Long-Term Projections of the Natural Expansion of the Pine Wood Nematode in the Iberian Peninsula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Pine Tree Species Susceptibility to Pine Wood Nematode
- Scenario A: All pine tree species on the Iberian Peninsula are equally susceptible to the PWN. Under this scenario, the PWN spread modelling does not distinguish between pine species.
- Scenario H: Only pine species with high (P. pinaster and P. radiata) or very high (P. sylvestris) susceptibility to the PWN, following Menéndez-Gutiérrez et al. [28], can get infected and infect other pine trees. The other pine species were considered as neither targets nor sources of infection in the PWN spread modelling.
2.3. Forest Tree Species Maps
2.4. Climatic Suitability to Pine Wilt Expression
- Current climate (Scenario C): Only 1 km2 cells in which the current mean summer temperature is above 19.31 °C are considered climatically suitable for wilt expression [31]. Excluded from the climatically suitable areas was 23% of the total pine forest extent in the Iberian Peninsula when considering all pine tree species (Scenario A) or 38% of the area covered in the Iberian Peninsula by the pine tree species that are highly susceptible to the PWN (Scenario H). In Scenario C, we simulated PWN spread assuming no dispersal of infected beetles from the areas where the disease does not express due to climate constraints.
- Future climate (Scenario F): All the Iberian Peninsula, except the isolated locations mentioned above, is climatically suitable for the expression of PWD. All 1 km2 cells can act as spreaders of the PWN once they are invaded by the disease.
2.5. PWN Spread Model
- d, the mean dispersal distance of the vector beetle, Monochamus galloprovincialis.
- N, the number of infected beetles emerging from each infected tree and thus available for dispersal.
- k, the number of infected beetles that need to reach an uninfected pine tree to actually transmit the disease to that tree.
- β, a coefficient determining the tail fatness (kurtosis) of the vector beetle dispersal kernel.
- c, the number of infected trees per hectare of pine forest in newly infected areas. This parameter was used to convert the pine forest area in a cell i (ai) to the number of pine trees expected to be infected in that cell when PWN transmission occurs.
2.6. PWN Spread Modelling Scenarios
- Scenario AC: all pine tree species (Scenario A) and current climate (Scenario C) considered.
- Scenario AF: all pine tree species (Scenario A) and future climate (Scenario F) considered.
- Scenario HC: only highly susceptible tree pine species (Scenario H) and current climate (Scenario C) considered.
- Scenario HF: only highly susceptible tree species (Scenario H) and future climate (Scenario F) considered.
3. Results
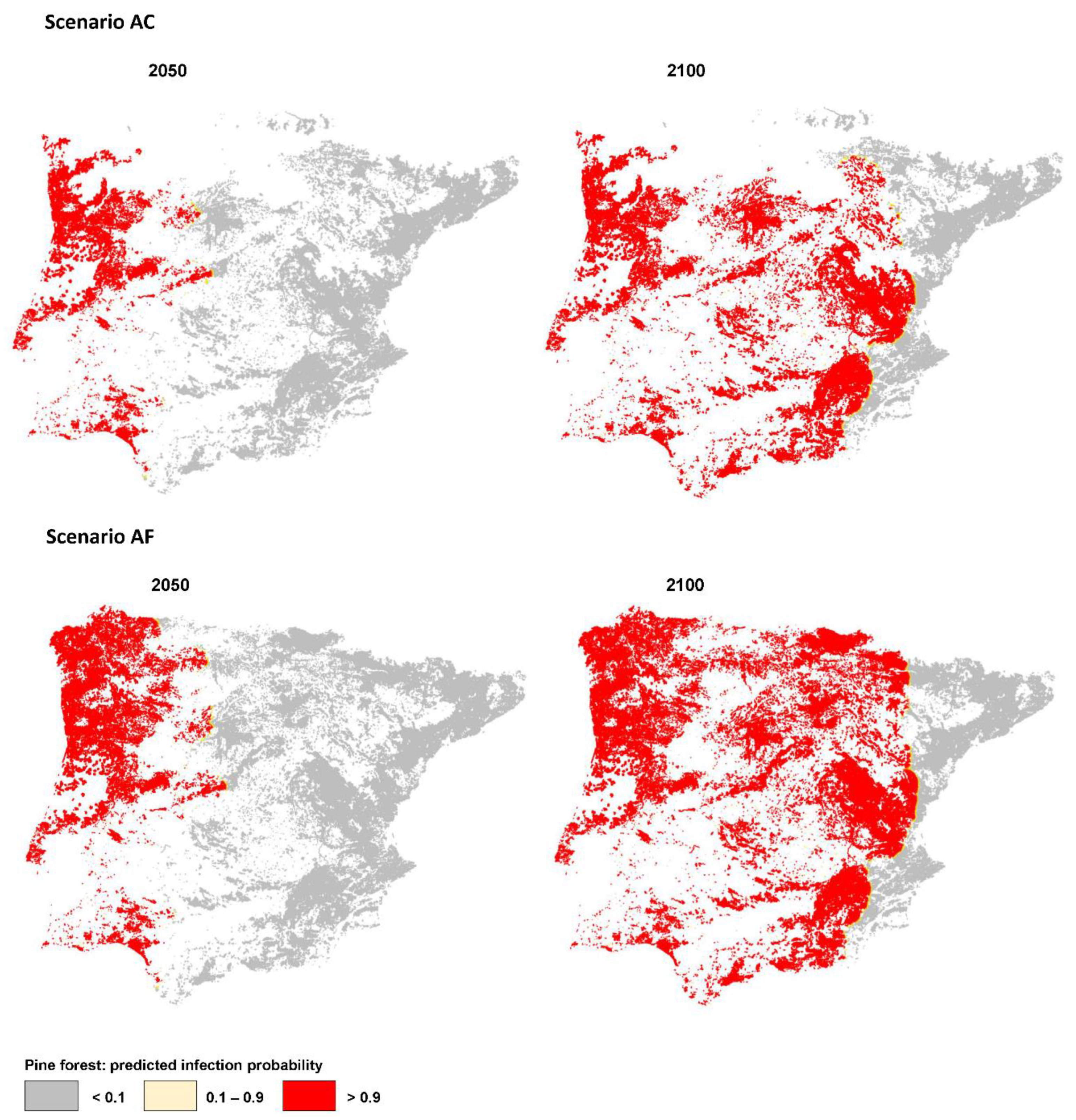
3.1. PWN Spread in Scenario AF: All Pine Forests and Future Climate
