Mechanisms Supporting the Use of Beta-Blockers for the Management of Breast Cancer Bone Metastasis
Simple Summary
Abstract
1. Introduction
2. Sympathetic Innervation of the Skeleton and Evidence for an Interplay with the Process of Bone Metastasis
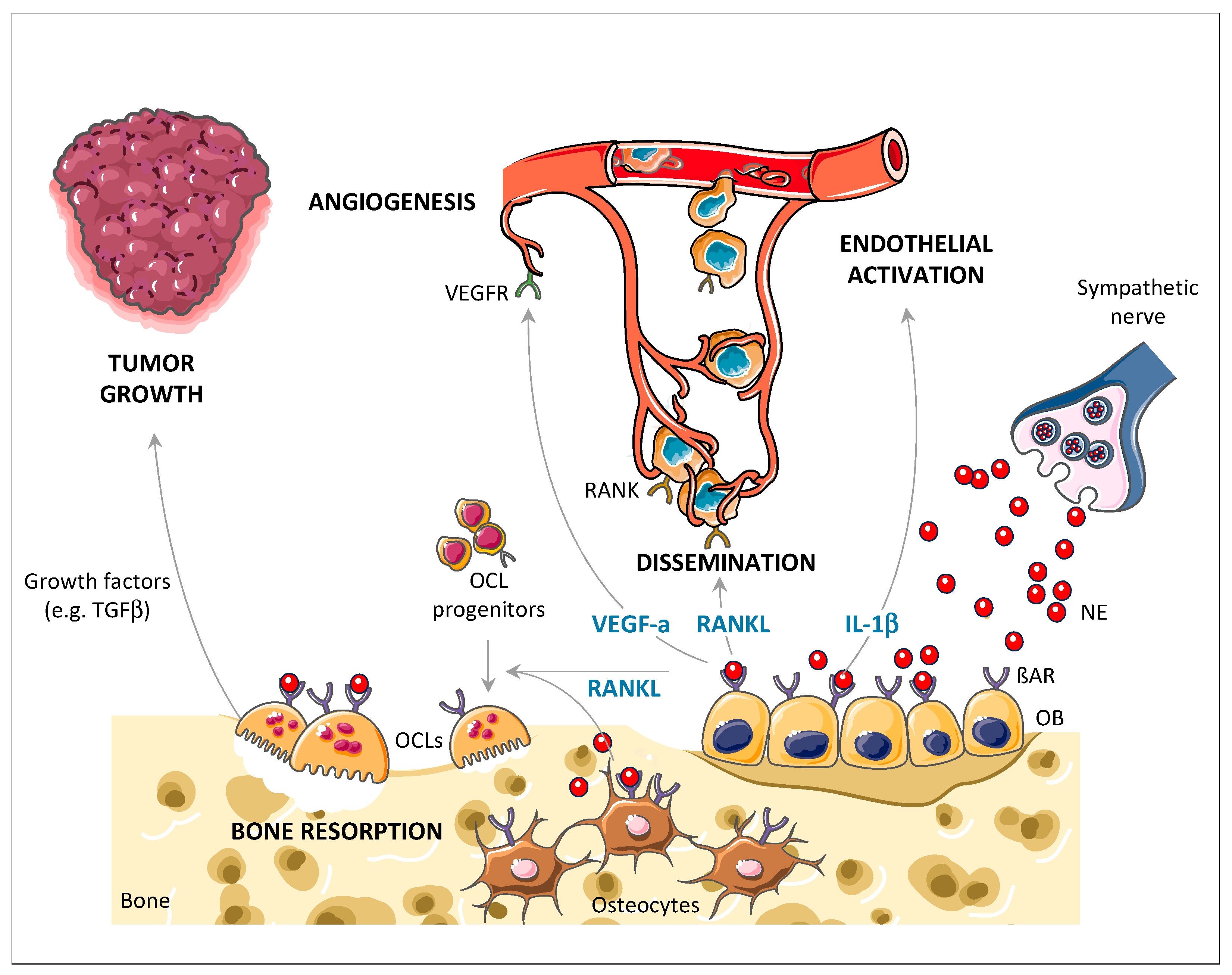
3. SNS-Induced Bone Stromal-Dependent Mechanisms Promote Skeletal Colonization by Cancer Cells
4. Treatment Strategies to Limit Metastatic Cancer Cell Engraftment into the Skeleton
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manders, K.; van de Poll-Franse, L.V.; Creemers, G.-J.; Vreugdenhil, G.; van der Sangen, M.J.; Nieuwenhuijzen, G.A.; Roumen, R.M.; Voogd, A.C. Clinical management of women with metastatic breast cancer: A descriptive study according to age group. BMC Cancer 2006, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Coleman, R.; Smith, P.; Rubens, R. Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer 1998, 77, 336–340. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Käkönen, S.-M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Bos, P.D.; Massagué, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef]
- Feller, L.; Kramer, B.; Lemmer, J. Pathobiology of cancer metastasis: A short account. Cancer Cell Int. 2012, 12, 24. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Shiri, S.; Farsinejad, S. Metastasis review: From bench to bedside. Tumour Biol. 2014, 35, 8483–8523. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Massagué, J. Genetic determinants of cancer metastasis. Nat. Rev. Genet. 2007, 8, 341–352. [Google Scholar] [CrossRef]
- Wels, J.; Kaplan, R.N.; Rafii, S.; Lyden, D. Migratory neighbors and distant invaders: Tumor-associated niche cells. Genes Dev. 2008, 22, 559–574. [Google Scholar] [CrossRef]
- Chiang, A.C.; Massagué, J. Molecular basis of metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef]
- Sikes, R.A.; Nicholson, B.E.; Koeneman, K.S.; Edlund, N.M.; Bissonette, E.A.; Bradley, M.J.; Thalmann, G.N.; Cecchini, M.G.; Pienta, K.J.; Chung, L.W.K. Cellular interactions in the tropism of prostate cancer to bone. Int. J. Cancer 2004, 110, 497–503. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Minn, A.J.; Kang, Y.; Serganova, I.; Gupta, G.P.; Giri, D.D.; Doubrovin, M.; Ponomarev, V.; Gerald, W.L.; Blasberg, R.; Massagué, J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Investig. 2005, 115, 44–55. [Google Scholar] [CrossRef]
- Hill, E.L.; Elde, R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991, 264, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F. Role of sympathetic nerves in the establishment of metastatic breast cancer cells in bone. J. Bone Oncol. 2016, 5, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Elefteriou, F. The new field of neuroskeletal biology. Calcif. Tissue Int. 2007, 80, 337–347. [Google Scholar] [CrossRef]
- Artico, M.; Bosco, S.; Cavallotti, C.; Agostinelli, E.; Giuliani-Piccari, G.; Sciorio, S.; Cocco, L.; Vitale, M. Noradrenergic and cholinergic innervation of the bone marrow. Int. J. Mol. Med. 2002, 10, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mach, D.B.; Rogers, S.D.; Sabino, M.C.; Luger, N.M.; Schwei, M.J.; Pomonis, J.D.; Keyser, C.P.; Clohisy, D.R.; Adams, D.J.; O’Leary, P.; et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience 2002, 113, 155–166. [Google Scholar] [CrossRef]
- Goblirsch, M.J.; Zwolak, P.P.; Clohisy, D.R. Biology of bone cancer pain. Clin. Cancer Res. 2006, 12, 6231s–6235s. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.R.; Brazill, J.M.; Beeve, A.T.; Shen, I.; Scheller, E.L. A neuroskeletal atlas: Spatial mapping and contextualization of axon subtypes innervating the long bones of C3H and B6 mice. J. Bone Miner. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sayilekshmy, M.; Hansen, R.B.; Delaissé, J.-M.; Rolighed, L.; Andersen, T.L.; Heegaard, A.-M. Innervation is higher above Bone Remodeling Surfaces and in Cortical Pores in Human Bone: Lessons from patients with primary hyperparathyroidism. Sci. Rep. 2019, 9, 5361. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Bjurholm, A.; Kreicbergs, A.; Schultzberg, M. Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine 1993, 18, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Bouwense, S.A.W.; Crawford, R.; Xiao, Y. Structural and cellular features in metaphyseal and diaphyseal periosteum of osteoporotic rats. J. Mol. Histol. 2010, 41, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, M.; Konttinen, Y.T.; Rees, R.G.; Santavirta, S.; Terenghi, G.; Polak, J.M. Distribution of nerve endings and sensory neuropeptides in rat synovium, meniscus and bone. Int. J. Tissue React. 1992, 14, 1–10. [Google Scholar]
- Imai, S.; Tokunaga, Y.; Maeda, T.; Kikkawa, M.; Hukuda, S. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: An immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J. Orthop. Res. 1997, 15, 133–140. [Google Scholar] [CrossRef]
- Chartier, S.R.; Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. The Changing Sensory and Sympathetic Innervation of the Young, Adult and Aging Mouse Femur. Neuroscience 2018, 387, 178–190. [Google Scholar] [CrossRef]
- Castañeda-Corral, G.; Jimenez-Andrade, J.M.; Bloom, A.P.; Taylor, R.N.; Mantyh, W.G.; Kaczmarska, M.J.; Ghilardi, J.R.; Mantyh, P.W. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011, 178, 196–207. [Google Scholar] [CrossRef]
- Martin, C.D.; Jimenez-Andrade, J.M.; Ghilardi, J.R.; Mantyh, P.W. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: Implications for the generation and maintenance of bone fracture pain. Neurosci. Lett. 2007, 427, 148–152. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Xu, H.; Ferng, A.S.; Dussor, G.; Vanderah, T.W.; Mantyh, P.W. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: Therapeutic opportunity for treating skeletal pain. Bone 2010, 46, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, R.E.; Li, Z.; Zhang, Q.; Goh, B.C.; Li, Z.; Thorek, D.L.J.; Rajbhandari, L.; Brushart, T.M.; Minichiello, L.; Zhou, F.; et al. NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Rep. 2016, 16, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, R.E.; Li, Z.; Li, Z.; Minichiello, L.; Riddle, R.C.; Venkatesan, A.; Clemens, T.L. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3632–E3641. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Lutgendorf, S.K.; Sood, A.K. Impact of stress on cancer metastasis. Future Oncol. 2010, 6, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Heffner, K.L.; Loving, T.J.; Robles, T.F.; Kiecolt-Glaser, J.K. Examining psychosocial factors related to cancer incidence and progression: In search of the silver lining. Brain. Behav. Immun. 2003, 17 (Suppl. 1), S109–S111. [Google Scholar] [CrossRef]
- Clouston, S.A.P.; Kuan, P.; Kotov, R.; Mukherjee, S.; Thompson-Carino, P.; Bromet, E.J.; Luft, B.J. Risk factors for incident prostate cancer in a cohort of world trade center responders. BMC Psychiatry 2019, 19, 389. [Google Scholar] [CrossRef]
- Kikuchi, N.; Nishiyama, T.; Sawada, T.; Wang, C.; Lin, Y.; Watanabe, Y.; Tamakoshi, A.; Kikuchi, S. Perceived Stress and Colorectal Cancer Incidence: The Japan Collaborative Cohort Study. Sci. Rep. 2017, 7, 40363. [Google Scholar] [CrossRef]
- Vahdaninia, M.; Omidvari, S.; Montazeri, A. What do predict anxiety and depression in breast cancer patients? A follow-up study. Soc. Psychiatry Psychiatr. Epidemiol. 2010, 45, 355–361. [Google Scholar] [CrossRef]
- Giese-Davis, J.; Wilhelm, F.H.; Conrad, A.; Abercrombie, H.C.; Sephton, S.; Yutsis, M.; Neri, E.; Taylor, C.B.; Kraemer, H.C.; Spiegel, D. Depression and stress reactivity in metastatic breast cancer. Psychosom. Med. 2006, 68, 675–683. [Google Scholar] [CrossRef]
- Ross, K. Mapping pathways from stress to cancer progression. J. Natl. Cancer Inst. 2008, 100, 914–915. [Google Scholar] [CrossRef]
- Burgess, C.; Cornelius, V.; Love, S.; Graham, J.; Richards, M.; Ramirez, A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ 2005, 330, 702. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.; Giese-Davis, J. Depression and cancer: Mechanisms and disease progression. Biol. Psychiatry 2003, 54, 269–282. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.-M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Voss, M.J.; Zänker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population- based study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Munzone, E.; Rotmensz, N.; Cipolla, C.; De Giorgi, V.; Santillo, B.; Zanelotti, A.; Adamoli, L.; Colleoni, M.; Viale, G.; et al. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 2013, 140, 567–575. [Google Scholar] [CrossRef]
- Choi, C.H.; Song, T.; Kim, T.H.; Choi, J.K.; Park, J.-Y.; Yoon, A.; Lee, Y.-Y.; Kim, T.-J.; Bae, D.-S.; Lee, J.-W.; et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J. Cancer Res. Clin. Oncol. 2014, 140, 1179–1188. [Google Scholar] [CrossRef]
- Elefteriou, F. Impact of the Autonomic Nervous System on the Skeleton. Physiol. Rev. 2018, 98, 1083–1112. [Google Scholar] [CrossRef]
- Ma, Y.; Nyman, J.S.; Tao, H.; Moss, H.H.; Yang, X.; Elefteriou, F. β2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 2011, 152, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Campbell, P.; Ma, Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif. Tissue Int. 2014, 94, 140–151. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Shimizu, N.; Hori, T.; Nakane, H. An interleukin-1 beta-induced noradrenaline release in the spleen is mediated by brain corticotropin-releasing factor: An in vivo microdialysis study in conscious rats. Brain Behav. Immun. 1994, 8, 14–23. [Google Scholar] [CrossRef]
- Madden, K.S.; Sanders, V.M.; Felten, D.L. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 417–448. [Google Scholar] [CrossRef]
- Badino, G.R.; Novelli, A.; Girardi, C.; Di Carlo, F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol. Res. 1996, 33, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Cole, B. Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef]
- Conceição, F.; Sousa, D.M.; Paredes, J.; Lamghari, M. Sympathetic activity in breast cancer and metastasis: Partners in crime. Bone Res. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Kubzansky, L.D.; Koenen, K.C.; Spiro, A.; Vokonas, P.S.; Sparrow, D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch. Gen. Psychiatry 2007, 64, 109–116. [Google Scholar] [CrossRef]
- Cohen, B.E.; Marmar, C.R.; Neylan, T.C.; Schiller, N.B.; Ali, S.; Whooley, M.A. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: Findings from the Heart and Soul Study. Arch. Gen. Psychiatry 2009, 66, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Block, J.P.; He, Y.; Zaslavsky, A.M.; Ding, L.; Ayanian, J.Z. Psychosocial stress and change in weight among US adults. Am. J. Epidemiol. 2009, 170, 181–192. [Google Scholar] [CrossRef]
- Gluck, M.E.; Geliebter, A.; Hung, J.; Yahav, E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom. Med. 2004, 66, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Jansson, C.; Wallander, M.-A.; Johansson, S.; Johnsen, R.; Hveem, K. Stressful psychosocial factors and symptoms of gastroesophageal reflux disease: A population-based study in Norway. Scand. J. Gastroenterol. 2010, 45, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Naliboff, B.D.; Fass, S.S.; Peleg, N.; Wendel, C.; Malagon, I.B.; Mayer, E.A. The effect of auditory stress on perception of intraesophageal acid in patients with gastroesophageal reflux disease. Gastroenterology 2008, 134, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.L.; Andreoli, A.; Luzi, C.; Arcà, M.; Berto, E.; Milite, G.; Marcheggiano, A. Stress and exacerbation in ulcerative colitis: A prospective study of patients enrolled in remission. Am. J. Gastroenterol. 2000, 95, 1213–1220. [Google Scholar] [CrossRef]
- Mittermaier, C.; Dejaco, C.; Waldhoer, T.; Oefferlbauer-Ernst, A.; Miehsler, W.; Beier, M.; Tillinger, W.; Gangl, A.; Moser, G. Impact of depressive mood on relapse in patients with inflammatory bowel disease: A prospective 18-month follow-up study. Psychosom. Med. 2004, 66, 79–84. [Google Scholar] [CrossRef]
- Maunder, R.G.; Greenberg, G.R.; Hunter, J.J.; Lancee, W.J.; Steinhart, A.H.; Silverberg, M.S. Psychobiological subtypes of ulcerative colitis: pANCA status moderates the relationship between disease activity and psychological distress. Am. J. Gastroenterol. 2006, 101, 2546–2551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erez, H.B.; Weller, A.; Vaisman, N.; Kreitler, S. The relationship of depression, anxiety and stress with low bone mineral density in post-menopausal women. Arch. Osteoporos. 2012, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Cizza, G.; Primma, S.; Coyle, M.; Gourgiotis, L.; Csako, G. Depression and osteoporosis: A research synthesis with meta-analysis. Horm. Metab. Res. 2010, 42, 467–482. [Google Scholar] [CrossRef]
- Huang, W.-S.; Hsu, J.-W.; Huang, K.-L.; Bai, Y.-M.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Chen, T.-J.; Tsai, S.-J.; Liou, Y.-J.; et al. Post-traumatic stress disorder and risk of osteoporosis: A nationwide longitudinal study. Stress Health 2018, 34, 440–445. [Google Scholar] [CrossRef] [PubMed]
- El-Gabalawy, R.; Blaney, C.; Tsai, J.; Sumner, J.A.; Pietrzak, R.H. Physical health conditions associated with full and subthreshold PTSD in U.S. military veterans: Results from the National Health and Resilience in Veterans Study. J. Affect. Disord. 2018, 227, 849–853. [Google Scholar] [CrossRef]
- Williams, L.J.; Pasco, J.A.; Jackson, H.; Kiropoulos, L.; Stuart, A.L.; Jacka, F.N.; Berk, M. Depression as a risk factor for fracture in women: A 10 year longitudinal study. J. Affect. Disord. 2016, 192, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Bab, I. Major depression is a risk factor for low bone mineral density: A meta-analysis. Biol. Psychiatry 2009, 66, 423–432. [Google Scholar] [CrossRef]
- Wong, S.Y.S.; Lau, E.M.C.; Lynn, H.; Leung, P.C.; Woo, J.; Cummings, S.R.; Orwoll, E. Depression and bone mineral density: Is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong). Osteoporos. Int. 2005, 16, 610–615. [Google Scholar] [CrossRef]
- Schweiger, U.; Deuschle, M.; Körner, A.; Lammers, C.H.; Schmider, J.; Gotthardt, U.; Holsboer, F.; Heuser, I. Low lumbar bone mineral density in patients with major depression. Am. J. Psychiatry 1994, 151, 1691–1693. [Google Scholar] [CrossRef]
- Esel, E.; Ozsoy, S.; Tutus, A.; Sofuoglu, S.; Kartalci, S.; Bayram, F.; Kokbudak, Z.; Kula, M. Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Michelson, D.; Stratakis, C.; Hill, L.; Reynolds, J.; Galliven, E.; Chrousos, G.; Gold, P. Bone mineral density in women with depression. N. Engl. J. Med. 1996, 335, 1176–1181. [Google Scholar] [CrossRef]
- Altindag, O.; Altindag, A.; Asoglu, M.; Gunes, M.; Soran, N.; Deveci, Z. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int. J. Clin. Pract. 2007, 61, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Ruffing, J.; Zion, M.; Uhorchak, J.; Ralston, S.; Tendy, S.; McGuigan, F.E.A.; Lindsay, R.; Nieves, J. Determinants of stress fracture risk in United States Military Academy cadets. Bone 2013, 55, 359–366. [Google Scholar] [CrossRef]
- Vandewalle, B.; Revillion, F.; Lefebvre, J. Functional beta-adrenergic receptors in breast cancer cells. J. Cancer Res. Clin. Oncol. 1990, 116, 303–306. [Google Scholar] [CrossRef]
- Madden, K.S.; Szpunar, M.J.; Brown, E.B. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 2011, 130, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Voss, M.J.; Habashy, H.O.; Zänker, K.S.; Green, A.R.; Ellis, I.O.; Entschladen, F. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: An immunohistochemical study. Breast Cancer Res. Treat. 2011, 130, 457–463. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, D.; Hinoi, E.; Ferron, M.; Kode, A.; Riley, K.J.; Zhou, B.; Guo, X.E.; Karsenty, G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J. Exp. Med. 2011, 208, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Schlienger, R.G.; Kraenzlin, M.E.; Jick, S.S.; Meier, C.R. Use of beta-blockers and risk of fractures. JAMA 2004, 292, 1326–1332. [Google Scholar] [CrossRef]
- Meisinger, C.; Heier, M.; Lang, O.; Döring, A. Beta-blocker use and risk of fractures in men and women from the general population: The MONICA/KORA Augsburg cohort study. Osteoporos. Int. 2007, 18, 1189–1195. [Google Scholar] [CrossRef]
- Toulis, K.A.; Hemming, K.; Stergianos, S.; Nirantharakumar, K.; Bilezikian, J.P. β-Adrenergic receptor antagonists and fracture risk: A meta-analysis of selectivity, gender, and site-specific effects. Osteoporos. Int. 2014, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lary, C.W.; Hinton, A.C.; Nevola, K.T.; Shireman, T.I.; Motyl, K.J.; Houseknecht, K.L.; Lucas, F.L.; Hallen, S.; Zullo, A.R.; Berry, S.D.; et al. Association of Beta Blocker Use With Bone Mineral Density in the Framingham Osteoporosis Study: A Cross-Sectional Study. JBMR Plus 2020, 4, e10388. [Google Scholar] [CrossRef]
- Turker, S.; Karatosun, V.; Gunal, I. Beta-blockers increase bone mineral density. Clin. Orthop. Relat. Res. 2006, 443, 73–74. [Google Scholar] [CrossRef]
- Pasco, J.A.; Henry, M.J.; Sanders, K.M.; Kotowicz, M.A.; Seeman, E.; Nicholson, G.C. Geelong Osteoporosis Study Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J. Bone Miner. Res. 2004, 19, 19–24. [Google Scholar] [CrossRef]
- Rejnmark, L.; Vestergaard, P.; Mosekilde, L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: A nationwide case-control study. J. Hypertens. 2006, 24, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Gadois, C.; McCloskey, E.; Lemineur, G.; Lespessailles, E.; Courteix, D.; Benhamou, C.L. Protective effect of beta blockers in postmenopausal women: Influence on fractures, bone density, micro and macroarchitecture. Bone 2007, 40, 1209–1216. [Google Scholar] [CrossRef]
- Khosla, S.; Drake, M.T.; Volkman, T.L.; Thicke, B.S.; Achenbach, S.J.; Atkinson, E.J.; Joyner, M.J.; Rosen, C.J.; Monroe, D.G.; Farr, J.N. Sympathetic β1-adrenergic signaling contributes to regulation of human bone metabolism. J. Clin. Investig. 2018, 128, 4832–4842. [Google Scholar] [CrossRef]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Taskén, K.A.; Håheim, L.L. Use of β-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 2013, 73, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Taskén, K.A. Association between use of β-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef]
- Watkins, J.L.; Thaker, P.H.; Nick, A.M.; Ramondetta, L.M.; Kumar, S.; Urbauer, D.L.; Matsuo, K.; Squires, K.C.; Coleman, R.L.; Lutgendorf, S.K.; et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 2015, 121, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Liao, Z.X.; Komaki, R.; Welsh, J.W.; O’Reilly, M.S.; Chang, J.Y.; Zhuang, Y.; Levy, L.B.; Lu, C.; Gomez, D.R. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Perron, L.; Bairati, I.; Harel, F.; Meyer, F. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control 2004, 15, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lemeshow, S.; Sørensen, H.T.; Phillips, G.; Yang, E.V.; Antonsen, S.; Riis, A.H.; Lesinski, G.B.; Jackson, R.; Glaser, R. β-Blockers and survival among Danish patients with malignant melanoma: A population-based cohort study. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Aydiner, A.; Ciftci, R.; Karabulut, S.; Kilic, L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac. J. Cancer Prev. 2013, 14, 6109–6114. [Google Scholar] [CrossRef]
- De Giorgi, V.; Gandini, S.; Grazzini, M.; Benemei, S.; Marchionni, N.; Geppetti, P. Effect of β-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin. Proc. 2013, 88, 1196–1203. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Coleman, H.G.; Murray, L.J.; Entschladen, F.; Powe, D.G. Beta-blocker usage and breast cancer survival: A nested case-control study within a UK clinical practice research datalink cohort. Int. J. Epidemiol. 2013, 42, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Reeder, A.; Attar, M.; Nazario, L.; Bathula, C.; Zhang, A.; Hochbaum, D.; Roy, E.; Cooper, K.L.; Oesterreich, S.; Davidson, N.E.; et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br. J. Cancer 2015, 112, 1461–1470. [Google Scholar] [CrossRef]
- Hara, M.R.; Sachs, B.D.; Caron, M.G.; Lefkowitz, R.J. Pharmacological blockade of a β(2)AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 2013, 12, 219–224. [Google Scholar] [CrossRef]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Armaiz-Pena, G.N.; Allen, J.K.; Cruz, A.; Stone, R.L.; Nick, A.M.; Lin, Y.G.; Han, L.Y.; Mangala, L.S.; Villares, G.J.; Vivas-Mejia, P.; et al. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat. Commun. 2013, 4, 1403. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Qian, L.; Wang, L.; Niu, L.; Zhang, H.; Yong, Z.; Gong, Z.; Song, L.; et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res. Treat. 2011, 125, 351–362. [Google Scholar] [CrossRef]
- Gu, L.; Lau, S.K.; Loera, S.; Somlo, G.; Kane, S.E. Protein kinase A activation confers resistance to trastuzumab in human breast cancer cell lines. Clin. Cancer Res. 2009, 15, 7196–7206. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Karpova, Y.; Baiz, D.; Yancey, D.; Pullikuth, A.; Flores, A.; Register, T.; Cline, J.M.; D’Agostino, R.; Danial, N.; et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Investig. 2013, 123, 874–886. [Google Scholar] [CrossRef]
- Sastry, K.S.R.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A.; Carson, J.P.; Weber, M.J.; Register, T.C.; Chen, Y.Q.; et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.J.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer 2013, 108, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Wolter, J.K.; Wolter, N.E.; Blanch, A.; Partridge, T.; Cheng, L.; Morgenstern, D.A.; Podkowa, M.; Kaplan, D.R.; Irwin, M.S. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 2014, 5, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Drell, T.L.; Joseph, J.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat. 2003, 80, 63–70. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Cole, S.; Costanzo, E.; Bradley, S.; Coffin, J.; Jabbari, S.; Rainwater, K.; Ritchie, J.M.; Yang, M.; Sood, A.K. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003, 9, 4514–4521. [Google Scholar] [PubMed]
- Nilsson, M.B.; Armaiz-Pena, G.; Takahashi, R.; Lin, Y.G.; Trevino, J.; Li, Y.; Jennings, N.; Arevalo, J.; Lutgendorf, S.K.; Gallick, G.E.; et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J. Biol. Chem. 2007, 282, 29919–29926. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Drell, T.L.; Lindecke, A.; Niggemann, B.; Kaltschmidt, C.; Zaenker, K.S.; Entschladen, F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer 2004, 112, 231–238. [Google Scholar] [CrossRef]
- Pérez Piñero, C.; Bruzzone, A.; Sarappa, M.G.; Castillo, L.F.; Lüthy, I.A. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Zhang, J.; Dancel, R.; Garcia, S.J.; Willis, C.; Seidler, F.J. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2000, 60, 153–166. [Google Scholar] [CrossRef]
- Gargiulo, L.; Copsel, S.; Rivero, E.M.; Galés, C.; Sénard, J.-M.; Lüthy, I.A.; Davio, C.; Bruzzone, A. Differential β₂-adrenergic receptor expression defines the phenotype of non-tumorigenic and malignant human breast cell lines. Oncotarget 2014, 5, 10058–10069. [Google Scholar] [CrossRef]
- Liu, D.; Deng, Q.; Sun, L.; Wang, T.; Yang, Z.; Chen, H.; Guo, L.; Liu, Y.; Ma, Y.; Guo, N.; et al. A Her2-let-7-β2-AR circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer 2015, 15, 832. [Google Scholar] [CrossRef]
- Rivero, E.M.; Martinez, L.M.; Bruque, C.D.; Gargiulo, L.; Bruzzone, A.; Lüthy, I.A. Prognostic significance of α- and β2-adrenoceptor gene expression in breast cancer patients. Br. J. Clin. Pharmacol. 2019, 85, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, S.; Kaira, K.; Matsumoto, H.; Hirakata, T.; Yokobori, T.; Inoue, K.; Horiguchi, J.; Katayama, A.; Koshi, H.; Shimizu, A.; et al. β2-Adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2019, 177, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Richard, F.; Brandão, M.; Awada, A.; Sotiriou, C.; de Azambuja, E. Prognostic and Predictive Impact of Beta-2 Adrenergic Receptor Expression in HER2-Positive Breast Cancer. Clin. Breast Cancer 2020, 20, 262–273.e7. [Google Scholar] [CrossRef] [PubMed]
- Pacholczyk, T.; Blakely, R.D.; Amara, S.G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 1991, 350, 350–354. [Google Scholar] [CrossRef]
- Zavosh, A.; Schaefer, J.; Ferrel, A.; Figlewicz, D.P. Desipramine treatment decreases 3H-nisoxetine binding and norepinephrine transporter mRNA in SK-N-SHSY5Y cells. Brain Res. Bull. 1999, 49, 291–295. [Google Scholar] [CrossRef]
- Tellioglu, T.; Robertson, D. Genetic or acquired deficits in the norepinephrine transporter: Current understanding of clinical implications. Expert Rev. Mol. Med. 2001, 2001, 1–10. [Google Scholar] [CrossRef]
- Blakely, R.D.; Bauman, A.L. Biogenic amine transporters: Regulation in flux. Curr. Opin. Neurobiol. 2000, 10, 328–336. [Google Scholar] [CrossRef]
- Ma, Y.; Krueger, J.J.; Redmon, S.N.; Uppuganti, S.; Nyman, J.S.; Hahn, M.K.; Elefteriou, F. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J. Biol. Chem. 2013, 288, 30105–30113. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Elefteriou, F. Cortical bone is an extraneuronal site of norepinephrine uptake in adult mice. Bone Rep. 2018, 9, 188–198. [Google Scholar] [CrossRef]
- Mohammadpour, H.; MacDonald, C.R.; Qiao, G.; Chen, M.; Dong, B.; Hylander, B.L.; McCarthy, P.L.; Abrams, S.I.; Repasky, E.A. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Investig. 2019, 129, 5537–5552. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Nagaraja, A.S.; Rodriguez-Aguayo, C.; Sadaoui, N.C.; Stone, R.L.; Matsuo, K.; Dalton, H.J.; Previs, R.A.; Jennings, N.B.; et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015, 6, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Korin, Y.D.; Fahey, J.L.; Zack, J.A. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J. Immunol. 1998, 161, 610–616. [Google Scholar] [PubMed]
- Collado-Hidalgo, A.; Sung, C.; Cole, S. Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav. Immun. 2006, 20, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Inbar, S.; Neeman, E.; Avraham, R.; Benish, M.; Rosenne, E.; Ben-Eliyahu, S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS ONE 2011, 6, e19246. [Google Scholar] [CrossRef]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Lee, J.-W.; Shahzad, M.M.K.; Lin, Y.G.; Armaiz-Pena, G.; Mangala, L.S.; Han, H.-D.; Kim, H.-S.; Nam, E.J.; Jennings, N.B.; Halder, J.; et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin. Cancer Res. 2009, 15, 2695–2702. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Campbell, J.P.; Karolak, M.R.; Ma, Y.; Perrien, D.S.; Masood-Campbell, S.K.; Penner, N.L.; Munoz, S.A.; Zijlstra, A.; Yang, X.; Sterling, J.A.; et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012, 10, e1001363. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Battista, M.; Kao, W.-M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Fu, G.; Dai, T.; Huang, H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007, 50, 274–280. [Google Scholar] [CrossRef]
- Elefteriou, F. Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 2008, 473, 231–236. [Google Scholar] [CrossRef]
- Yao, Q.; Liang, H.; Huang, B.; Xiang, L.; Wang, T.; Xiong, Y.; Yang, B.; Guo, Y.; Gong, P. Beta-adrenergic signaling affect osteoclastogenesis via osteocytic MLO-Y4 cells’ RANKL production. Biochem. Biophys. Res. Commun. 2017, 488, 634–640. [Google Scholar] [CrossRef]
- Jiao, K.; Niu, L.-N.; Li, Q.; Ren, G.; Zhao, C.; Liu, Y.; Tay, F.R.; Wang, M. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci. Rep. 2015, 5, 12593. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Mäkinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef]
- Ogawa, S.; Oku, A.; Sawano, A.; Yamaguchi, S.; Yazaki, Y.; Shibuya, M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem. 1998, 273, 31273–31282. [Google Scholar] [CrossRef]
- Meyer, M.; Clauss, M.; Lepple-Wienhues, A.; Waltenberger, J.; Augustin, H.G.; Ziche, M.; Lanz, C.; Büttner, M.; Rziha, H.J.; Dehio, C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis—Correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef]
- Yang, E.V.; Kim, S.; Donovan, E.L.; Chen, M.; Gross, A.C.; Webster Marketon, J.I.; Barsky, S.H.; Glaser, R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav. Immun. 2009, 23, 267–275. [Google Scholar] [CrossRef]
- Liu, J.; Deng, G.-H.; Zhang, J.; Wang, Y.; Xia, X.-Y.; Luo, X.-M.; Deng, Y.-T.; He, S.-S.; Mao, Y.-Y.; Peng, X.-C.; et al. The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 2015, 52, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, C.; He, Y.; Griffin, R.; Ye, Q.; Li, L. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 2015, 51, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Itkin, T.; Mäe, M.A.; Langen, U.H.; Betsholtz, C.; Lapidot, T.; Adams, R.H. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 2016, 532, 380–384. [Google Scholar] [CrossRef]
- Mulcrone, P.L.; Campbell, J.P.; Clément-Demange, L.; Anbinder, A.L.; Merkel, A.R.; Brekken, R.A.; Sterling, J.A.; Elefteriou, F. Skeletal Colonization by Breast Cancer Cells Is Stimulated by an Osteoblast and β2AR-Dependent Neo-Angiogenic Switch. J. Bone Miner. Res. 2017, 32, 1442–1454. [Google Scholar] [CrossRef]
- Clément-Demange, L.; Mulcrone, P.L.; Tabarestani, T.Q.; Sterling, J.A.; Elefteriou, F. β2ARs stimulation in osteoblasts promotes breast cancer cell adhesion to bone marrow endothelial cells in an IL-1β and selectin-dependent manner. J. Bone Oncol. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Gopinathan, G.; Milagre, C.; Pearce, O.M.T.; Reynolds, L.E.; Hodivala-Dilke, K.; Leinster, D.A.; Zhong, H.; Hollingsworth, R.E.; Thompson, R.; Whiteford, J.R.; et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015, 75, 3098–3107. [Google Scholar] [CrossRef]
- Catar, R.; Witowski, J.; Zhu, N.; Lücht, C.; Derrac Soria, A.; Uceda Fernandez, J.; Chen, L.; Jones, S.A.; Fielding, C.A.; Rudolf, A.; et al. IL-6 Trans-Signaling Links Inflammation with Angiogenesis in the Peritoneal Membrane. J. Am. Soc. Nephrol. 2017, 28, 1188–1199. [Google Scholar] [CrossRef]
- Shinriki, S.; Jono, H.; Ueda, M.; Ota, K.; Ota, T.; Sueyoshi, T.; Oike, Y.; Ibusuki, M.; Hiraki, A.; Nakayama, H.; et al. Interleukin-6 signalling regulates vascular endothelial growth factor-C synthesis and lymphangiogenesis in human oral squamous cell carcinoma. J. Pathol. 2011, 225, 142–150. [Google Scholar] [CrossRef]
- Jiang, X.-P.; Yang, D.C.; Elliott, R.L.; Head, J.F. Down-regulation of expression of interleukin-6 and its receptor results in growth inhibition of MCF-7 breast cancer cells. Anticancer Res. 2011, 31, 2899–2906. [Google Scholar]
- Studebaker, A.W.; Storci, G.; Werbeck, J.L.; Sansone, P.; Sasser, A.K.; Tavolari, S.; Huang, T.; Chan, M.W.Y.; Marini, F.C.; Rosol, T.J.; et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008, 68, 9087–9095. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Biancone, L.; Araki, M.; Araki, K.; Vassalli, P.; Stamenkovic, I. Redirection of tumor metastasis by expression of E-selectin in vivo. J. Exp. Med. 1996, 183, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Borsig, L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef]
- Sökeland, G.; Schumacher, U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol. Cancer 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Makó, V.; Czúcz, J.; Weiszhár, Z.; Herczenik, E.; Matkó, J.; Prohászka, Z.; Cervenak, L. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytometry A 2010, 77, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.M.; Zhou, L.; Karasek, M.A.; Paturi, S.G.; Chaudhuri, V. Regulation of skin microvasculature angiogenesis, cell migration, and permeability by a specific inhibitor of PKCalpha. J. Investig. Dermatol. 2006, 126, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Idan, C.; Romzova, M.; Kaplanov, I.; et al. Unique Versus Redundant Functions of IL-1α and IL-1β in the Tumor Microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef]
- Khatib, A.-M.; Auguste, P.; Fallavollita, L.; Wang, N.; Samani, A.; Kontogiannea, M.; Meterissian, S.; Brodt, P. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am. J. Pathol. 2005, 167, 749–759. [Google Scholar] [CrossRef]
- Okahara, H.; Yagita, H.; Miyake, K.; Okumura, K. Involvement of very late activation antigen 4 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) in tumor necrosis factor alpha enhancement of experimental metastasis. Cancer Res. 1994, 54, 3233–3236. [Google Scholar] [PubMed]
- Giavazzi, R.; Garofalo, A.; Bani, M.R.; Abbate, M.; Ghezzi, P.; Boraschi, D.; Mantovani, A.; Dejana, E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990, 50, 4771–4775. [Google Scholar] [PubMed]
- Lauri, D.; Bertomeu, M.C.; Orr, F.W.; Bastida, E.; Sauder, D.; Buchanan, M.R. Interleukin-1 increases tumor cell adhesion to endothelial cells through an RGD dependent mechanism: In vitro and in vivo studies. Clin. Exp. Metastasis 1990, 8, 27–32. [Google Scholar] [CrossRef]
- Arguello, F.; Baggs, R.B.; Graves, B.T.; Harwell, S.E.; Cohen, H.J.; Frantz, C.N. Effect of IL-1 on experimental bone/bone-marrow metastases. Int. J. Cancer 1992, 52, 802–807. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Jimi, E.; Matsuzaki, K.; Tsurukai, T.; Itoh, K.; Nakagawa, N.; Yasuda, H.; Goto, M.; Tsuda, E.; et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: Receptor activator of NF-kappa B ligand. Bone 1999, 25, 517–523. [Google Scholar] [CrossRef]
- Tatsumi, S.; Ishii, K.; Amizuka, N.; Li, M.; Kobayashi, T.; Kohno, K.; Ito, M.; Takeshita, S.; Ikeda, K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007, 5, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Horwood, N.J.; Kartsogiannis, V.; Quinn, J.M.; Romas, E.; Martin, T.J.; Gillespie, M.T. Activated T lymphocytes support osteoclast formation in vitro. Biochem. Biophys. Res. Commun. 1999, 265, 144–150. [Google Scholar] [CrossRef]
- Komuro, H.; Olee, T.; Kühn, K.; Quach, J.; Brinson, D.C.; Shikhman, A.; Valbracht, J.; Creighton-Achermann, L.; Lotz, M. The osteoprotegerin/receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand system in cartilage. Arthritis Rheum. 2001, 44, 2768–2776. [Google Scholar] [CrossRef]
- Kwan Tat, S.; Amiable, N.; Pelletier, J.-P.; Boileau, C.; Lajeunesse, D.; Duval, N.; Martel-Pelletier, J. Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology 2009, 48, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef]
- Chen, L.-M.; Kuo, C.-H.; Lai, T.-Y.; Lin, Y.-M.; Su, C.-C.; Hsu, H.-H.; Tsai, F.-J.; Tsai, C.-H.; Huang, C.-Y.; Tang, C.-H. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J. Cell. Biochem. 2011, 112, 933–941. [Google Scholar] [CrossRef]
- Armstrong, A.P.; Miller, R.E.; Jones, J.C.; Zhang, J.; Keller, E.T.; Dougall, W.C. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate 2008, 68, 92–104. [Google Scholar] [CrossRef]
- Zhang, L.; Teng, Y.; Zhang, Y.; Liu, J.; Xu, L.; Qu, J.; Hou, K.; Yang, X.; Liu, Y.; Qu, X. Receptor activator for nuclear factor κ B expression predicts poor prognosis in breast cancer patients with bone metastasis but not in patients with visceral metastasis. J. Clin. Pathol. 2012, 65, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Sacanna, E.; Gaudio, M.; Mercatali, L.; Scarpi, E.; Zoli, W.; Serra, P.; Ricci, R.; Serra, L.; Kang, Y.; et al. Role of RANK, RANKL, OPG, and CXCR4 tissue markers in predicting bone metastases in breast cancer patients. Clin. Breast Cancer 2011, 11, 369–375. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Axmann, R.; Böhm, C.; Krönke, G.; Zwerina, J.; Smolen, J.; Schett, G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009, 60, 2747–2756. [Google Scholar] [CrossRef]
- Kudo, O.; Sabokbar, A.; Pocock, A.; Itonaga, I.; Fujikawa, