Impact of Type of Parturition on Colostrum Microbiota Composition and Puppy Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cesarean Section (CS)
2.3. Vaginal Delivery (VD)
2.4. Collection of the Colostrum
2.5. Collection of the Meconium
2.6. Monitoring Puppy’s Growth
2.7. Bacteriological Examination
2.8. Statistical Analyses
3. Results
3.1. Collection of Samples
3.2. Bacteria Isolation
3.2.1. Bacteria Isolated from Meconium and Colostrum
3.2.2. Meconium Samples
3.2.3. Bacteria Isolated from the Colostrum Samples According to the Type of Parturition
Relation between Colostrum Microbiota and the Type of Parturition
Prevalence of Bacteria in Colostrum Samples According to Their Oxygen Requirement
3.3. Bacteria Isolated from the Colostrum and the Meconium Samples
Comparison between the Colostrum and the Meconium Microbiota According to the Type of Parturition
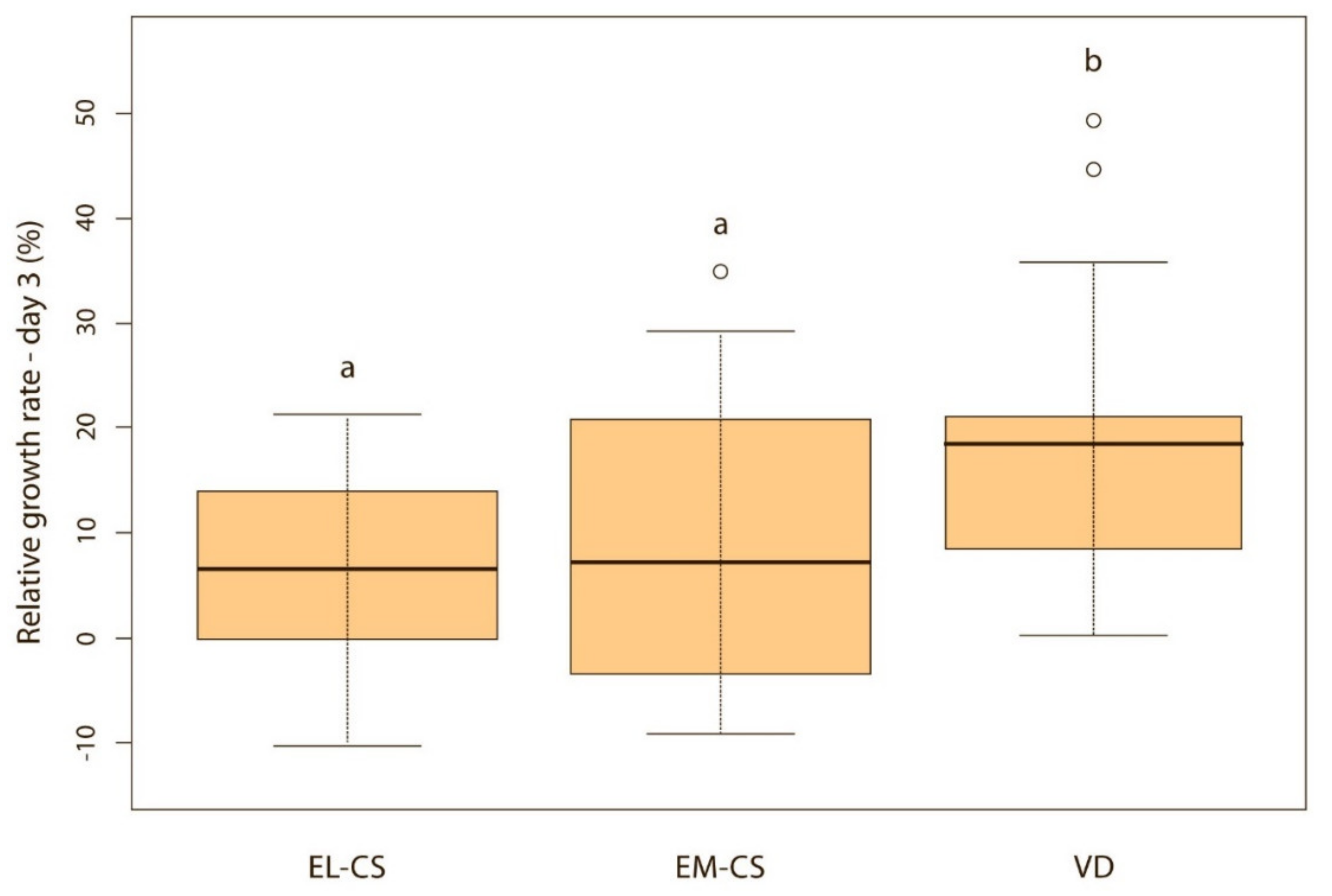
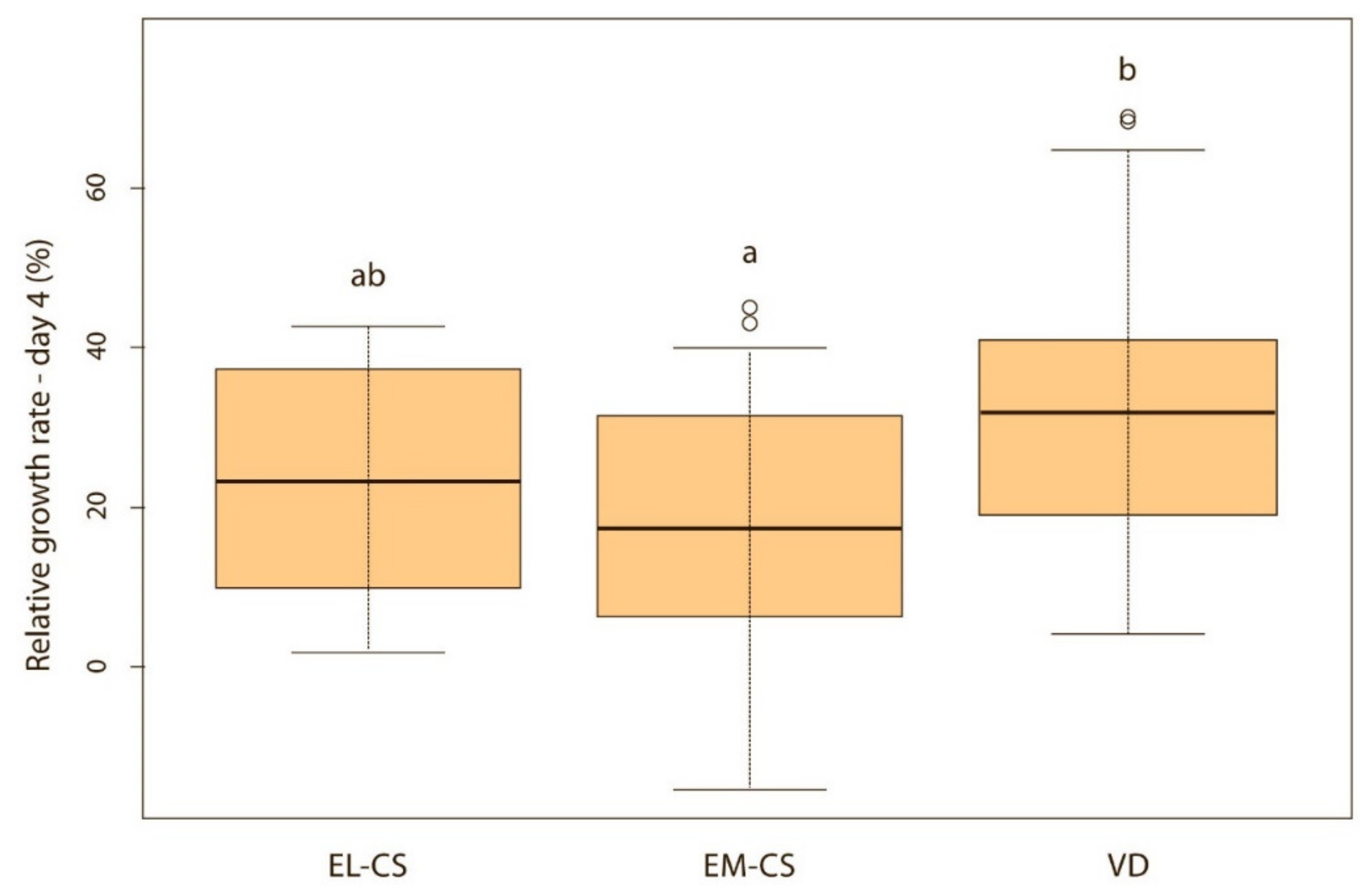
3.4. Growth Rate
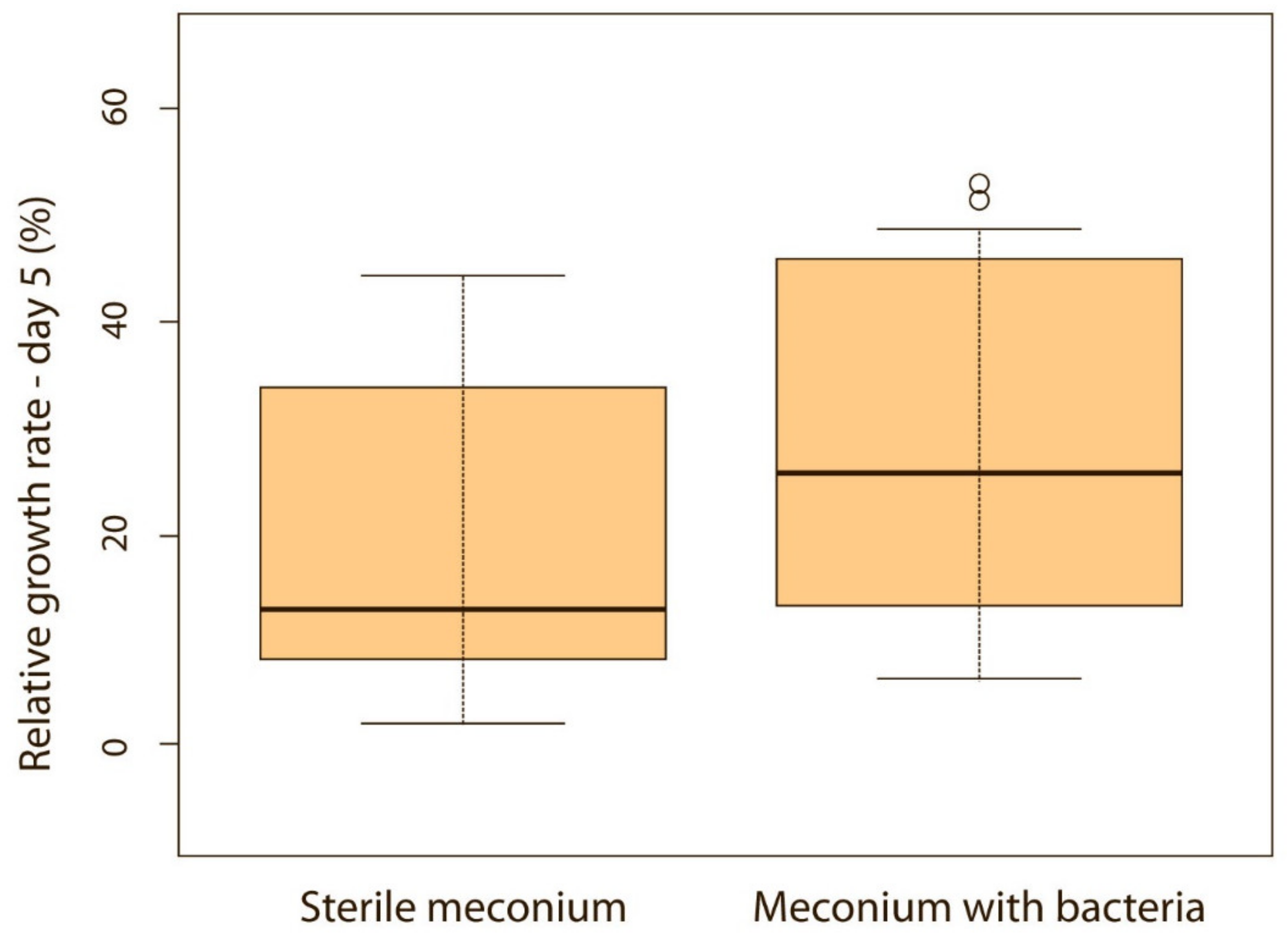
The Correlation between the Relative Weight Gain between the Puppies That Had Bacteria Present in the Meconium and Those without Bacteria in the Meconium
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drago, L.; Toscano, M.; De Grandi, R.; Grossi, E.; Padovani, E.M.; Peroni, D.G. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy versus Burundi. ISME J. 2017, 11, 875–884. [Google Scholar] [CrossRef]
- Borghesi, J.; Mario, L.; Rodrigues, M.; Favaron, P.; Miglino, M. Immunoglobulin Transport during Gestation in Domestic Animals and Humans—A Review. Open J. Anim. Sci. 2014, 4, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Chastant-Maillard, S.; Aggouni, C.; Albaret, A.; Fournier, A.; Mila, H. Canine and feline colostrum. Reprod. Domest. Anim. 2017, 52, 148–152. [Google Scholar] [CrossRef]
- Satyaraj, E.; Reynolds, A.; Pelker, R.; Labuda, J.; Zhang, P.; Sun, P. Supplementation of diets with bovine colostrum influences immune function in dogs. Br. J. Nutr. 2013, 110, 2216–2221. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishna, K.P.; Hand, T.W. Influence of Maternal Milk on the Neonatal Intestinal Microbiome. Nutrients 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic review of the human milk microbiota. Nutr. Clin. Pract. 2016, 32, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. 2017, 17, 205. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, U.; Amoudruz, P.; Johansson, M.A.; Haileselassie, Y.; Ongoiba, A.; Kayentao, K.; Traore, B.; Doumbo, S.; Schollin, J.; Doumbo, O.; et al. Maternal country of origin, breast milk characteristicsand potential influences on immunity in offspring. Clin. Exp. Immunol. 2010, 162, 500–509. [Google Scholar] [CrossRef]
- Amoudruz, P.; Holmlund, U.; Schollin, J.; Sverremark-Ekström, E.; Montgomery, S.M. Maternal country of birth and previous pregnancies are associated with breastmilk characteristics. Pediatr. Allergy Immunol. 2009, 20, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Kalanetra, K.M.; Mills, D.A.; Underwood, M.A. Buccal administration of human colostrum: Impact on the oral microbiota of premature infants. J. Perinatol. 2016, 36, 106–111. [Google Scholar] [CrossRef]
- Indrebø, A.; Trangerud, C.; Moe, L. Canine neonatal mortality in four large breeds. Acta Vet. Scand. 2007, 49, S2. [Google Scholar] [CrossRef] [Green Version]
- Mila, H.; Grellet, A.; Chastant-Maillard, S. Prognostic value of birth weight and early weight gain on neonatal and pediatric mortality: A longitudinal study on 870 puppies. In Proceedings of the Program and Presented at the 7th International Symposium on Canine and Feline Reproduction 2012, Whistler, BC, Canada, 26–29 July 2012; pp. 163–164. [Google Scholar]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addis, M.F.; Tanca, A.; Uzzau, S.; Oikonomou, G.; Bicalho, R.C.; Moroni, P. The bovine milk microbiota: Insights and perspectives from -omics studies. Mol. Biosyst. 