3.2. PWN Spread in Scenario AC: All Pine Forests and Current Climate
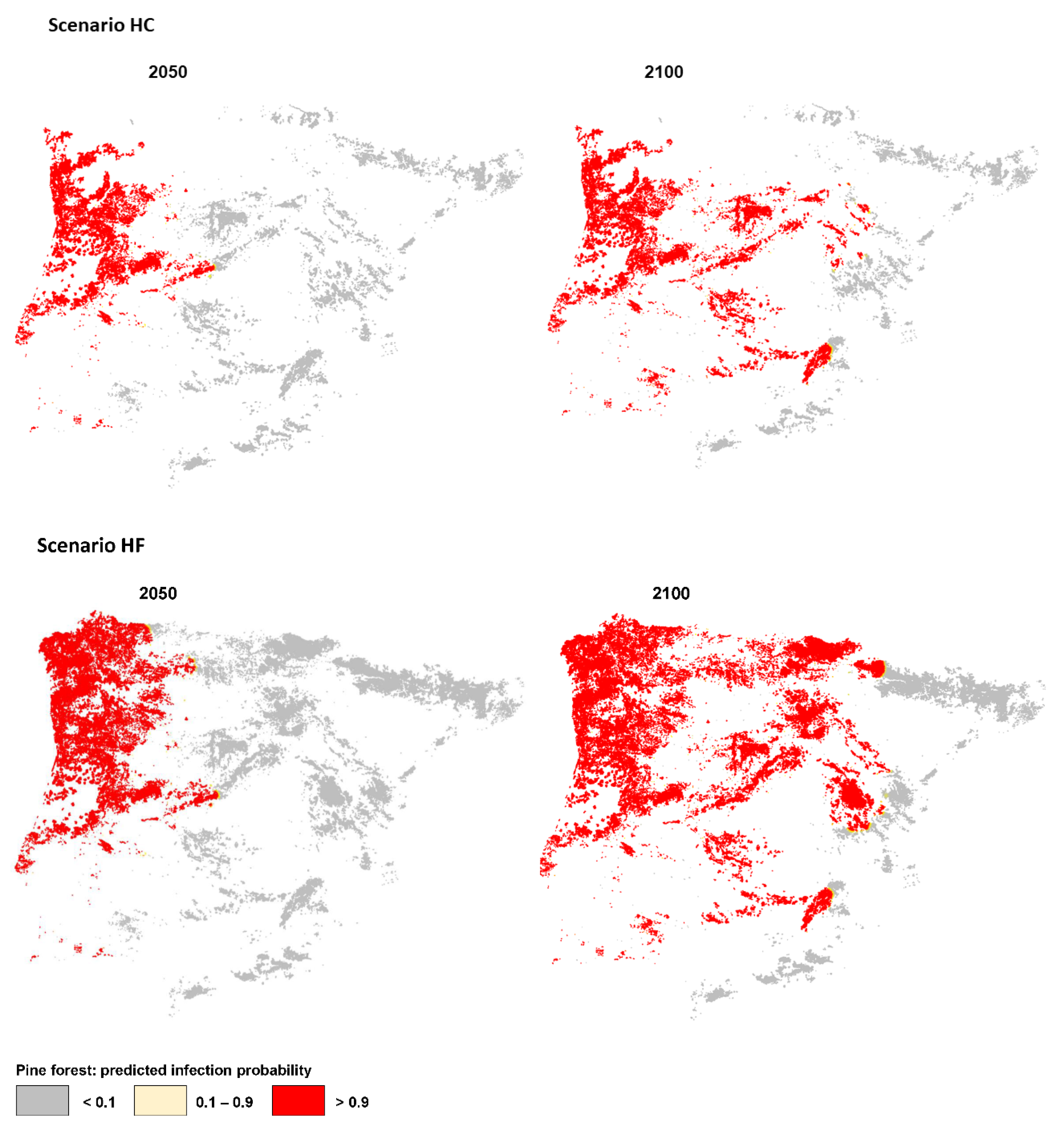
3.3. PWN Spread in Scenario HF: Highly Susceptible Pine Forests and Future Climate
3.4. PWN Spread in Scenario HC: Highly Susceptible Pine Forests and Current Climate
4. Discussion
4.1. The Large Spread Potential of the PWN in the Iberian Peninsula
4.2. The Impact of Current and Future Climate on PWN Spread
4.3. The Importance of Pine Tree Species Susceptibility to the PWN
4.4. Measures to Contain PWN Spread
5. Conclusions
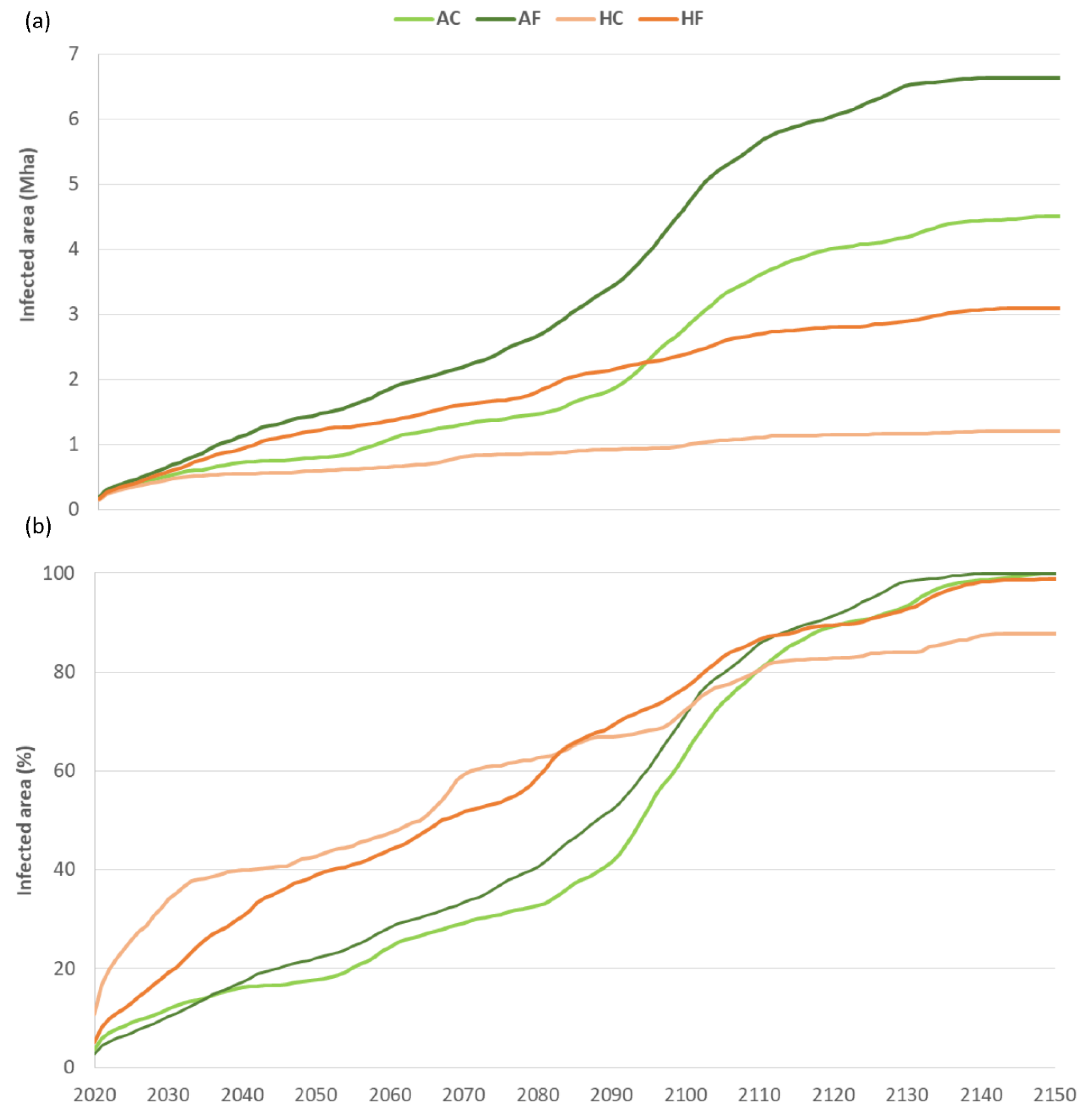
- In the absence of effective containment measures, the PWN will spread naturally, through the dispersal of its vector beetle, to the entire Iberian Peninsula, including the Pyrenees, which would provide a gateway for further PWN expansion into France and the rest of Europe.
- The natural spread of the PWN will be relatively gradual, with an average rate of 0.83% of the total Iberian forest area infected yearly, so that it will take a century or longer for all the susceptible pine forests in Spain to be invaded by the PWN.
- Climate is not an important limiting factor for long-term spread of the PWN, because (i) there is ample availability of alternative pathways for PWN dispersal through the areas that are already suitable for the PWN in the current climatic conditions; and (ii) climate change projections, even conservative ones, indicate that future temperatures will make the whole Iberian Peninsula suitable for PWN transmission by or before the end of this century, except for some isolated mountain peaks.
- Unlike climatic conditions, the susceptibility of different pine tree species to PWN is by far the main determinant of PWN spread rates and of the extent of forest area affected by the wilt disease. Our findings highlight the need and importance of integrating data on individual pine tree species into predictive models on the spread and magnitude of damage caused by the PWN. Additional research that can refine our knowledge on tree species susceptibility is hence of particular importance for more accurate modelling of the PWN spread and for better guiding related containment efforts.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Rodrigues, J.M. National eradication programme for the pinewood nematode. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008; pp. 5–14. [Google Scholar]
- EPPO. Reporting Service (2010/051), Reporting Service (2010/058): Detected on a Single Pinus Tree Near Caceres (Extremadura) in 2008; Reporting Service (2010/202): Detected in November 2010 on Seven Trees (P. pinaster) in a Forest Area in the Municipality of ‘As Neves’ (Province of Pontevedra), in Galicia; European and Mediterranean Plant Protection Organization (EPPO): Paris, France, 2010. Available online: https://gd.eppo.int/taxon/BURSXY/reporting (accessed on 3 May 2021).
