ± 0.11 | 10.1 | 6.72 | 8.38 | 8.58 | 7.29 | ||||||||
| Tyrosine | 38.32 ± 0.11 | 34.15 ± 0.09 | 16.4 ± 2.69 | 25.8 ± 1.08 | 5.20 | 5.62 | 5.70 | 6.11 | 9.86 | ||||||||
| Phenylalanine | 38.67 ± 0.01 | 40.92 ± 0.22 | 52.0 ± 2.43 | 59.1 ± 2.81 | 4.52 | 3.08 | 2.61 | 2.66 | 10.79 | ||||||||
| Histidine | 24.81 ± 1.38 | 24.69 ± 0.10 | - | - | 11.2 | 5.49 | 3.62 | 2.98 | 1.93 | ||||||||
| Lysine | 96.07 ± 0.36 | 97.98 ± 0.05 | 50.3 ± 0.04 | 75.9 ± 1.11 | 13.9 | 8.45 | 8.35 | 8.72 | 9.76 | ||||||||
| Arginine | 61.01 ± 0.46 | 59.62 ± 0.04 | 34.4 ± 2.42 | 49.1 ± 0.50 | 14.0 | 2.64 | 3.50 | 3.88 | 5.79 | ||||||||
| Tryptophan | - | - | - | - | - | - | - | - | 3.55 | ||||||||
| Cystine | 4.16 ± 0.01 | 4.96 ± 0.04 | - | - | 0.69 | 1.43 | 1.47 | 1.53 | 2.49 | ||||||||
| HAA | 365.44 ± 0.34 | 354.92 ± 0.15 | 241.6 | 307.2 | 40.76 | 38.66 | 41.79 | 41.92 | 49.91 | ||||||||
| AAA | 77.00 ± 0.11 | 75.07 ± 0.13 | 68.4 | 84.9 | 9.72 | 8.7 | 8.31 | 8.77 | 24.2 | ||||||||
| NCAA | 325.53 ± 1.75 | 332.92 ± 0.92 | 186.4 | 218.2 | 5.51 | 19.89 | 23.32 | 23.29 | 12.76 | ||||||||
| PCAA | 181.74 ± 0.68 | 182.29 ± 0.09 | 84.7 | 125 | 39.1 | 16.58 | 15.47 | 15.58 | 17.48 | ||||||||
| EAA | 390.23 ± 0.28 | 389.00 ± 0.40 | 245.7 | 327.2 | 59.24 | 35.5 | 38.64 | 38.08 | 54.24 | ||||||||
| Whitemouth Croaker (mg/g) [61] | Atlantic Salmon (mg/100 g) [74] | Coho Salmon (mg/100 g) [74] | Alaska Pollock (mg/100 g) [74] | Southern Blue Whiting (mg/100 g) [74] | |||||||||||||
| Enzyme | Alcalase | Pepsin | Pepsin + pancreatin | Pepsin | Pepsin + pancreatin | Pepsin | Pepsin + pancreatin | Pepsin | Pepsin + pancreatin | ||||||||
| Size | 2–34 kDa | - | - | - | - | - | - | - | - | ||||||||
| Protein content | 82.3 ± 0.8 | a633–1020 | a1610–2530 | a633–1020 | a1610–2530 | a633–1020 | a1610–2530 | a633–1020 | a1610–2530 | ||||||||
| Aspartic acid | 105.23 ± 0.86 | 0.4 ± 0.2 | 11.6 ± 1.1 | 1.4 ± 0.2 | 17.8 ± 1.3 | 11.8 ± 0.7 | 16.9 ± 5.3 | 5.9 ± 1.2 | 1.1 ± 0.6 | ||||||||
| Threonine | 48.58 ± 0.58 | 2.9 ± 0.2 | 6.0 ± 0.3 | 2.8 ± 0.1 | 7.3 ± 0.4 | 13.5 ± 0.2 | 24.2 ± 6.0 | 4.9 ± 0.4 | 2.8 ± 0.9 | ||||||||
| Serine | 39.47 ± 1.63 | 4.8 ± 0.2 | 6.4 ± 0.3 | 6.1 ± 0.1 | 8.3 ± 0.4 | 18.3 ± 1.1 | 6.2 ± 1.4 | 7.6 ± 1.1 | 0.6 ± 0.4 | ||||||||
| Glutamic acid | 173.11 ± 4.93 | 3.1 ± 0.1 | 29.0 ± 1.2 | 6.4 ± 0.2 | 45.9 ± 2.2 | 3.1 ± 0.1 | 29.0 ± 1.2 | 32.9 ± 5.5 | 5.8 ± 1.7 | ||||||||
| Proline | 39.33 ± 0.94 | - | - | - | - | - | - | - | - | ||||||||
| Glycine | 40.54 ± 0.19 | 13.7 ± 0.8 | 15.2 ± 1.0 | 28.3 ± 0.1 | 26.0 ± 3.6 | 36.7 ± 1.2 | 55.4 ± 11.6 | 19.1 ± 2.5 | 19.0 ± 3.7 | ||||||||