2016, 12, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falentin, H.; Rault, L.; Nicolas, A.; Bouchard, D.S.; Lassalas, J.; Lamberton, P.; Aubry, J.M.; Marnet, P.G.; Le Loir, Y.; Even, S. Bovine teat microbiome analysis revealed reduced alpha diversity and significant changes in taxonomic profiles in quarters with a history of mastitis. Front. Microbiol. 2016, 7, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [Green Version]
- McInnis, E.A.; Kalanetra, K.M.; Mills, D.A.; Maga, E.A. Analysis of raw goat milk microbiota: Impact of stage of lactation and lysozyme on microbial diversity. Food Microbiol. 2015, 46, 121–131. [Google Scholar] [CrossRef]
- Treven, P.; Mrak, V.; Bogović Matijašić, B.; Horvat, S.; Rogelj, I. Administration of probiotics Lactobacillus rhamnosus GG and Lactobacillus gasseri K7 during pregnancy and lactation changes mouse mesenteric lymph nodes and mammary gland microbiota. J. Dairy Sci. 2015, 98, 2114–2128. [Google Scholar] [CrossRef] [Green Version]
- Catozzi, C.; Sanchez Bonastre, A.; Francino, O.; Lecchi, C.; De Carlo, E.; Vecchio, D.; Martucciello, A.; Fraulo, P.; Bronzo, V.; Cuscó, A.; et al. The microbiota of water buffalo milk during mastitis. PLoS ONE 2017, 12, e0184710. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wright, A.-D.G.; Yang, Y.; Si, H.; Li, G. Unique bacteria community composition and co-occurrence in the milk of different ruminants. Sci. Rep. 2017, 7, 40950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto Del Rio, M.L.D.; Dalmasso, A.; Civera, T.; Bottero, M.T. Characterization of bacterial communities of donkey milk by high-throughput sequencing. Int. J. Food Microbiol. 2017, 251, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aakko, J.; Kumar, H.; Rautava, S.; Wise, A.; Autran, C.; Bode, L.; Isolauri, E.; Salminen, S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef. Microbes 2017, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, H.; Plaizier, J.C.; De Buck, J.; Barkema, H.W.; Khafipour, E. Composition of the teat canal and intramammary microbiota of dairy cows subjected to antimicrobial dry cow therapy and internal teat sealant. J. Dairy Sci. 2018, 101, 10191–10205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakošek Pipan, M.; Švara, T.; Zdovc, I.; Papić, B.; Avberšek, J.; Kušar, D.; Mrkun, J. Staphylococcus pseudintermedius septicemia in puppies after elective cesarean section: Confirmed transmission via dam’s milk. BMC Vet. Res. 2019, 15, 41. [Google Scholar] [CrossRef]
- Paul, M.; Bishara, J.; Yahav, D.; Goldberg, E.; Neuberger, A.; Ghanem-Zoubi, N.; Dickstein, Y.; Nseir, W.; Dan, M.; Leibovici, L. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: Randomised controlled trial. BMJ 2015, 350, h2219. [Google Scholar] [CrossRef] [Green Version]
- Saijonmaa-Koulumies, L.E.; Lloyd, D.H. Colonization of neonatal puppies by Staphylococcus intermedius. Vet. Dermatol. 2002, 13, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermediusin the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253-52. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Del Carro, A.; Bertero, A.; Del Carro, A.; Starvaggi Cucuzza, A.; Banchi, P.; Corrò, M. Does Bacteria Colonization of Canine Newborns Start in the Uterus? Animals 2021, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerdó, T.; Ruiz, A.; Acuña, I.; Jáuregui, R.; Jehmlich, N.; Haange, S.B.; von Bergen, M.; Suárez, A.; Campoy, C. Gut microbial functional maturation and succession during human early life. Environ. Microbiol. 2018, 20, 2160–2177. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Luoto, R.; Salminen, S.; Isolauri, E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Ishaq, S.L.; Bichi, E.; Olivo, S.K.; Lowe, J.; Aldridge, B.M. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci. Rep. 2018, 8, 3197. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tønnessen, R.; Borge, K.S.; Nødtvedt, A.; Indrebø, A. Canine perinatal mortality: A cohort study of 224 breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef] [PubMed]


| Breed size | Breed | Number and Proportion (%) of Parturitions | Number and Proportion (%) of Puppies | Number and Proportion (%) of Female Puppies | Number and Proportion (%) of Male Puppies |
|---|---|---|---|---|---|
| Small breeds (≤10 kg) | Miniature Schnauzer | 1 (6.25) | 5 (6.3) | 2 (2.6) | 3 (3.8) |
| Jack Russell Terrier | 1 (6.25) | 4 (5.1) | 1 (1.3) | 3 (3.8) | |
| Pomeranian | 1 (6.25) | 3 (3.8) | 1 (1.3) | 2 (2.6) | |
| Miniature Poodle | 1 (6.25) | 2 (2.6) | 1 (1.3) | 1 (1.3) | |
| Boston Terrier | 4 (25.0) | 16 (20.2) | 11 (13.9) | 5 (6.3) | |
| Total | 8 (50.0) | 30 (38.0) | 16 (20.26) | 14 (17.7) | |
| Medium-large breeds | Whippet | 1 (6.25) | 7 (8.9) | 4 (5.1) | 3 (3.8) |
| (10.1–25 kg) | Pembroke Welsh Corgi | 2 (12.5) | 10 (12.6) | 3 (3.8) | 7 (8.9) |
| English Bulldog | 1 (6.25) | 4 (5.1) | 2 (2.6) | 2 (2.5) | |
| French Bulldog | 2 (12.5) | 13 (16.5) | 5 (6.3) | 8 (10.1) | |
| Total | 6 (37.5) | 34 (43.0) | 14 (17.7) | 20 (25.3) | |
| Large to giant breeds (>25.1 kg) | Greater Swiss Mountain Dog | 1 (6.25) | 6 (7.6) | 1 (1.3) | 5 (6.3) |
| Newfoundland | 1 (6.25) | 9 (11.4) | 4 (5.1) | 5 (6.3) | |
| Total | 2 (12.5) | 15 (19.0) | 5 (6.3) | 10 (12.7) |
| Number of Puppies (n) | Proportion of Puppies [%] | |||
|---|---|---|---|---|
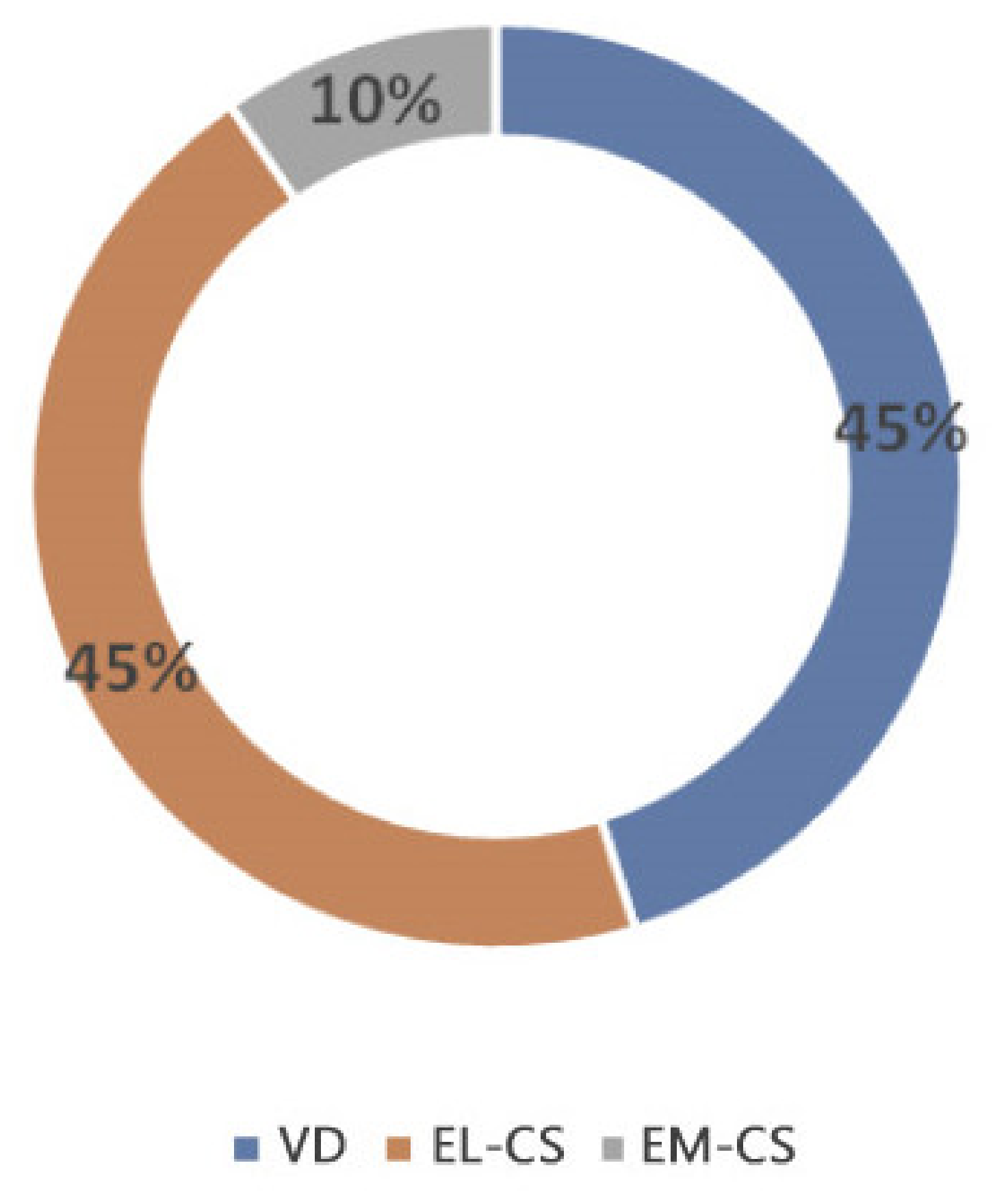
| Type of parturition | Vaginal delivery | 18 | 22.8 | |
| Cesarean section | Elective | 41 | 51.9 | |
| Emergency | 20 | 25.3 | ||
| Sex of the puppies | Female | 35 | 44.3 | |
| Male | 44 | 55.7 | ||
| Survival | Born alive | 77 | 97.5 | |
| Stillborn puppies | 2 | 2.5 | ||
| Gram-Positive Bacteria | COL | MEC |
|---|---|---|
| Staphylococcus | ||
| Staph. epidermidis | + | + |
| Staph. pseudintermedius | + | + |
| Staph. hominis | + | + |
| Staph. aureus | + | |
| Staph. haemolyticus | + | + |
| Staph. warneri | + | + |
| Staph. capitis | + | + |
| Staph. pettenkoferi | + | |
| Staph. caprae | + | |
| Staph. simulans | + | |
| Staph. felis | + | |
| Staphylococcus spp. | + | + |
| Streptococcus | ||
| Strep. salivarius | + | |
| Strep. oralis | + | |
| Strep. pluranimalium | + | |
| Strep. mitis | + | |
| Strep. sanguinis | + | |
| Strep. parasanguinis | + | |
| Strep. pneumoniae | + | |
| Streptococcus spp. | + | |
| Enterococcus faecalis | + | |
| Actinomyces | ||
| A. odontolyticum | + | |
| A. weissii | + | |
| Corynebacterium spp. | + | |
| Rothia R. dentocariosa R. mucilaginosa | + + | |
| Lactobacillus | ||
| L. johnsonii | + | |
| Lactobacillus lacti | + | + |
| Lactobacillus spp. | + | + |
| Macrococcus caseolyticus | + | |
| Cutibacterium (Propionibacterium) | ||
| C. acnes | + | + |
| C. granulosum | + | |
| Bacillus | ||
| B. flexus | + | |
| B. megaterium | + | |
| B. pumilus | + | |
| B. simplex | + | |
| Bacillus spp. | + | + |
| Kocuria | ||
| K. kristinae | + | |
| K. palustris | + | |
| Kocuria spp. | + | + |
| Streptomyces spp. | + | |
| Macrococcus | ||
| M. caseolyticus | + | |
| Macrococcus spp. | + | |
| Gram-Negative Bacteria | COL | MEC |
| Neisseria | ||
| N. weaveri | + | |
| N. zoodegmatis | + | |
| Pasteurella | ||
| P. canis | + | |
| P. stomatis | + | |
| Pasteurella spp. | + | |
| Haemophilus | ||
| H. haemoglobinophilus | + | + |
| Haemophilus spp. | + | |
| Moraxella | ||
| M. osloensis | + | |
| Moraxella spp. | + | |
| Acinetobacter | ||
| A. schindleri | + | |
| A. junni | + | |
| A. lwoffii | + | |
| Klebsiella pneumoniae | + | |
| Paracoccus spp. | + | |
| Escherichia coli | + | |
| Pantoea spp. | + | |
| Brevundimonas aurantiaca | + |
| Gram-Positive Bacteria | TOP | |
|---|---|---|
| Staphylococcus | ||
| Staph. epidermidis | VD, EL-CS, EM-CS | |
| Staph. pseudintermdius | VD, EL-CS | |
| Staph. hominis | VD, EL-CS | |
| Staph. aureus | VD, EL-CS | |
| Staph. haemolyticus | VD, EL-CS | |
| Staph. warneri | VD, EL-CS, EM-CS | |
| Staph. hominis | VD, EL-CS | |
| Staph. capitis | EL-CS, EM-CS | |
| Staph. pettenkoferi | EL-CS | |
| Staph. caprae | EL-CS | |
| Staphylococcus spp. | VD, EL-CS, EM-CS | |
| Streptococcus | ||
| Strep. salivarius | EL-CS | |
| Strep. oralis | EL-CS, EM-CS | |
| Strep. pluranimalium | EM-CS | |
| Strep. mitis | VD | |
| Strep. sanguinis | EM-CS, EL-CS | |
| Strep. parasanguinis | EL-CS | |
| Strep. pneumoniae | EL-CS | |
| Streptococcus spp. | VD | |
| Actinomyces odontolyticum | EL-CS | |
| Corynebacterium spp. | VD | |
| Rothia | ||
| R. dentocariosa | EL-CS | |
| R. mucilaginosa | EM-CS | |
| Lactobacillus | ||
| L. johnsonii | VD, EL-CS | |
| L. lacti | VD, EL-CS | |
| Lactobacillus spp. | EL-CS | |
| Bacillus | ||
| B. flexus | VD | |
| B. megaterium | VD | |
| B. pumilus | VD | |
| B. simplex | EM-CS | |
| Bacillus spp. | VD | |
| Kocuria | ||
| K. kristinae | EL-CS | |
| Kocuria spp. | VD | |
| Macrococcus caseolyticus | VD | |
| Streptomyces spp | VD | |
| Cutibacterium | ||
| C. acne | EL-CS, VD, EM-CS | |
| C. granulosum | EM-CS | |
| Gram-Negative Bacteria | COL | MEC |
| Neisseria | ||
| N. weaveri | + | |
| N. zoodegmatis | + | |
| Pasteurella | ||
| P. canis | + | |
| P. stomatis | + | |
| Pasteurella spp. | + | |
| Haemophilus | ||
| H. haemoglobinophilus | + | + |
| Haemophilus spp. | + | |
| Moraxella | ||
| M. osloensis | + | |
| Moraxella spp. | + | |
| Acinetobacter | ||
| A. schindleri | + | |
| A. junni | + | |
| A. lwoffii | + | |
| Klebsiella pneumoniae | + | |
| Paracoccus spp. | + | |
| Escherichia coli | + | |
| Pantoea spp. | + | |
| Brevundimonas aurantiaca | + | |
| Gram-Positive Bacteria | Type of Parturition | Gram-Negative Bacteria | Type of Parturition |
|---|---|---|---|
| Staphylococcus | Neisseria weaveri | VD | |
| Staph. epidermidis | EL-CS | ||
| Staph. pseudintermedius | EL-CS, VD | ||
| Staph. haemolyticus | EL-CS | ||
| Staph. warneri | EL-CS | ||
| Staph. hominis | EL-CS | ||
| Staph. capitis | EL-CS | ||
| Staph. pettenkoferi | EL-CS | ||
| Staph. simulans | EL-CS, VD | ||
| Enterococcus faecalis | VD, EL-CS | Pasteurella spp. | VD |
| Actinomyces meissii | EL-CS | Haemophilus haemoglobinophilus | VD |
| Lactobacillus spp. | VD | Moraxella osloensis | EM-CS |
| Cutibacterium acnes | EL-CS, EM-CS | Acinetobacter schindleri | VD |
| Bacillus cereus | EM-CS | Klebsiella pneumoniae | VD |
| Kocuria | Panthoea spp. | VD | |
| K. palustris | EL-CS | ||
| Kocuria spp. | EL-CS, VD | ||
| Macrococcus spp. | VD |
| Vaginal Delivery | Cesarean Section | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EL-CS | EM-CS | |||||||||||||||
| N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| C→M | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | Y | N | Y | N | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajdič, L.; Plavec, T.; Zdovc, I.; Kalin, A.; Zakošek Pipan, M. Impact of Type of Parturition on Colostrum Microbiota Composition and Puppy Survival. Animals 2021, 11, 1897. https://doi.org/10.3390/ani11071897
Kajdič L, Plavec T, Zdovc I, Kalin A, Zakošek Pipan M. Impact of Type of Parturition on Colostrum Microbiota Composition and Puppy Survival. Animals. 2021; 11(7):1897. https://doi.org/10.3390/ani11071897
Chicago/Turabian StyleKajdič, Leonida, Tanja Plavec, Irena Zdovc, Anja Kalin, and Maja Zakošek Pipan. 2021. "Impact of Type of Parturition on Colostrum Microbiota Composition and Puppy Survival" Animals 11, no. 7: 1897. https://doi.org/10.3390/ani11071897