- EPPO. Reporting Service (2012/047): Detected in One Tree (P. pinaster) in a Forest of the ‘Monte Barroco Toiriña’ (Province of Cáceres in Extremadura; European and Mediterranean Plant Protection Organization (EPPO): Paris, France, 2012. Available online: https://gd.eppo.int/taxon/BURSXY/reporting (accessed on 3 May 2021).
- EPPO. Reporting Service (2014/020): Detected in December 2013 in a Sample Collected from Symptomatic Pine Trees in a Forest Stand Located in the Municipality of ‘Sancti-Spíritus’, Province of Salamanca (Castilla y León); European and Mediterranean Plant Protection Organization (EPPO): Paris, France, 2014. Available online: https://gd.eppo.int/taxon/BURSXY/reporting (accessed on 3 May 2021).
- EPPO. Reporting Service (2018/140): Detected in one Pine Tree in a Forest Area in the Municipality of Lagunilla (Salamanca Province, Castilla y Léon); European and Mediterranean Plant Protection Organization (EPPO): Paris, France, 2018. Available online: https://gd.eppo.int/taxon/BURSXY/reporting (accessed on 3 May 2021).
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Miranda, J.; Álvarez, B.; González-Casas, A.; Mayor, E.; et al. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Xunta de Galicia. Resolución de 28 de Diciembre de 2018 de la Dirección General de Ganadería Agricultura e Industrias Agroalimentarias por la que se Declara en el Territorio de la Comunidad Autónoma de Galicia la Presencia de cinco Nuevos Positivos del Organismo de Cuarentena Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle et al. (Nematodo del pino) y se Ordena Comenzar las Medidas Para su Erradicación; Diario Oficial de Galicia: Galicia, Spain, 2019; p. 1159. [Google Scholar]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Vector–Host Tree Relationships and the Abiotic Environment. In Pine Wilt Disease; Springer: Tokyo, Japan, 2008; pp. 144–161. [Google Scholar]
- Kobayashi, F.; Yamane, A.; Ikeda, T. The Japanese pine sawyer beetle as the vector of pine wilt disease. Annu. Rev. Entomol. 1984, 29, 115–135. [Google Scholar] [CrossRef]
- Sousa, E.; Rodrígues, J.M.; Bonifácio, L.F.; Naves, P.M.; Rodrígues, A. Management and control of the pine Wood nematode, Bursaphelenchus xylophilus, in Portugal. In Nematodes: Morphology, Functions and Management Strategies; Nova Science Publishers Inc.: New York, NY, USA, 2011; Chapter 6. [Google Scholar]
- Sousa, E.; Bravo, M.A.; Pires, J.; Naves, P.; Penas, A.C. Bursaphelenchus xylophilus (Nematoda; Aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology 2001, 3, 89–91. [Google Scholar]
- Linit, M.J. Nematode-vector relationships in the pine wilt system. J. Nematol. 1988, 20, 227–235. [Google Scholar]
- Naves, P.M.; Camacho, S.; De Sousa, E.M.; Quartau, J.A. Transmission of the pine wood nematode Bursaphelenchus xylophilus through feeding activity of Monochamus galloprovincialis (Col., Cerambycidae). J. Appl. Entomol. 2007, 131, 21–25. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Webster, J.; Mota, M. Pine Wilt Disease: Global Issues, Trade and Economic Impact; Springer: Berlin/Heidelberg, Germany, 2008; pp. 315–316. [Google Scholar]
- Soliman, T.; Mourits, M.C.; Van Der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.O. Framework for modelling economic impacts of invasive species, applied to pine wood nematode in Europe. PLoS ONE 2012, 7, e45505. [Google Scholar]
- Hu, G.; Xu, X.; Wang, Y.; Lu, G.; Feeley, K.J.; Yu, M. Regeneration of different plant functional types in a Masson pine forest following pine wilt disease. PLoS ONE 2012, 7, e36432. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, B.; Beck, P.S. Invasive species may disrupt protected area networks: Insights from the pine wood nematode spread in Portugal. Forests 2018, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Miyahara, F.; Tsutsumi, Y.; Kondo, R. Relationship between resistance to pine wilt disease and the migration or proliferation of pine wood nematodes. Eur. J. Plant Pathol. 2018, 122, 529–538. [Google Scholar] [CrossRef]
