17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats
Abstract
1. Introduction
2. Results
2.1. E2 Exacerbated EOI-Induced Masseter Hyperalgesia in a Dose-Dependent Manner in OVX Rats
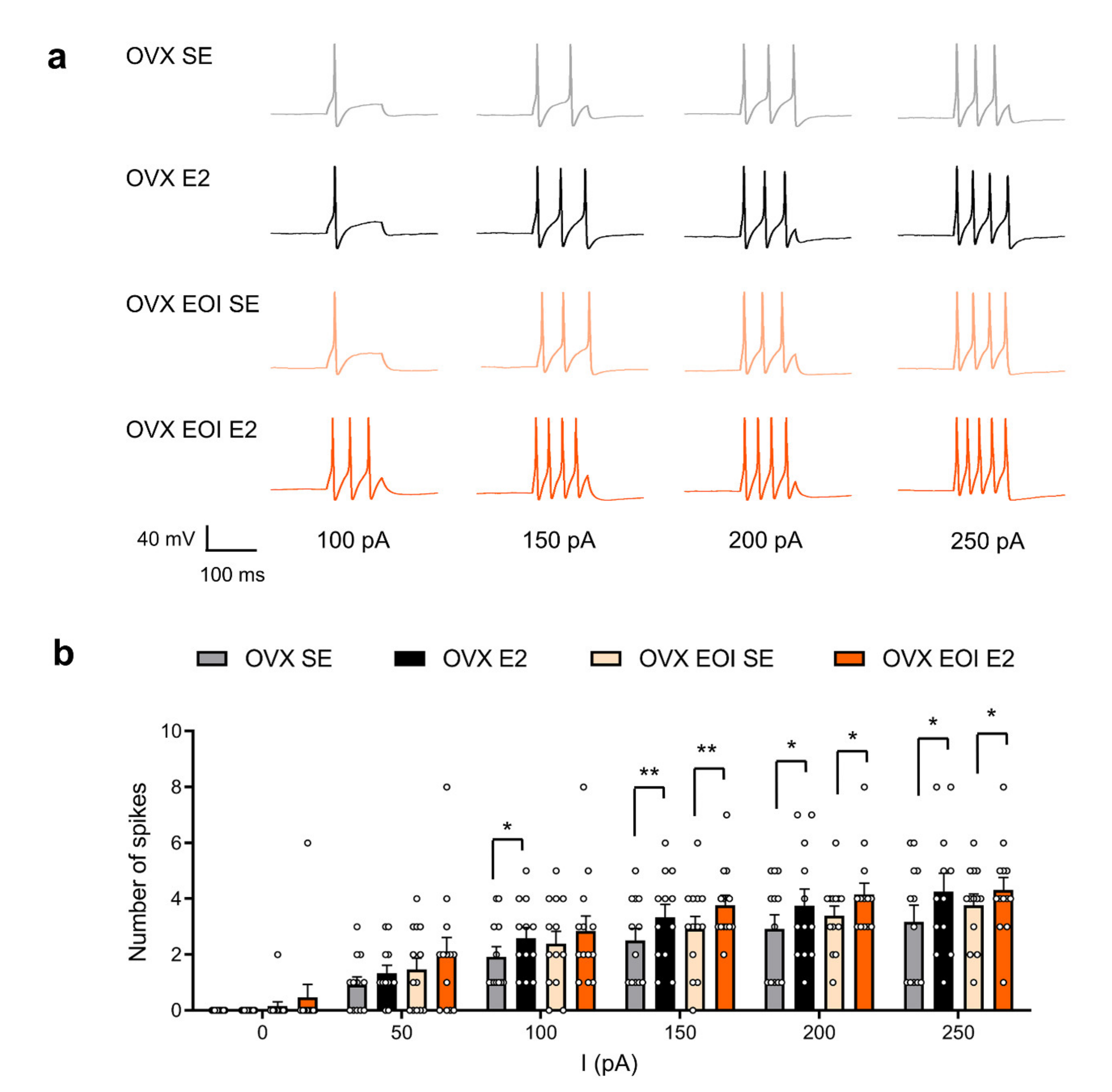
2.2. E2 Enhanced TG Neuronal Excitability in OVX and OVX EOI Rats
2.3. EOI Induced Functional TRPV1 Upregulation in TG Neurons from OVX Rats
2.4. E2 Potentiated EOI-Induced TRPV1 Upregulation in TGs
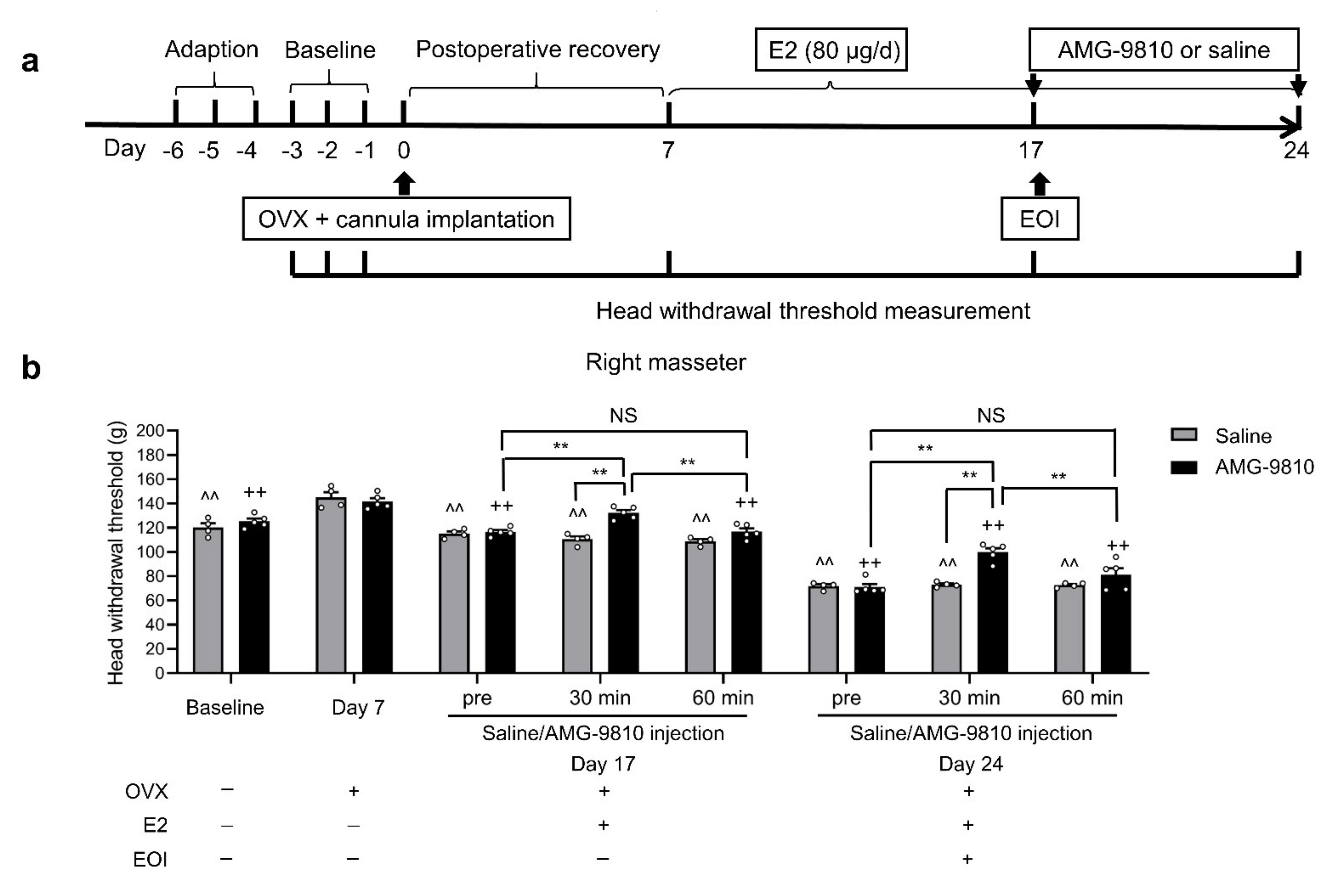
2.5. Blocking TRPV1 in TGs Attenuated E2-Mediated Masseter Sensitivity before and after EOI
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ovariectomized (OVX) Surgery
4.3. Administration of Drugs
4.4. Establishment of the EOI-Induced Chronic Masseter Hyperalgesia Model
4.5. Retrograde Labeling of Masseter Afferent Neurons
4.6. Guide Cannula Implantation for Intratrigeminal Ganglionic Microinjection
4.7. Evaluation of the Masseter Mechanical Sensitivity
4.8. Behavioral Experiments
4.8.1. Effects of E2 on EOI-Induced Masseter Hyperalgesia
4.8.2. Effects of the TRPV1 Antagonist AMG-9810 on E2-Potentiated EOI-Induced Masseter Hyperalgesia
4.9. E2 Determination
4.10. Western Blot Analysis
4.11. Immunofluorescence Staining
4.12. Isolation of TG Neurons
4.13. Patch-Clamp Recording
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OVX | Ovariectomized |
| E2 | 17β-estradiol |
| EOI | Experimental occlusal interference |
| TRPV1 | Transient receptor potential vanilloid-1 |
| WT | Wild-type |
| KO | Knockout |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethylsulfoxide |
| DiI | 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbo cyanine perchlorate |
| FITC | Fluorescein isothiocyanate |
| SD | Sprague–Dawley |
| AP | Action potential |
| CFA | Complete Freund’s adjuvant |
| TMD | Temporomandibular disorders |
| TMJ | Temporomandibular joints |
References
- List, T.; Jensen, R.H. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia Int. J. Headache 2017, 37, 692–704. [Google Scholar] [CrossRef]
- Carlsson, G.E. Epidemiology and treatment need for temporomandibular disorders. J. Orofac. Pain 1999, 13, 232. [Google Scholar]
- Warren, M.P.; Fried, J.L. Temporomandibular disorders and hormones in women. Cells Tissues Organs 2001, 169, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, N.; Racic, M.; Lecic, R.; Bozovic, D.; Kulic, M. Relationship Between Symptoms of Temporomandibular Disorders and Estrogen Levels in Women with Different Menstrual Status. J. Oral Facial Pain Headache 2018, 32, 151–158. [Google Scholar] [CrossRef] [PubMed]
- LeResche, L.; Mancl, L.; Sherman, J.J.; Gandara, B.; Dworkin, S.F. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain 2003, 106, 253–261. [Google Scholar] [CrossRef]
- Leresche, L.; Saunders, K.; Korff, M.; Barlow, W.; Dworkin, S.F. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain 1997, 69, 153. [Google Scholar] [CrossRef]
- Wu, Y.W.; Kou, X.X.; Bi, R.Y.; Xu, W.; Wang, K.W.; Gan, Y.H.; Ma, X.C. Hippocampal nerve growth factor potentiated by 17beta-estradiol and involved in allodynia of inflamed TMJ in rat. J. Pain Off. J. Am. Pain Soc. 2012, 13, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.Y.; Meng, Z.; Zhang, P.; Wang, X.D.; Ding, Y.; Gan, Y.H. Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ. PLoS ONE 2017, 12, e0178589. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Bi, Y.P.; Kou, X.X.; Xu, W.; Ma, L.Q.; Wang, K.W.; Gan, Y.H.; Ma, X.C. 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8710–8719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, W.X.; Sun, J.R.; Zhu, T.T.; Fan, J.; Yu, L.H.; Burnstock, G.; Yang, H.; Ma, B. Inhibitory effect of estrogen receptor beta on P2X3 receptors during inflammation in rats. Purinergic Signal. 2017, 13, 105–117. [Google Scholar] [CrossRef]
- Sarajari, S.; Oblinger, M.M. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp. Neurol. 2010, 224, 163–169. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Ju, B.G.; Yune, T.Y. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, Q.F.; Li, K.; Light, A.R.; Fu, K.Y. Experimental occlusal interference induces long-term masticatory muscle hyperalgesia in rats. Pain 2009, 144, 287–293. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Cioffi, J.L.; Bereiter, D.F. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch. Oral Biol. 2005, 50, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Flake, N.M.; Bonebreak, D.B.; Gold, M.S. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J. Neurophysiol. 2005, 93, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Liverman, C.S.; Brown, J.W.; Sandhir, R.; Klein, R.M.; McCarson, K.; Berman, N.E. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: Evidence for a peripheral mechanism of allodynia. Cephalalgia Int. J. Headache 2009, 29, 520–531. [Google Scholar] [CrossRef]
- Cao, E.; Cordero-Morales, J.F.; Liu, B.; Qin, F.; Julius, D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013, 77, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.Y.; Lee, J.S.; Zhang, Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 2009, 144, 270–277. [Google Scholar] [CrossRef]
- Cho, T.; Chaban, V.V. Expression of P2X3 and TRPV1 receptors in primary sensory neurons from estrogen receptors-alpha and estrogen receptor-beta knockout mice. Neuroreport 2012, 23, 530–534. [Google Scholar] [CrossRef]
- Xu, X.X.; Cao, Y.; Ding, T.T.; Fu, K.Y.; Li, Y.; Xie, Q.F. Role of TRPV1 and ASIC3 channels in experimental occlusal interference-induced hyperalgesia in rat masseter muscle. Eur. J. Pain 2016, 20, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.O.; Theodorsson, A.; Ingberg, E.; Isaksson, I.M.; Theodorsson, E. Ovariectomy and 17beta-estradiol replacement in rats and mice: A visual demonstration. J. Vis. Exp. JoVE 2012, 64, e4013. [Google Scholar]
- Kumar, V.; Sur, V.P.; Guha, R.; Konar, A.; Hazra, S. Estrogen Modulates Corneal Nociception and Maintains Corneal Homeostasis in Rat Eye. Cornea 2018, 37, 508–514. [Google Scholar] [CrossRef]
- Yan, T.; Liu, B.; Du, D.; Eisenach, J.C.; Tong, C. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth. Analg. 2007, 104, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.F.; Yang, G.J.; Bi, R.Y.; Mo, S.Y.; Gan, Y.H.; Xie, Q.F. Genistein Antagonizes 17beta-Estradiol Effects on Glutamate-Evoked Masseter Muscle Hypernociception in Rats. Front. Neurol. 2018, 9, 649. [Google Scholar] [CrossRef]
- Wise, E.A.; Riley, J.L., III; Robinson, M.E. Clinical pain perception and hormone replacement therapy in postmenopausal women experiencing orofacial pain. Clin. J. Pain 2000, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Saczko, J.; Michel, O.; Chwilkowska, A.; Sawicka, E.; Maczynska, J.; Kulbacka, J. Estrogen Receptors in Cell Membranes: Regulation and Signaling. Adv. Anat. Embryol. Cell Biol. 2017, 227, 93–105. [Google Scholar]
- Tashiro, A.; Okamoto, K.; Bereiter, D.A. Rapid estrogenic effects on TMJ-responsive brainstem neurons. J. Dent. Res. 2012, 91, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Saleeon, W.; Jansri, U.; Srikiatkhachorn, A.; Bongsebandhu-Phubhakdi, S. The estrous cycle modulates voltage-gated ion channels in trigeminal ganglion neurons. J. Physiol. Sci. JPS 2015, 65 (Suppl. S2), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Carrer, H.F.; Araque, A.; Buno, W. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6338–6344. [Google Scholar] [CrossRef]
- Yong, C.B.; Oh, J.M.; Hwang, S.J.; Shigenaga, Y.; Valtschanoff, J.G. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J. Comp. Neurol. 2010, 478, 62–71. [Google Scholar]
- Quartu, M.; Serra, M.P.; Boi, M.; Poddighe, L.; Picci, C.; Demontis, R.; Fiacco, M.D. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: Immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J. Anat. 2016, 229, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Hao, T.; Kou, X.X.; Gan, Y.H.; Ma, X.C. Synovial TRPV1 is upregulated by 17-beta-estradiol and involved in allodynia of inflamed temporomandibular joints in female rats. Arch. Oral Biol. 2015, 60, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Capsaicin mimics mechanical load-induced intracellular signaling events Involvement of TRPV1-mediated calcium signaling in induction of skeletal muscle hypertrophy. Channels 2013, 7, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B.; et al. Oxytocin Modulates Nociception as an Agonist of Pain-Sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Asgar, J.; Joseph, J.; Ro, J.Y.; Wei, F.; Campbell, J.N.; Chung, M.-K. Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J. Biol. Chem. 2017, 292, 8291–8303. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Q.; Mendell, L.M. Neurotrophins and hyperalgesia. Proc. Natl. Acad. Sci. USA 1999, 96, 7693–7696. [Google Scholar] [CrossRef]
- Touska, F.; Marsakova, L.; Teisinger, J.; Vlachova, V. A “Cute” Desensitization of TRPV1. Curr. Pharm. Biotechnol. 2011, 12, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Vyklicky, L.; Novakova-Tousova, K.; Benedikt, J.; Samad, A.; Touska, F.; Vlachova, V. Calcium-Dependent Desensitization of Vanilloid Receptor TRPV1: A Mechanism Possibly Involved in Analgesia Induced by Topical Application of Capsaicin. Physiol. Res. 2008, 57, S59–S68. [Google Scholar] [CrossRef]
- Devesa, I.; Ferrándiz-Huertas, C.; Mathivanan, S.; Wolf, C.; Luján, R.; Changeux, J.-P.; Ferrer-Montiel, A. αCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc. Natl. Acad. Sci. USA 2014, 111, 18345–18350. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, Y.; Keast, J.R.; Osborne, P.B. 17beta-estradiol activates estrogen receptor beta-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology 2008, 149, 5540–5548. [Google Scholar] [CrossRef]
- Liu, B. Functional Recovery from Desensitization of Vanilloid Receptor TRPV1 Requires Resynthesis of Phosphatidylinositol 4,5-Bisphosphate. J. Neurosci. 2005, 25, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Koplas, P.A.; Rosenberg, R.L.; Oxford, G.S. The Role of Calcium in the Desensitization of Capsaicin Responses in Rat Dorsal Root Ganglion Neurons. J. Neurosci. 1997, 17, 3525–3537. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.S.; Cheng, X.; Jeffry, J.A.; Disney, K.E.; Premkumar, L.S. Sumatriptan inhibits TRPV1 channels in trigeminal neurons. Headache 2012, 52, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Herndon, C. New roles for neuronal estrogen receptors. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2017, 29, e13121. [Google Scholar] [CrossRef]
- Payrits, M.; Saghy, E.; Cseko, K.; Pohoczky, K.; Bolcskei, K.; Ernszt, D.; Barabas, K.; Szolcsanyi, J.; Abraham, I.M.; Helyes, Z.; et al. Estradiol Sensitizes the Transient Receptor Potential Vanilloid 1 Receptor in Pain Responses. Endocrinology 2017, 158, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Sugimura, M.; Yoshida, M.; Sekine, S.; Kawano, A.; Oyamaguchi, A.; Maegawa, H.; Niwa, H. Estrogens Exacerbate Nociceptive Pain via Up-Regulation of TRPV1 and ANO1 in Trigeminal Primary Neurons of Female Rats. Endocrinology 2016, 157, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Saloman, J.L.; Weiland, G.; Auh, Q.S.; Chung, M.K.; Ro, J.Y. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012, 153, 1514–1524. [Google Scholar] [CrossRef]
- Honda, K.; Shinoda, M.; Kondo, M.; Shimizu, K.; Yonemoto, H.; Otsuki, K.; Akasaka, R.; Furukawa, A.; Iwata, K. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain 2017, 158, 1754–1764. [Google Scholar] [CrossRef]
- Fehrenbacher, J.C.; Loverme, J.; Clarke, W.; Hargreaves, K.M.; Piomelli, D.; Taylor, B.K. Rapid pain modulation with nuclear receptor ligands. Brain Res. Rev. 2009, 60, 114–124. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Liu, Y.; Zhu, K.; Zhang, Z.; Qiao, H.; Lu, Z.; Zhong, T.; Liu, Y.; Zhou, H. Blocking of TRPV-1 in the parodontium relieves orthodontic pain by inhibiting the expression of TRPV-1 in the trigeminal ganglion during experimental tooth movement in rats. Neurosci. Lett. 2016, 628, 67–72. [Google Scholar] [CrossRef]
- Araya, E.I.; Nones, C.F.M.; Ferreira, L.E.N.; Kopruszinski, C.M.; Cunha, J.M.D.; Chichorro, J.G. Role of peripheral and central TRPV1 receptors in facial heat hyperalgesia in streptozotocin-induced diabetic rats. Brain Res. 2017, 1670, 146–155. [Google Scholar] [CrossRef]
- Hiroki, O.; Kimiaki, K.; Shiori, M.; Makiko, K.; Makoto, T.; Kazue, M.; Masabumi, M. TRPV1 and TRPV4 Play Pivotal Roles in Delayed Onset Muscle Soreness. PLoS ONE 2013, 8, e65751. [Google Scholar]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chu, Y.; Han, L.; Li, M.; Li, Z.; LaVinka, P.C.; Sun, S.; Tang, Z.; Park, K.; Caterina, M.J.; et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014, 81, 873–887. [Google Scholar] [CrossRef]
- Chung, M.K.; Park, J.; Asgar, J.; Jin, Y.R. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol. Pain 2016, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Yang, Y.; Ji, P.; Kong, J.; Wu, Q.; Si, H. Upregulation of the Purinergic Receptor Subtype P2X3 in the Trigeminal Ganglion Is Involved in Orofacial Pain Induced by Occlusal Interference in Rats. J. Oral Facial Pain Headache 2016, 30, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, Y.; Ding, T.; Fu, K.; Xie, Q. Trigeminal purinergic P2X4 receptor involved in experimental occlusal interference-induced hyperalgesia in rat masseter muscle. Chin. J. Stomatol. 2016, 51, 176–181. [Google Scholar]
- De Angelis, F.; Marinelli, S.; Fioretti, B.; Catacuzzeno, L.; Franciolini, F.; Pavone, F.; Maria Tata, A. M2 Receptors Exert Analgesic Action on DRG Sensory Neurons by Negatively Modulating VR1 Activity. J. Cell. Physiol. 2014, 229, 783–790. [Google Scholar] [CrossRef]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, X.-X.; Cao, Y.; Mo, S.-Y.; Bai, S.-S.; Fan, Y.-Y.; Zhang, X.-Y.; Xie, Q.-F. 17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats. Int. J. Mol. Sci. 2021, 22, 6945. https://doi.org/10.3390/ijms22136945
Liu Y, Xu X-X, Cao Y, Mo S-Y, Bai S-S, Fan Y-Y, Zhang X-Y, Xie Q-F. 17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats. International Journal of Molecular Sciences. 2021; 22(13):6945. https://doi.org/10.3390/ijms22136945
Chicago/Turabian StyleLiu, Yun, Xiao-Xiang Xu, Ye Cao, Si-Yi Mo, Shan-Shan Bai, Ying-Ying Fan, Xiao-Yu Zhang, and Qiu-Fei Xie. 2021. "17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats" International Journal of Molecular Sciences 22, no. 13: 6945. https://doi.org/10.3390/ijms22136945
APA StyleLiu, Y., Xu, X.-X., Cao, Y., Mo, S.-Y., Bai, S.-S., Fan, Y.-Y., Zhang, X.-Y., & Xie, Q.-F. (2021). 17β-Estradiol Exacerbated Experimental Occlusal Interference-Induced Chronic Masseter Hyperalgesia by Increasing the Neuronal Excitability and TRPV1 Function of Trigeminal Ganglion in Ovariectomized Rats. International Journal of Molecular Sciences, 22(13), 6945. https://doi.org/10.3390/ijms22136945