| Alanine | 57.10 ± 1.49 | 24.7 ± 1.5 | 35.5 ± 0.9 | 26.7 ± 0.6 | 35.9 ± 2.8 | 37.2 ± 1.2 | 58.9 ± 13.5 | 36.9 ± 5.3 | 10.4 ± 1.9 | ||||||||
| Valine | 52.52 ± 1.13 | 2.5 ± 0.1 | 15.2 ± 0.3 | 2.3 ± 0.2 | 20.1 ± 3.2 | 5.1 ± 0.2 | 20.0 ± 5.2 | 5.4 ± 0.8 | 10.3 ± 2.2 | ||||||||
| Methionine | 39.63 ± 0.58 | 1.3 ± 0.1 | 11.8 ± 0.4 | 1.9 ± 0.1a | 14.2 ± 0.6 | 5.8 ± 0.4 | 21.6 ± 4.9 | 5.5 ± 1.0 | 17.6 ± 3.3 | ||||||||
| Isoleucine | 42.77 ± 2.48 | 1.2 ± 0.1 | 21.9 ± 2.2 | 1.1 ± 0.1 | 17.4 ± 4.7 | 3.4 ± 0.1 | 17.4 ± 3.9 | 4.4 ± 0.7 | 10.2 ± 1.8 | ||||||||
| Leucine | 96.42 ± 2.75 | 2.4 ± 0.1 | 84.3 ± 1.4 | 2.7 ± 0.2 | 88.8 ± 2.2 | 7.0 ± 0.2 | 90.3 ± 23 | 6.7 ± 0.9 | 63.4 ± 13.3 | ||||||||
| Tyrosine | - | 1.9 ± 0.1 | 76.9 ± 4.3 | 3.1 ± 0.1 | 67.4 ± 8.1 | 3.9 ± 0.1 | 62.8 ± 17 | 3.2 ± 0.5 | 50.0 ± 11.3 | ||||||||
| Phenylalanine | 44.43 ± 0.33 | 3.1 ± 0.3 | 76.3 ± 5.3 | 2.4 ± 0.1 | 82.0 ± 5.1 | 3.8 ± 0.3 | 62.6 ± 16 | 3.7 ± 0.5 | 47.5 ± 11.4 | ||||||||
| Histidine | 23.74 ±2.46 | 16.4 ± 0.9 | 21.0 ± 0.8 | 24.9 ± 0.1 | 27.8 ± 1.3 | 9.1 ± 0.4 | 11.9 ± 3.0 | 2.2 ± 0.4 | 3.1 ± 0.8 | ||||||||
| Lysine | 97.85 ± 1.41 | 1.9 ± 0.2 | 130.0 ± 8.0 | 2.7 ± 0.2 | 141 ± 8.0 | 20.5 ± 0.8 | 86.5 ± 24 | 0.6 ± 0.1 | 45.9 ± 11 | ||||||||
| Arginine | 59.36 ± 0.52 | 1.3 ± 0.6 | 196 ± 14 | 1.5 ± 0.2 | 204 ± 13 | 9.3 ± 0.3 | 215 ± 57 | 3.2 ± 0.4 | 132 ± 30 | ||||||||
| Tryptophan | 34.81 ± 0.66 | n.d. | 18.3 ± 0.5 | 1.4 ± 0.1 | 19.4 ± 0.5 | n.d. | n.d. | n.d. | n.d. | ||||||||
| Cystine | 5.09 ± 0.31 | n.d. | 1.7 ± 0.1 | n.d | 1.9 ± 0.1 | n.d. | n.d. | n.d. | n.d. | ||||||||
| HAA | 377.29 | 37.1 | 323.6 | 40.2 | 327.7 | 66.2 | 333.6 | 65.8 | 209.4 | ||||||||
| AAA | 79.24 | 5 | 171.5 | 6.9 | 168.8 | 7.7 | 125.4 | 6.9 | 97.5 | ||||||||
| NCAA | 278.34 | 3.5 | 40.6 | 7.8 | 63.7 | 14.9 | 45.9 | 38.8 | 6.9 | ||||||||
| PCAA | 180.94 | 19.6 | 347 | 29.1 | 372.8 | 38.9 | 313.4 | 6 | 181 | ||||||||
| EAA | 445.88 | 31.7 | 366.5 | 40.8 | 398.6 | 68.2 | 334.5 | 33.4 | 200.8 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.; Shin, K.-H.; Kim, S.-K. Muscle Protein Hydrolysates and Amino Acid Composition in Fish. Mar. Drugs 2021, 19, 377. https://doi.org/10.3390/md19070377
Ryu B, Shin K-H, Kim S-K. Muscle Protein Hydrolysates and Amino Acid Composition in Fish. Marine Drugs. 2021; 19(7):377. https://doi.org/10.3390/md19070377
Chicago/Turabian StyleRyu, Bomi, Kyung-Hoon Shin, and Se-Kwon Kim. 2021. "Muscle Protein Hydrolysates and Amino Acid Composition in Fish" Marine Drugs 19, no. 7: 377. https://doi.org/10.3390/md19070377