- Zhang, F.; Kajiwara, J.; Mori, Y.; Ohira, M.; Tsutsumi, Y.; Kondo, R. Metabolites from resistant and susceptible Pinus thunbergii after inoculation with pine wood nematode. Am. J. Plant Sci. 2013, 4, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.F.; McNamara, D.G.; Braasch, H.; Chadoeuf, J.; Magnusson, C. Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Da Silva, M.N.; Solla, A.; Sampedro, L.; Zas, R.; Vasconcelos, M.W. Susceptibility to the pinewood nematode of four pine species involved in potential range expansion across Europe. Tree Physiol. 2015, 35, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, C.S.; Gonçalves, E.V.; Firmino, P.N.; Calvão, T.; Fonseca, L.; Abrantes, I.; Máguas, C. Differences in constitutive and inducible defences in pine species determining susceptibility to pinewood nematode. Plant Pathol. 2017, 66, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Menéndez-Gutiérrez, M.; Alonso, M.; Jiménez, E.; Toval, G.; Mansilla, P.; Abelleira, A.; Abelleira-Sanmartín, A.; Díaz, R. Interspecific variation of constitutive chemical compounds in Pinus spp. xylem and susceptibility to pinewood nematode (Bursaphelenchus xylophilus). Eur. J. Plant Pathol. 2018, 150, 939–953. [Google Scholar] [CrossRef]
- Inácio, M.L.; Nóbrega, F.; Vieira, P.; Bonifácio, L.; Naves, P.; Sousa, E.; Mota, M. First detection of Bursaphelenchus xylophilus associated with Pinus nigra in Portugal and in Europe. For. Pathol. 2017, 45, 235–238. [Google Scholar] [CrossRef]
- De la Fuente, B.; Saura, S.; Beck, P.S.A. Predicting the spread of an invasive tree pest: The pine wood nematode in Southern Europe. J. Appl. Ecol. 2018, 55, 2374–2385. [Google Scholar] [CrossRef]
- Robinet, C.; Van Opstal, N.; Baker, R.; Roques, A. Applying a spread model to identify the entry points from which the pine wood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol. Invasions 2011, 13, 2981–2995. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, T.A.; Webster, J.M. Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Evans, H.F. Plant Health Risk and Monitoring Evaluation (PHRAME) Final Report. In PHRAME Final Report and Conclusions. 2007. Available online: https://www.forestry.gov.uk/fr/infd-7xrfx9 (accessed on 25 April 2020).
- Gruffudd, H.R.; Jenkins, T.A.R.; Evans, H.F. Using an evapo-transpiration model to predict the risk and expression of symptoms of pine wilt disease across Europe. Biol. Invasions 2016, 18, 2823–2840. [Google Scholar] [CrossRef]
- Haran, J.; Roques, A.; Bernard, A.; Robinet, C.; Roux, G. Altitudinal Barrier to the Spread of an Invasive Species: Could the Pyrenean Chain Slow the Natural Spread of the Pinewood Nematode? PLoS ONE 2015, 10, e0134126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, G.; Díez, J.J.; Ibeas, F.; Pajares, J.A. Assessing pine wilt disease risk under a climate change scenario in Northwestern Spain. In Managing Forest Ecosystems: The Challenge of Climate Change; Springer: Dordrecht, The Netherlands, 2008; pp. 269–282. [Google Scholar]
- Roques, A.; Zhao, L.; Sun, J.; Robinet, C. Pine wood nematode, pine wilt disease, vector beetle and pine tree: How a multiplayer system could reply to climate change. Clim. Chang. Insect Pests 2015, 7, 220–234. [Google Scholar]
- Pukkala, T.; Möykkynen, T.; Robinet, C. Comparison of the potential spread of pinewood nematode (Bursaphelenchus xylophilus) in Finland and Iberia simulated with a cellular automaton model. For. Pathol. 2014, 44, 341–352. [Google Scholar] [CrossRef]
