Crosslinking of Gelatin in Bicomponent Electrospun Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Crosslinking Methods
2.4. Fibre Biodegradation Test
2.5. Scanning Electron Microscope (SEM) Imaging
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
3. Results and Discussion
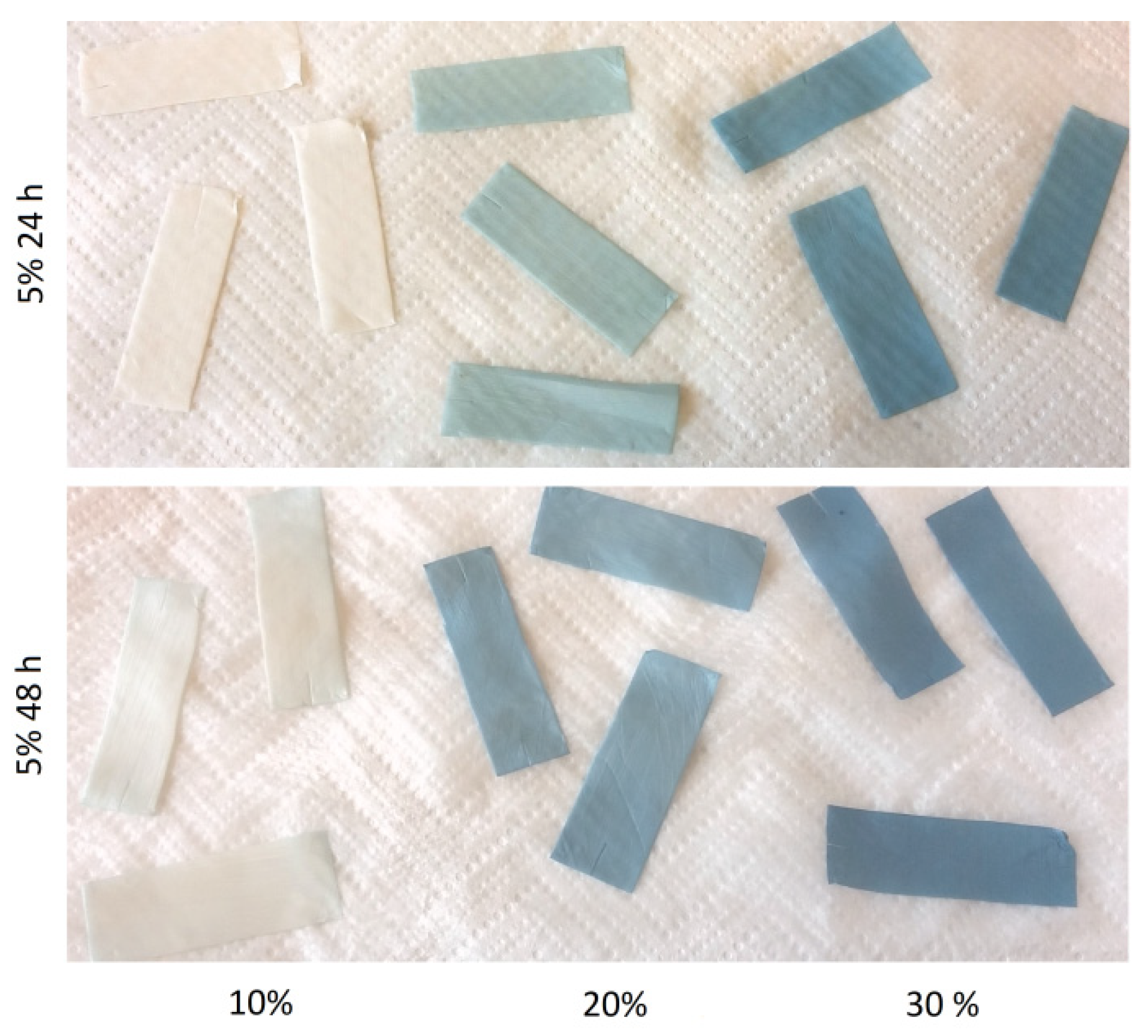
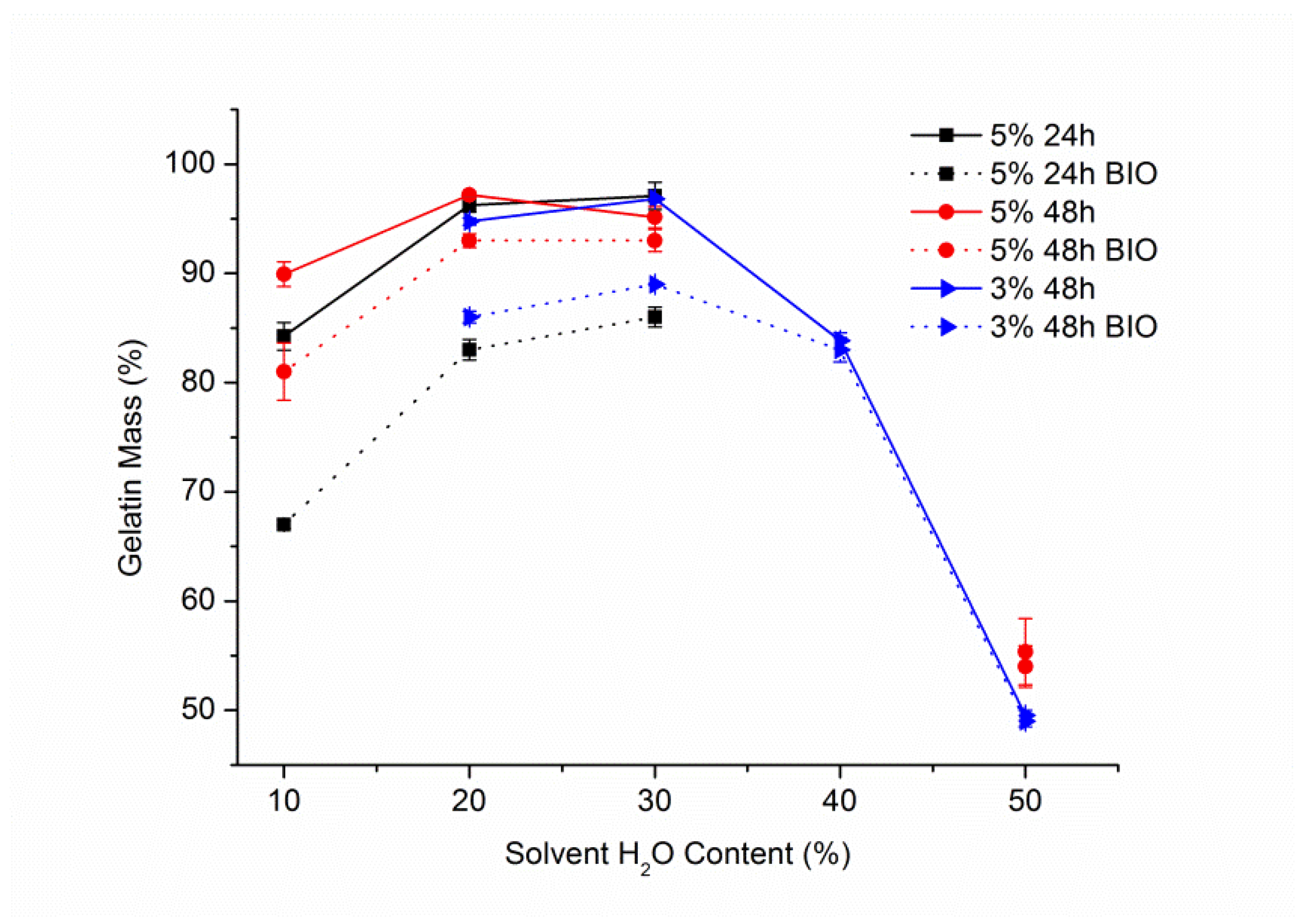
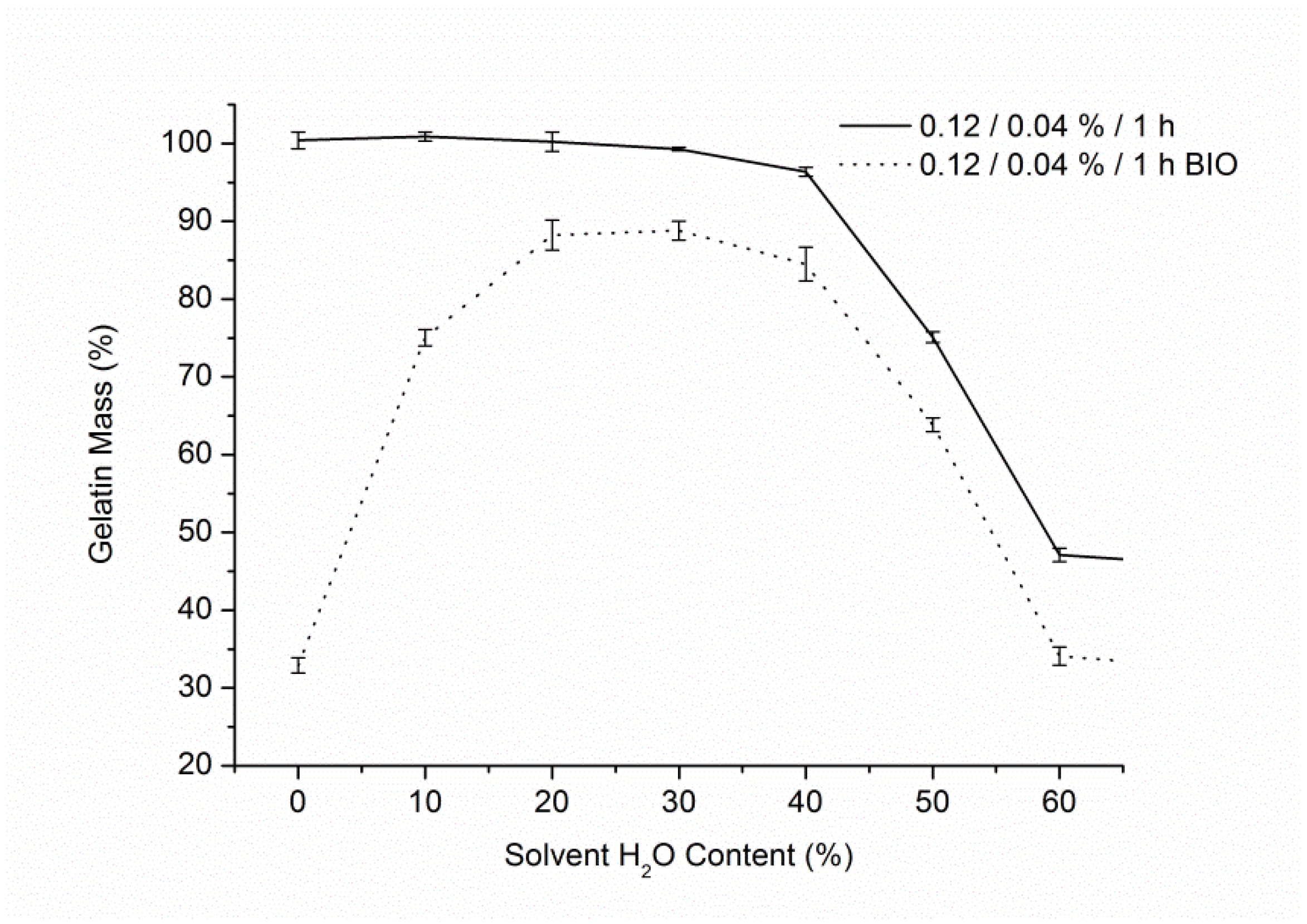
3.1. Solvent Optimisation
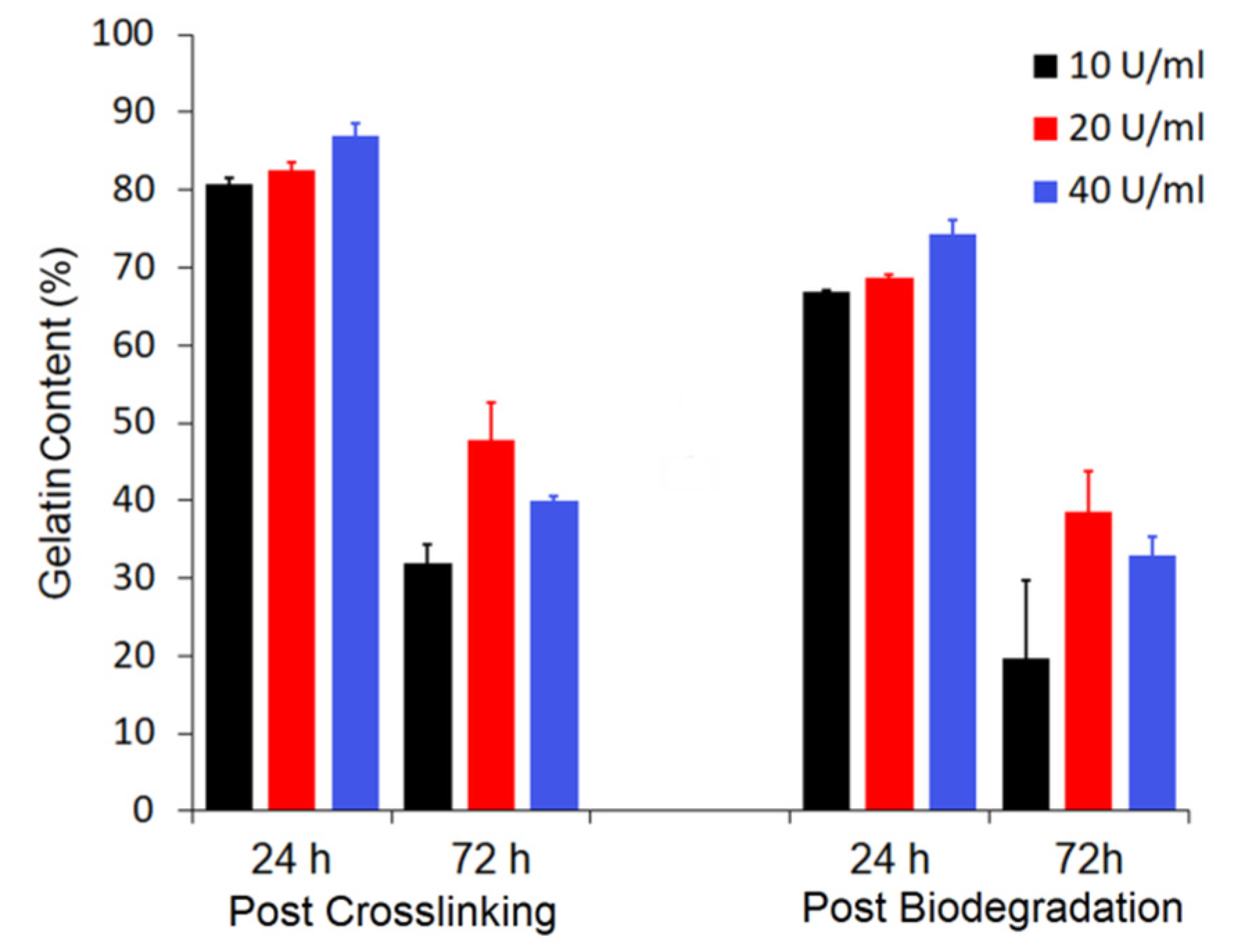
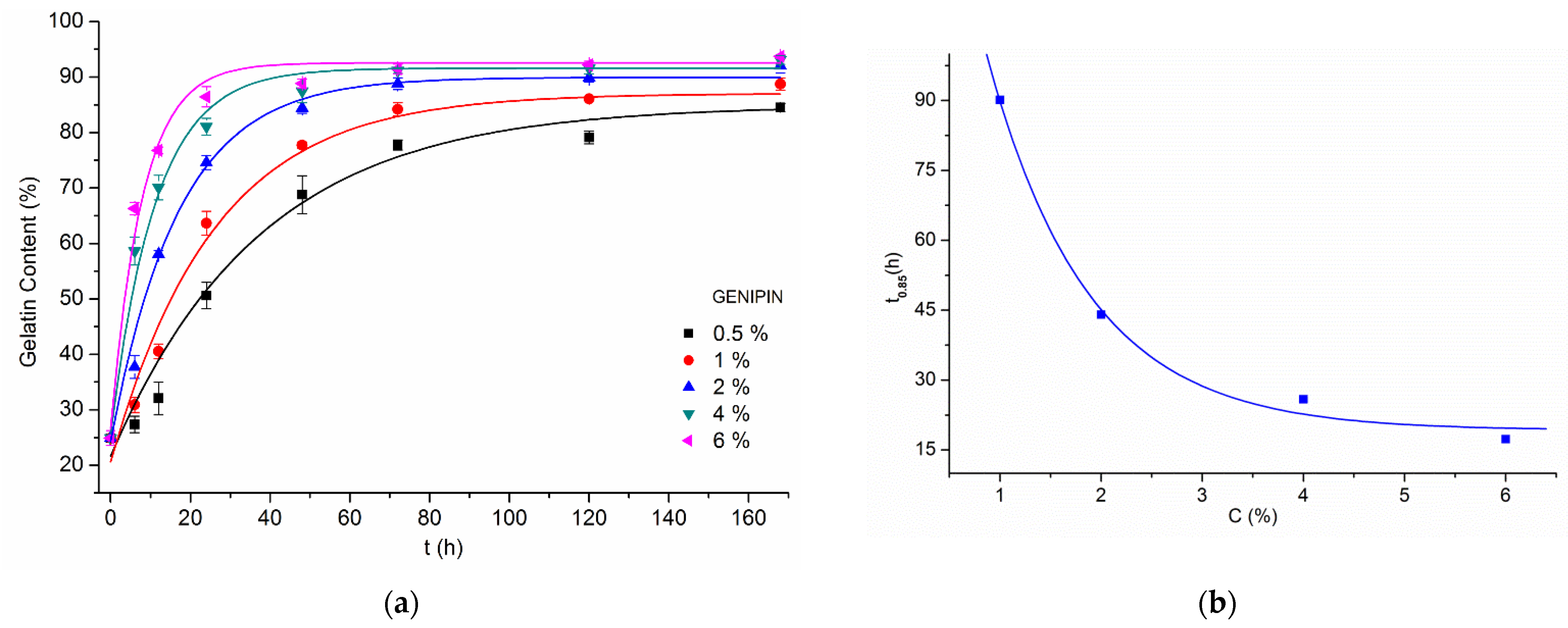
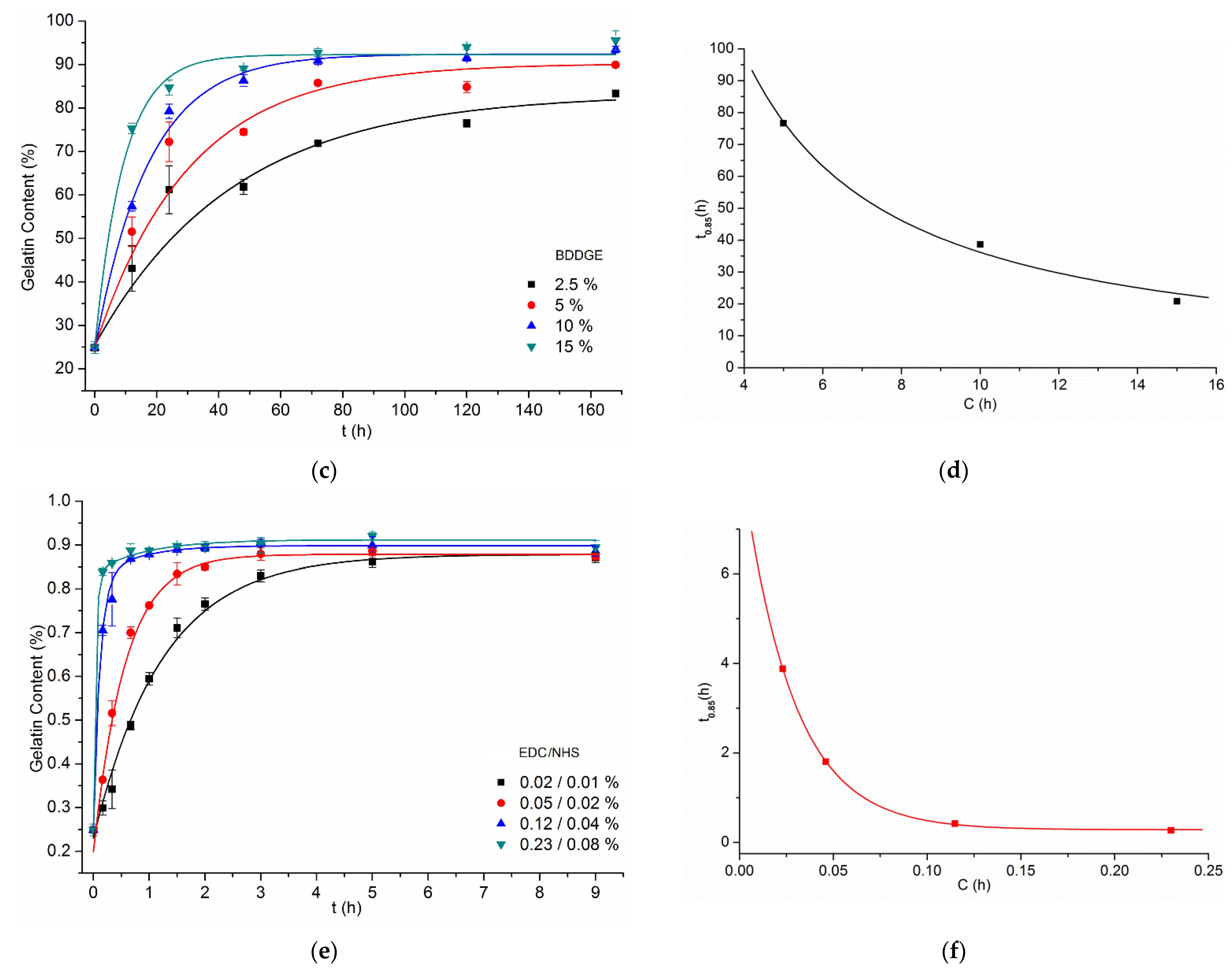
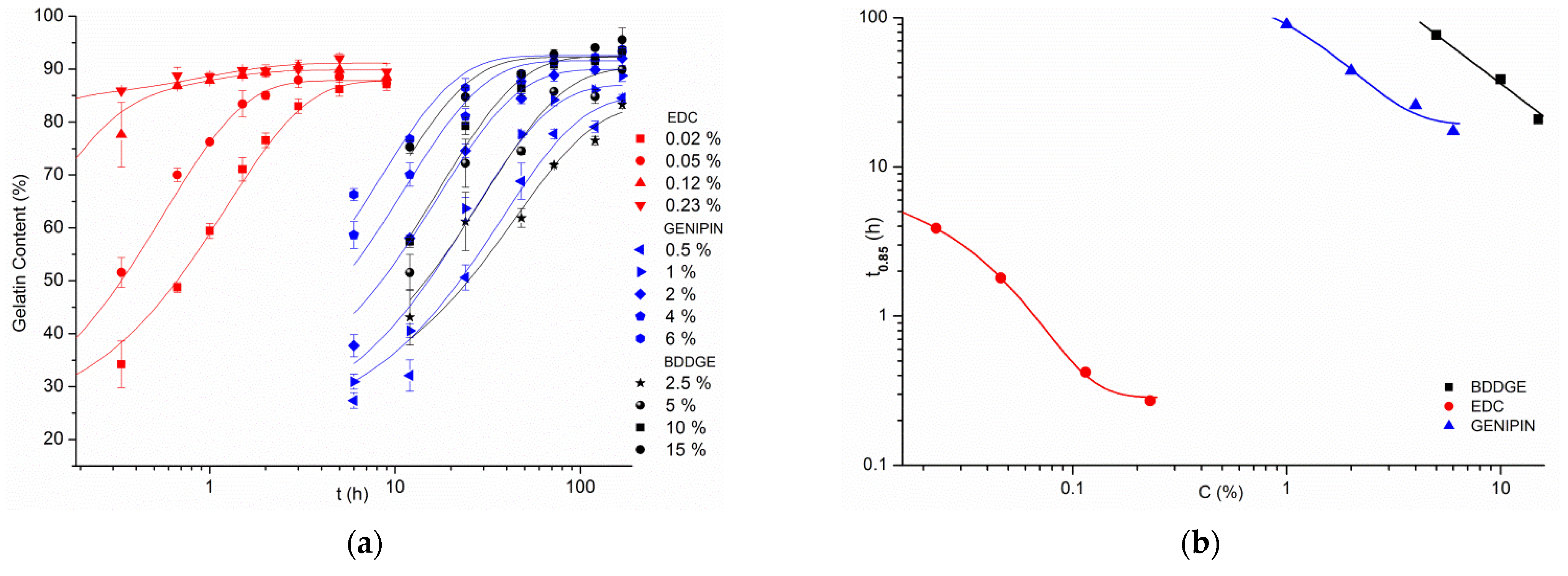
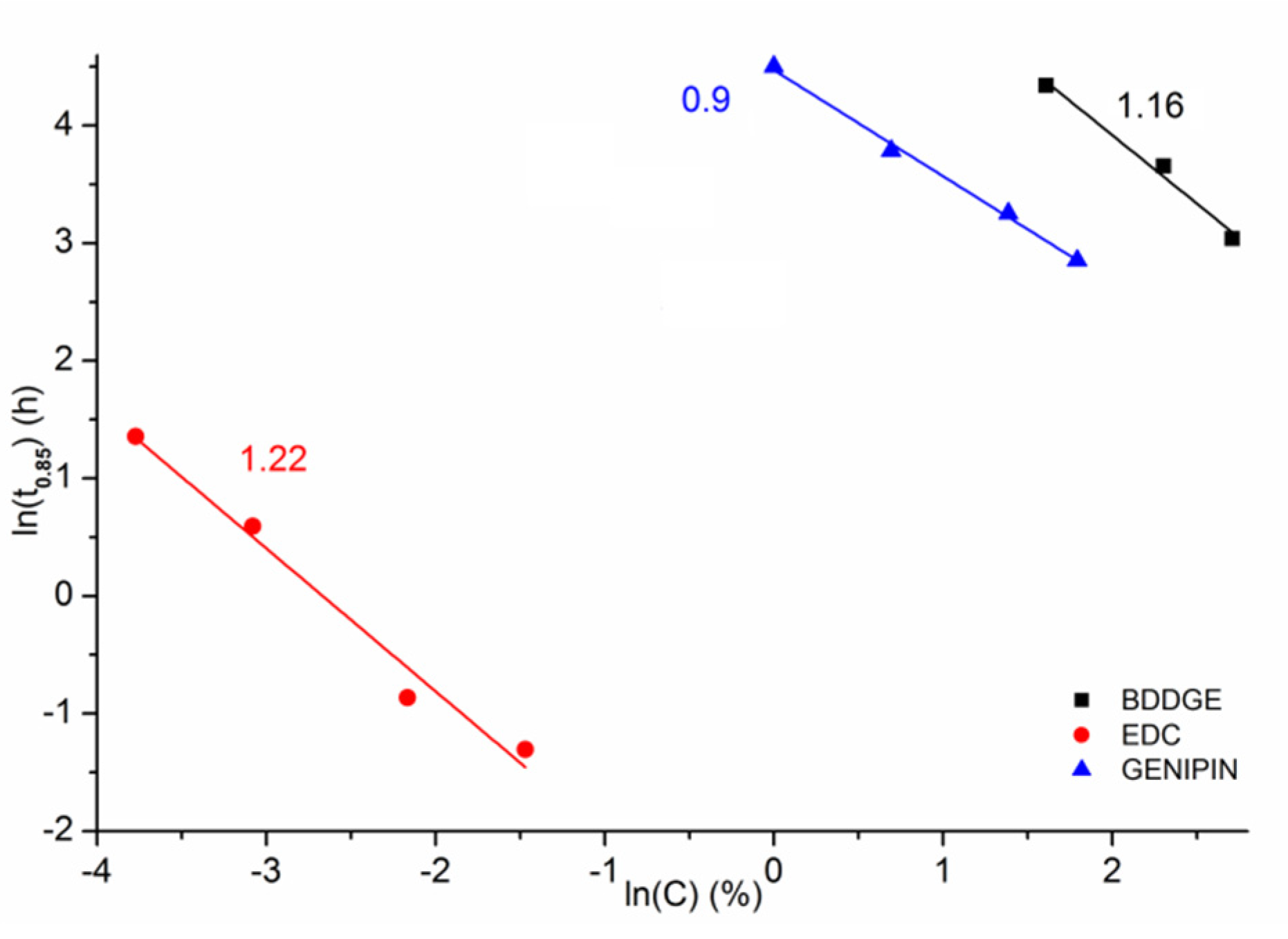
3.2. Gelatin Mass Change
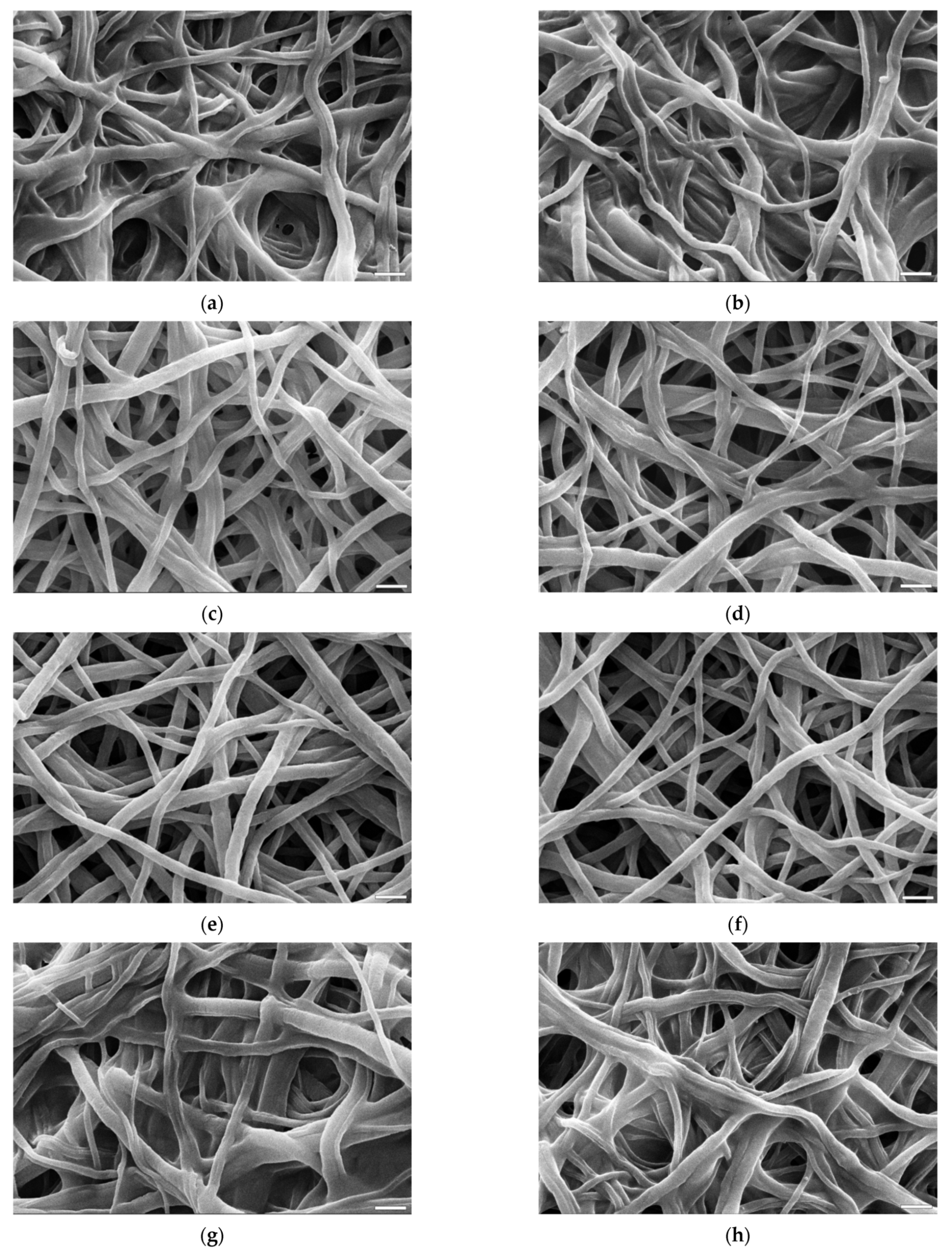
3.3. Morphology of the Fibres
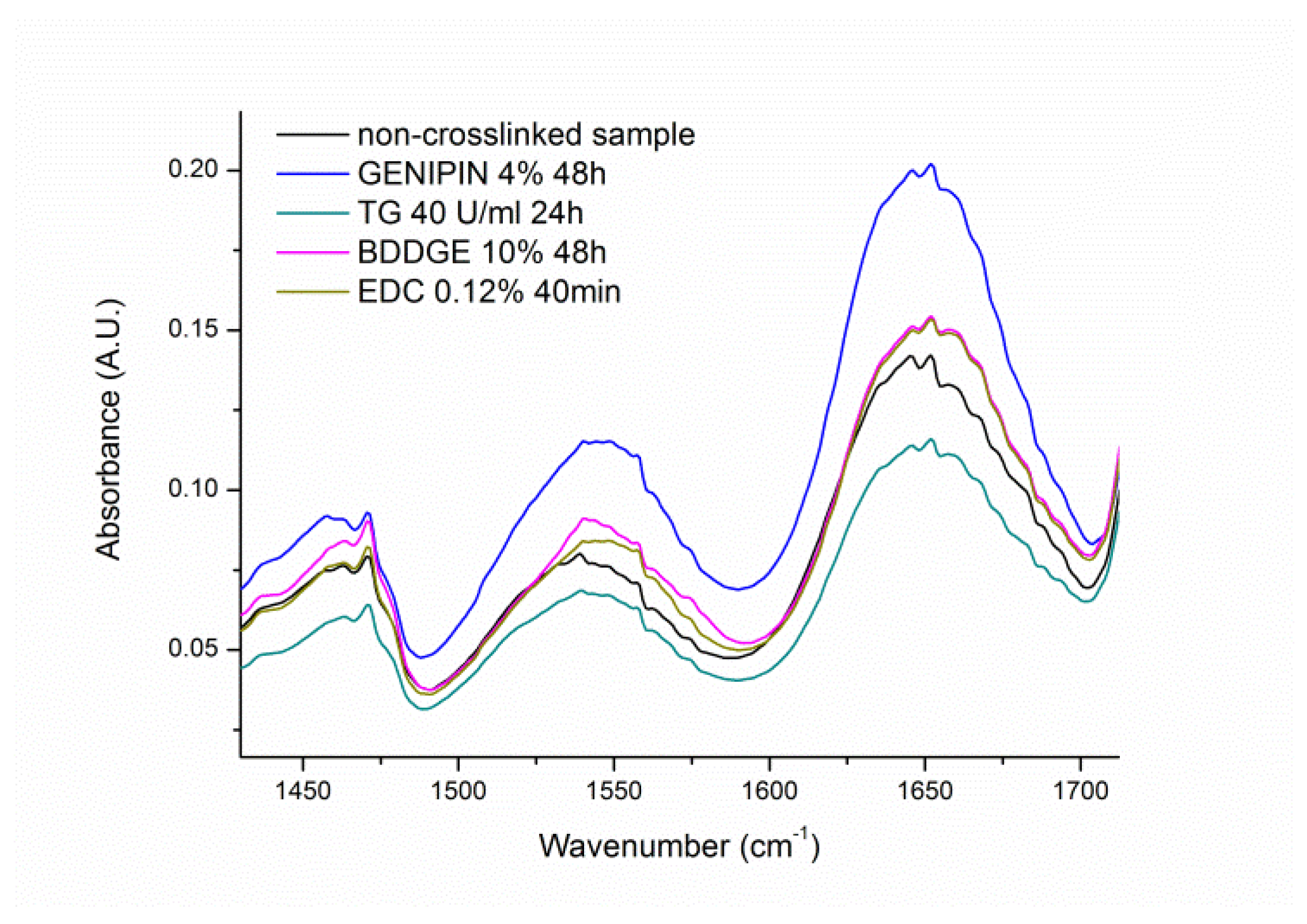
3.4. Gelatin FT-IR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Adv. Technol. 2011, 22, 350–365. [Google Scholar] [CrossRef]
- Denis, P.; Dulnik, J.; Sajkiewicz, P. Electrospinning and Structure of Bicomponent Polycaprolactone/Gelatin Nanofibers Obtained Using Alternative Solvent System. Int. J. Polym. Mater. 2015, 64, 354–364. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Dulnik, J.; Kołbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Beamish, J.A.; Fu, A.Y.; Choi, A.; Haq, N.A.; Kottke-Marchant, K.; Marchant, R.E. The influence of RGD-bearing hydrogels on the re-expression of contractile vascular smooth muscle cell phenotype. Biomaterials 2009, 30, 4127–4135. [Google Scholar] [CrossRef] [Green Version]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Masutani, E.M.; Kinoshita, C.K.; Tanaka, T.T.; Ellison AK, D.; Yoza, B.A. Increasing Thermal Stability of Gelatin by UV-Induced Cross-Linking with Glucose. Int. J. Biomater. 2014, 2014, 979636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, J.; Lan, P. The Review on Electrospun Gelatin Fiber Scaffold. J. Res. Updates Polym. Sci. 2012, 1, 59–71. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Keuffel, E.L.; Dunn, M.G. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. J. Biomed. Mater. Res. 1996, 32, 221–226. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef] [PubMed]
- OldeDamink, L.H.H.; Dijkstra, P.J.; van Luyn, M.J.A.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef] [Green Version]
- Thi-Hiep, N.; Byong-Taek, L. Fabrication and characterization of cross-linked gelatin electro-spun nano-fibers. J. Biomed. Sci. Eng. 2010, 3, 1117–1124. [Google Scholar] [CrossRef]
- Lien, S.-M.; Li, W.-T.; Huang, T.-J. Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Mater. Sci. Eng. C 2008, 28, 36–43. [Google Scholar] [CrossRef]
- Clercq, K.D.; Schelfhout, C.; Bracke, M.; Wever, O.D.; Bockstal, M.V.; Ceelen, W.; Remon, J.P.; Vervaet, C. Genipin-crosslinked gelatin microspheres as a strategy to prevent postsurgical peritoneal adhesions: In vitro and in vivo characterization. Biomaterials 2016, 96, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Hajiabbas, M.; Alemzadeh, I.; Vossoughi, M. A porous hydrogel-electrospun composite scaffold made of oxidized alginate/gelatin/silk fibroin for tissue engineering application. Carbohydr. Polym. 2020, 245, 116465. [Google Scholar] [CrossRef]
- Fiorani, A.; Gualandi, C.; Panseri, S.; Montesi, M.; Marcacci, M.; Focarete, M.L.; Bigi, A. Comparative performance of collagen nanofibers electrospun from different solvents and stabilized by different crosslinkers. J. Mater. Sci. Mater. Med. 2014, 25, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Baptista-Silva, S.; Oliveira CM, T.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Chau, D.Y.S.; Collighan, R.J.; Verderio, E.A.M.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.; Barbani, N.; Giusti, P.; Ciardelli, G. Transglutaminase reactivity with gelatine: Perspective applications in tissue engineering. Biotechnol. Lett. 2006, 28, 697–702. [Google Scholar] [CrossRef]
- Collighan, R.J.; Griffin, M. Transglutaminase 2 cross-linking of matrix proteins: Biological significance and medical applications. Amino Acids 2009, 36, 659–670. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen Structural Hierarchy and Susceptibility to Degradation by Ultraviolet Radiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2008, 28, 1420–1429. [Google Scholar] [CrossRef] [Green Version]

| Crosslinking Agent | Concentration (% w/w) | Process Duration | Solvent (w/w Ratio) |
|---|---|---|---|
| Genipin | 0.5–6% | 6–168 h | EtOH:H2O (7:3) |
| Transglutaminase | 10–40 U/mL | 24–72 h | H2O |
| EDC/ NHS | 0.02–0.23% 0.01–0.08% | 10 min–9 h | EtOH:H2O (7:3) |
| BDDGE | 2.5–15% | 12–168 h | EtOH:H2O (7:3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulnik, J.; Sajkiewicz, P. Crosslinking of Gelatin in Bicomponent Electrospun Fibers. Materials 2021, 14, 3391. https://doi.org/10.3390/ma14123391
Dulnik J, Sajkiewicz P. Crosslinking of Gelatin in Bicomponent Electrospun Fibers. Materials. 2021; 14(12):3391. https://doi.org/10.3390/ma14123391
Chicago/Turabian StyleDulnik, Judyta, and Paweł Sajkiewicz. 2021. "Crosslinking of Gelatin in Bicomponent Electrospun Fibers" Materials 14, no. 12: 3391. https://doi.org/10.3390/ma14123391






