How Does Glycation Affect Binding Parameters of the Albumin-Gliclazide System in the Presence of Drugs Commonly Used in Diabetes? In Vitro Spectroscopic Study
Abstract
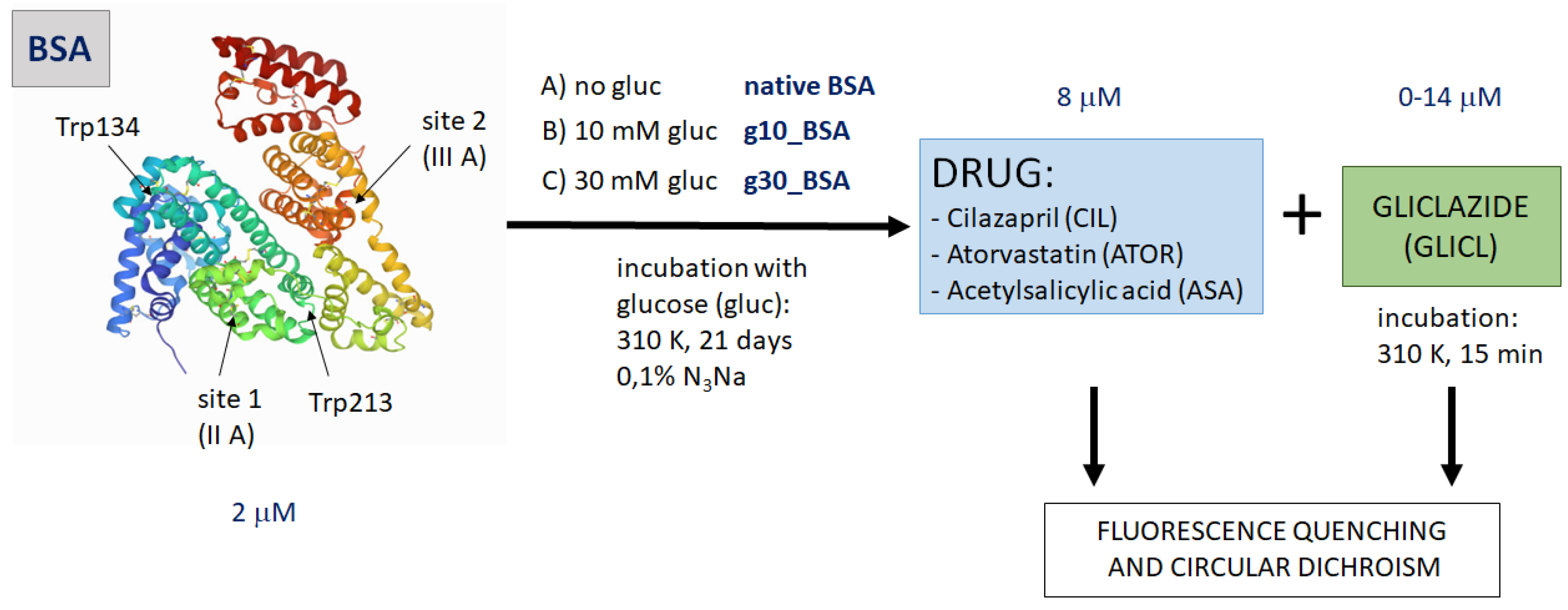
:1. Introduction
2. Results and Discussion
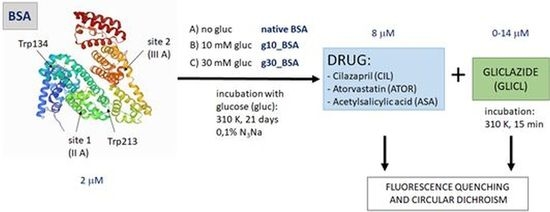
2.1. BSA Glycation
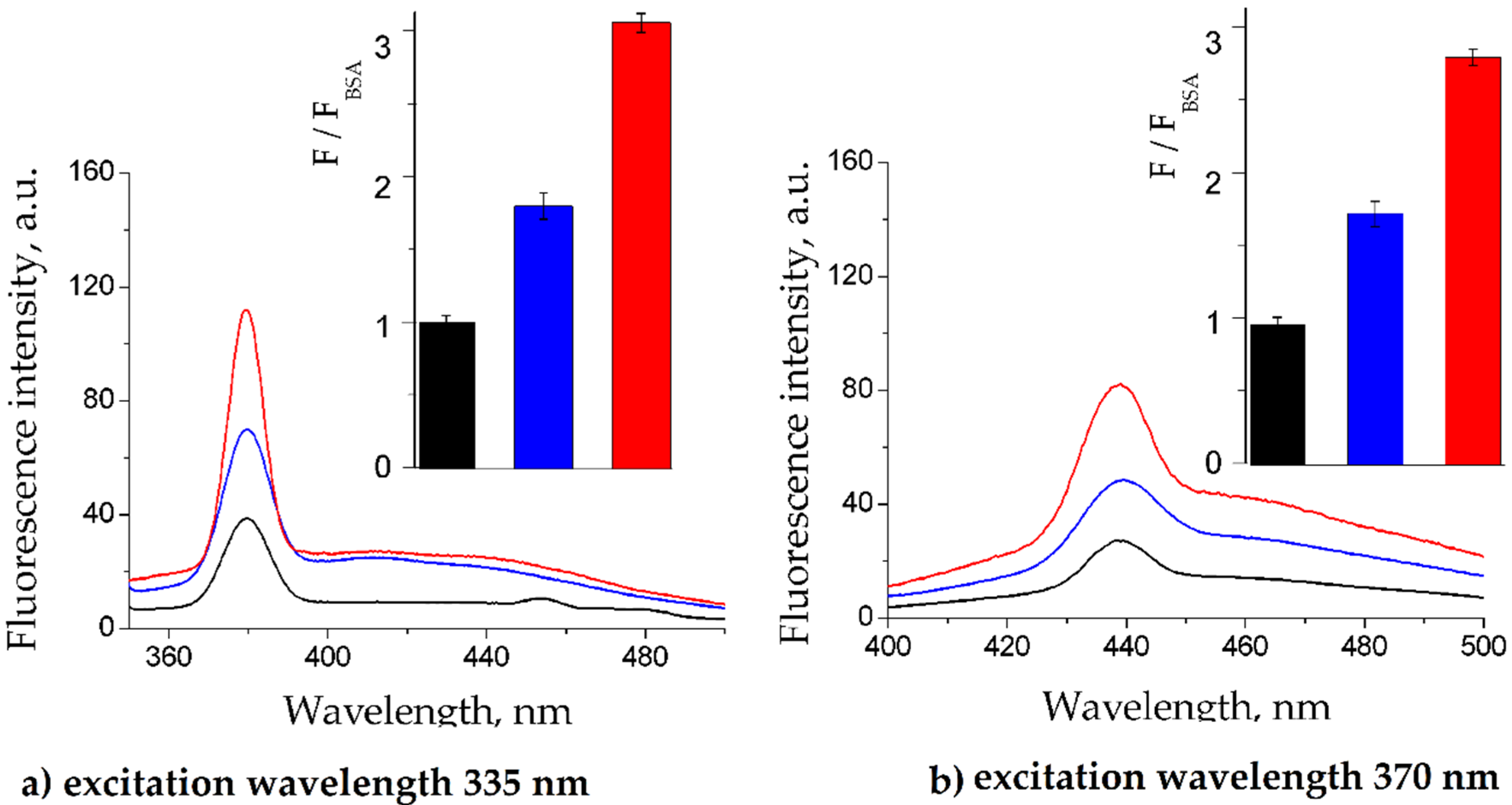
2.2. Fluorescence Quenching of BSA by Gliclazide in the Presence of Cilazapril, Atorvastatin, and Acetylsalicylic Acid
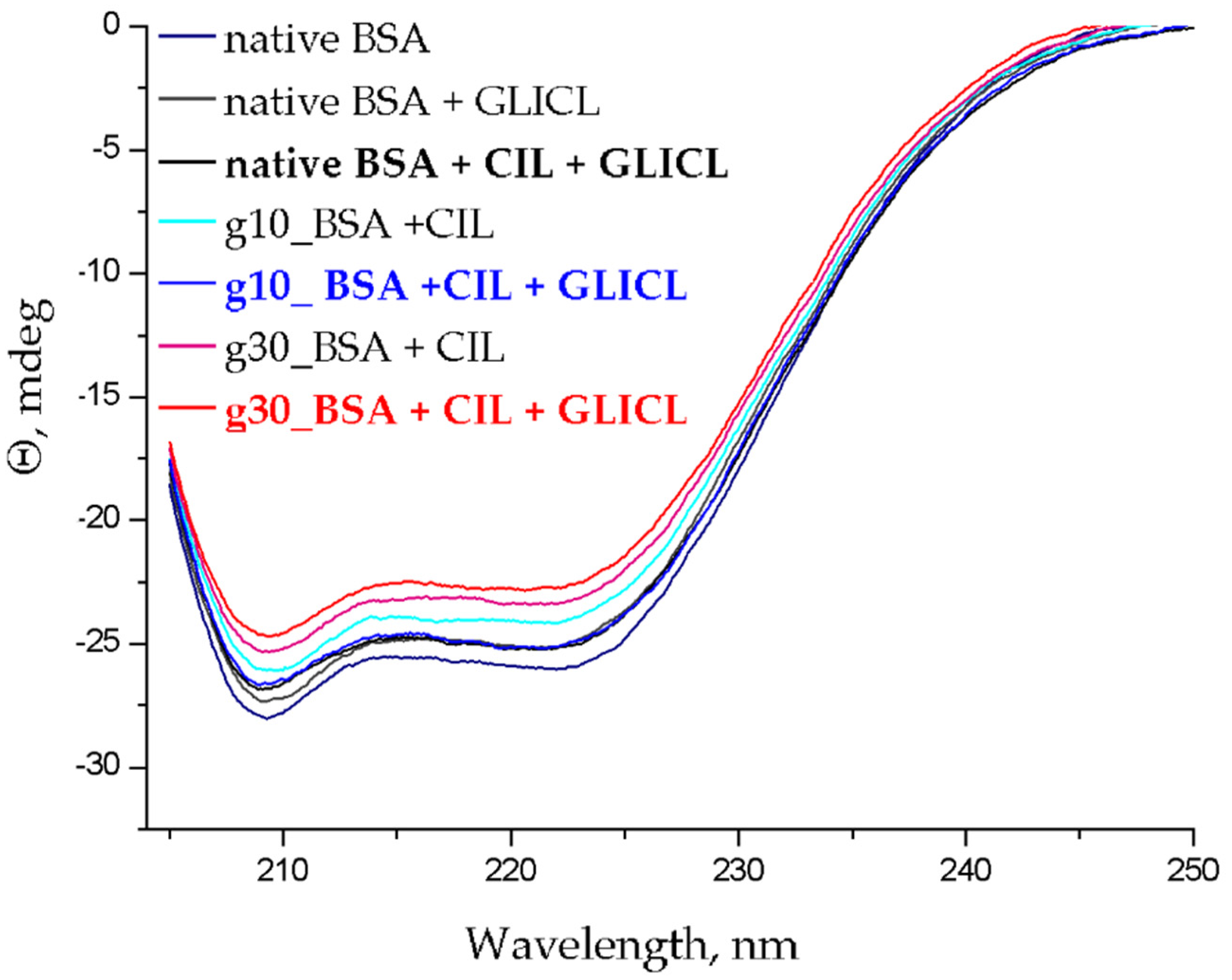
2.3. Changes to α-Helical Contents
3. Materials and Methods
3.1. BSA Glycation
3.2. Binding Experiments
3.3. Fluorescence Quenching and Calculation of Binding Parameters
3.4. α-Helical Content of Albumin Molecules
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 31 March 2021).
- American Diabetes Association, Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S125–S150. [CrossRef]
- May, M.; Schindler, C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther. Adv. Endocrinol. Metab. 2016, 7, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Blache, D.; Bourdon, E.; Salloignon, P.; Lucchi, G.; Ducoroy, P.; Petit, J.M.; Verges, B.; Lagrost, L. Glycated albumin with loss of fatty acid binding capacity contributes to enhanced arachidonate oxygenation and platelet hyperactivity: Relevance in patients with type 2 diabetes. Diabetes 2015, 64, 960–972. [Google Scholar] [CrossRef] [Green Version]
- Warwas, M.; Żurawska-Płaksej, E.; Ciężka, D.; Piwowar, A. Glycated albumin as a marker of glycemia in diabetes and its vascular complications. Postepy Hig. Med. Dosw. 2015, 69, 638–648. [Google Scholar]
- Arasteh, A.; Farahi, S.; Habibi-Rezaei, M.; Moosavi-Movahedi, A.A. Glycated albumin: An overview of the In Vitro models of an In Vivo potential disease marker. J. Diabetes Metab. Disord. 2014, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, T.; Gan, L.S. Plasma protein binding: From discovery to development. J. Pharm. Sci. 2013, 102, 2953–2994. [Google Scholar] [CrossRef]
- Gajahi Soudahome, A.; Catan, A.; Giraud, P.; Assouan Kouao, S.; Guerin-Dubourg, A.; Debussche, X.; Le Moullec, N.; Bourdon, E.; Bravo, S.B.; Paradela-Dobarro, B.; et al. Glycation ofhuman serum albumin impairs binding to the glucagon-like peptide-1 analogue liraglutide. J. Biol. Chem. 2018, 293, 4778–4791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudlarek, A.; Pożycka, J.; Maciążek-Jurczyk, M. Influence of Piracetam on Gliclazide-Glycated Human Serum Albumin Interaction. A Spectrofluorometric Study. Molecules 2018, 29, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, D.R.; Davies, M.J.; Jarvis, J.; Seidu, S.; Khunti, K. The right place for Sulphonylureas today. Diabetes Res. Clin. Pract. 2019, 157, 107836. [Google Scholar] [CrossRef]
- Żurawska-Płaksej, E.; Rorbach-Dolata, A.; Wiglusz, K.; Piwowar, A. The effect of glycation on bovine serum albumin conformation and ligand binding properties with regard to gliclazide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 625–633. [Google Scholar] [CrossRef] [PubMed]
- MacManus-Spencer, L.A.; Tse, M.L.; Hebert, P.C.; Bischel, H.N.; Luthy, R.G. Binding of perfluorocarboxylates to serum albumin: A comparison of analytical methods. Anal. Chem. 2010, 82, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, V.D.; Walekar, L.S.; Gore, A.H.; Anbhule, P.V.; Kolekar, G.B. Spectroscopic analysis on the binding interaction of biologically active pyrimidine derivative with bovine serum albumin. J. Pharm. Anal. 2016, 6, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Siligardi, G.; Hussain, R.; Patching, S.G.; Phillips-Jones, M.K. Ligand- and drug-binding studies of mem-brane proteins revealed through circular dichroism spectroscopy. Biochim. Biophys. Acta 2014, 1838, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3475, Gliclazide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gliclazide (accessed on 31 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 56330, Cilazapril. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cilazapril (accessed on 31 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 60823, Atorvastatin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Atorvastatin (accessed on 31 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2244, Aspirin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin (accessed on 31 March 2021).
- Voziyan, P.A.; Khalifah, R.G.; Thibaudeau, C.; Yildiz, A.; Jacob, J.; Serianni, A.S.; Hudson, B.G. Modification of proteins in vitro by physiological levels of glucose: Pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J. Biol. Chem. 2003, 278, 46616–46624. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef]
- Śliwińska-Hill, U.; Wiglusz, K. The interaction between human serum albumin and antidiabetic agent—Exenatide: Determination of the mechanism binding and effect on the protein conformation by fluorescence and circular dichroism techniques—Part I. J. Biomol. Struct. Dyn. 2020, 38, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Tabrez, S.; Islam, B.; Rehman, M.T.; Alsenaidy, A.M.; AlAjmi, M.F.; Khan, R.A.; Alsenaidy, M.A.; Khan, M.S. Characterization of colchicine binding with normal and glycated albumin: In vitro and molecular docking analysis. J. Biomol. Struct. Dyn. 2018, 36, 3453–3462. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Aiping, X.; Shiyong, M.; Yixi, X. Influence of Human Serum Albumin Glycation on the Binding Affinities for Natural Flavonoids. Open Chem. 2019, 17, 806–812. [Google Scholar] [CrossRef]
- Sarkar, A.; Tiwari, A.; Bhasin, P.S.; Mitra, M. Pharmacological and Pharmaceutical Profile of Gliclazide: A Review. J. Appl. Pharm. Sci. 2011, 1, 11–19. [Google Scholar]
- Seedher, N.; Kanojia, M. Effect of competitive binding of hypoglycemic agents to human serum albumin on drug pharmacology. Int. J. Pharm. 2015, 5, 1261–1271. [Google Scholar]
- Pawar, S.K.; Jaldappagari, S. Interaction of repaglinide with bovine serum albumin: Spectroscopic and molecular docking approaches. J. Pharm. Anal. 2019, 9, 274–283. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ohta, D.; Hachiya, K.; Mitsui, Y.; Takeda, K. Fluorescence behavior of tryptophan residues of bovine and human serum albumins in ionic surfactant solutions: A comparative study of the two and one tryptophan(s) of bovine and human albumins. J. Protein Chem. 1996, 15, 265–272. [Google Scholar] [CrossRef]
- Ghisaidoobe, A.B.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on Förster resonance energy transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef] [PubMed]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Anguizola, J.; Joseph, K.S.; Barnaby, O.S.; Matsuda, R.; Alvarado, G.; Clarke, W.; Cerny, R.L.; Hage, D.S. Development of affinity microcolumns for drug–protein binding studies in personalized medicine: Interactions of sulfonylurea drugs with in vivo glycated human serum albumin. Anal. Chem. 2013, 85, 4453–60110. [Google Scholar] [CrossRef] [Green Version]
- Szkudlarek, A.; Pentak, D.; Ploch, A.; Pożycka, J.; Maciążek-Jurczyk, M. In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States. Molecules 2017, 22, 2249. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, R.; Li, Z.; Zheng, X.; Hage, D.S. Analysis of glipizide binding to normal and glycated human serum albumin by high-performance affinity chromatography. Anal. Bioanal. Chem. 2015, 407, 5309–5321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, C.R.; Jiang, M.; Zhu, Y.Y.; Wang, J.; Chen, J.; Shi, J.H. Binding interaction of atorvastatin with bovine serum albumin: Spectroscopic methods and molecular docking. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 156, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Anguizola, J.; Matsuda, R.; Barnaby, O.S.; Hoy, K.S.; Wa, C.; DeBolt, E.; Koke, M.; Hage, D.S. Review: Glycation of human serum albumin. Clin. Chim. Acta 2013, 425, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Melander, A.; Lebovitz, H.E.; Faber, O.K. Sulfonylureas. Why, which, and how? Diabetes Care 1990, 3, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Matsoukis, I.; Paraskevas, P.; Ganotopoulou, A.; Gova, E.; Kostanti, X.; Asimis, A.; Tsivilis, N.; Zaxariadi, Z.; Liouris, I. The combination of aspirin with antidiabetics drugs increases the number of hypoglycaemic events in patients with DM type II. Eur. J. Intern. Med. 2013, 1, E100. [Google Scholar] [CrossRef]
- Bag, S.; Das, S.; Bagchi, C.; Tripathi, S.K. Aspirin potentiates blood glucose lowering effect of glimepiride-pioglitazone combination in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2014, 46, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Dobrică, E.C.; Găman, M.-A.; Cozma, M.-A.; Bratu, O.G.; Stoian, A.P.; Diaconu, C.C. Polypharmacy in Type 2 Diabetes Mellitus: Insights from an Internal Medicine Department. Medicina 2019, 55, 446. [Google Scholar] [CrossRef] [Green Version]
- Sabau, M.; Bungau, S.; Buhas, C.L.; Carp, G.; Daina, L.G.; Judea-Pusta, C.T.; Buhas, B.A.; Jurca, C.M.; Daina, C.M.; Tit, D.M. Legal medicine implications in fibrinolytic therapy of acute ischemic stroke. BMC Med. Ethics 2019, 20, 70. [Google Scholar] [CrossRef]
- Azimi, O.; Emami, Z.; Salari, H.; Chamani, J. Probing the interaction of human serum albumin with norfloxacin in the presence of high-frequency electromagnetic fields: Fluorescence spectroscopy and circular dichroism investigations. Molecules 2011, 16, 9792–9818. [Google Scholar] [CrossRef]
- GhoshMoulick, R.; Bhattacharya, J.; Roy, S.; Basak, S.; Dasgupta, A.K. Compensatory secondary structure alterations in protein glycation. Biochim. Biophys. Acta 2007, 1774, 233–242. [Google Scholar] [CrossRef]
- Quiming, N.S.; Vergel, R.B.; Nicolas, M.G.; Villanueva, J.A. Interaction of Bovine Serum Albumin and Metallothionein. J. Health Sci. 2005, 51, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Protein Data Bank. Crystal Structure of Bovine Serum Albumin. Available online: https://www.rcsb.org/structure/4F5S (accessed on 31 March 2021). [CrossRef]
- Zhao, X.C.; Liu, R.T.; Chi, Z.X. New insights into the behavior of bovine serum albumin adsorbed onto carbon nanotubes compre-hensive spectroscopic studies. J. Phys. Chem. B 2010, 114, 5625–5631. [Google Scholar] [CrossRef]
- Bi, S.Y.; Sun, Y.T.; Qiao, C.Y.; Zhang, H.Q.; Liu, C.M. Binding of several anti-tumor drugs to bovine serum albumin: Fluorescence study. J. Lumin. 2009, 129, 541–547. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]



| Drug Model | Sample Type | KSV 104 [M−1] | r | Ka 104 [M−1] | n | r |
|---|---|---|---|---|---|---|
| CIL-GLICL | native BSA | 2.45 ± 0.25 | 0.9929 | 0.48 ± 0.05 | 0.85 ± 0.03 | 0.9933 |
| g10_BSA | 2.14 ± 0.18 | 0.9961 | 0.25 ± 0.02 | 0.81 ± 0.02 | 0.9975 | |
| g30_BSA | 2.01 ± 0.14 | 0.9948 | 0.23 ± 0.04 | 0.81 ± 0.04 | 0.9777 | |
| ATOR-GLICL | native BSA | 1.93 ± 0.28 | 0.9904 | 0.11 ± 0.02 | 0.82 ± 0.03 | 0.9904 |
| g10_BSA | 2.09 ± 0.10 | 0.9918 | 0.15 ± 0.01 | 0.76 ± 0.02 | 0.9972 | |
| g30_BSA | 2.21 ± 0.08 | 0.9976 | 0.25 ± 0.02 | 0.73 ± 0.02 | 0.9966 | |
| ASA-GLICL | native BSA | 3.20 ± 0.31 | 0.9958 | 1.07 ± 0.09 | 0.90 ± 0.05 | 0.9983 |
| g10_BSA | 2.21 ± 0.22 | 0.9984 | 0.88 ± 0.07 | 0.92 ± 0.04 | 0.9946 | |
| g30_BSA | 2.35 ± 0.44 | 0.9897 | 0.37 ± 0.05 | 0.84 ± 0.04 | 0.9835 |
| Drug Model | Sample Type | α-Helical Content Decrease (%) |
|---|---|---|
| CIL-GLICL | BSA | 2.14 ± 0.11 |
| g10_BSA | 3.84 ± 0.18 | |
| g30_BSA | 3.84 ± 0.20 | |
| ATOR-GLICL | BSA | 1.88 ± 0.08 |
| g10_BSA | 2.45 ± 0.14 | |
| g30_BSA | 3.61 ± 0.14 | |
| ASA-GLICL | BSA | 4.79 ± 0.21 |
| g10_BSA | 2.77 ± 0.19 | |
| g30_BSA | 2.05 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiglusz, K.; Żurawska-Płaksej, E.; Rorbach-Dolata, A.; Piwowar, A. How Does Glycation Affect Binding Parameters of the Albumin-Gliclazide System in the Presence of Drugs Commonly Used in Diabetes? In Vitro Spectroscopic Study. Molecules 2021, 26, 3869. https://doi.org/10.3390/molecules26133869
Wiglusz K, Żurawska-Płaksej E, Rorbach-Dolata A, Piwowar A. How Does Glycation Affect Binding Parameters of the Albumin-Gliclazide System in the Presence of Drugs Commonly Used in Diabetes? In Vitro Spectroscopic Study. Molecules. 2021; 26(13):3869. https://doi.org/10.3390/molecules26133869
Chicago/Turabian StyleWiglusz, Katarzyna, Ewa Żurawska-Płaksej, Anna Rorbach-Dolata, and Agnieszka Piwowar. 2021. "How Does Glycation Affect Binding Parameters of the Albumin-Gliclazide System in the Presence of Drugs Commonly Used in Diabetes? In Vitro Spectroscopic Study" Molecules 26, no. 13: 3869. https://doi.org/10.3390/molecules26133869