- Ninyerola, M.; Pons, X.; Roure, J.M. Atlas Climático Digital de la Península Ibérica: Metodología y Aplicaciones en Bioclimatología y Geobotánica; Universidad Autónoma de Barcelona: Barcelona, Spain, 2005. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Sheffield, J.; Wood, E.F. Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim. Dyn. 2008, 31, 79–105. [Google Scholar] [CrossRef]
- Gao, R.; Shi, J.; Huang, R.; Wang, Z.; Luo, Y. Effects of pine wilt disease invasion on soil properties and Masson pine forest communities in the Three Gorges reservoir region, China. Ecol. Evol. 2015, 5, 1702–1716. [Google Scholar] [CrossRef] [PubMed]
- Gallego, D.; Sánchez-García, F.J.; Mas, H.; Campo, M.T.; Lencina, J.L. Estudio de la capacidad de vuelo a larga distancia de Monochamus galloprovincialis en un mosaico agro-forestal. Boletín Sanid. Veg. Plagas 2012, 38, 109–123. [Google Scholar]
- Álvarez, G.; Etxebeste, I.; Gallego, D.; David, G.; Bonifacio, L.; Jactel, H.; Pajares, J.A. Optimization of traps for live trapping of pine wood nematode vector Monochamus galloprovincialis. J. Appl. Entomol. 2015, 139, 618–626. [Google Scholar] [CrossRef]
- Etxebeste, I.; Sanchez-Husillos, E.; Álvarez, G.; Gisbert, H.M.i.; Pajares, J. Dispersal of Monochamus galloprovincialis as recorded by mark–release–recapture using pheromone traps. J. Appl. Entomol. 2015, 140, 485–499. [Google Scholar] [CrossRef]
- Firmino, P.N.; Calvão, T.; Ayres, M.P.; Pimentel, C.S. Monochamus galloprovincialis and Bursaphelenchus xylophilus life history in an area severely affected by pine wilt disease: Implications for forest management. For. Ecol. Manag. 2017, 389, 105–115. [Google Scholar] [CrossRef]
- De la Fuente, B.; Beck, P.S. Management measures to control pine wood nematode spread in Europe. J. Appl. Ecol. 2019, 56, 2577–2580. [Google Scholar] [CrossRef]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt in Japan: From First Incidence to the Present. In Pine Wilt Disease; Springer: Tokyo, Japan, 2008; pp. 5–12. [Google Scholar]
- Jactel, H.; Menassieu, P.; Vetillard, F.; Gaulier, A.; Samalens, J.C.; Brockerhoff, E.G. Tree species diversity reduces the invasibility of maritime pine stands by the bast scale, Matsucoccus feytaudi. Can. J. For. Res. 2006, 36, 314–323. [Google Scholar] [CrossRef]
- Muzika, R.M.; Liebhold, A.M. A critique of silvicultural approaches to managing defoliating insects in North America. Agric. For. Entomol. 2000, 2, 97–105. [Google Scholar] [CrossRef]
- Liebhold, A.M. Forest pest management in a changing world. Int. J. Pest Manag. 2012, 58, 289–295. [Google Scholar] [CrossRef]
- Gaspar, D.; Trindade, C.; Usié, A.; Meireles, B.; Barbosa, P.; Fortes, A.M.; Ramos, A.M. Expression profiling in Pinus pinaster in response to infection with the pine wood nematode Bursaphelenchus xylophilus. Forests 2017, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A.; Van Opstal, N.; Baker, R.; Pan, H.; Fang, G.; Sun, J. Anthropogenic pathways in the spread of the pinewood nematode and predictions of future expansion. In International Congress on Biological Invasions; HAL-INRAE: Fuzhou, China, 2009; p. 1. [Google Scholar]
- Hudgins, E.J.; Liebhold, A.M.; Leung, B. Predicting the spread of all invasive forest pests in the United States. Ecol. Lett. 2017, 20, 426–435. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente, B.; Saura, S. Long-Term Projections of the Natural Expansion of the Pine Wood Nematode in the Iberian Peninsula. Forests 2021, 12, 849. https://doi.org/10.3390/f12070849
de la Fuente B, Saura S. Long-Term Projections of the Natural Expansion of the Pine Wood Nematode in the Iberian Peninsula. Forests. 2021; 12(7):849. https://doi.org/10.3390/f12070849
Chicago/Turabian Stylede la Fuente, Begoña, and Santiago Saura. 2021. "Long-Term Projections of the Natural Expansion of the Pine Wood Nematode in the Iberian Peninsula" Forests 12, no. 7: 849. https://doi.org/10.3390/f12070849





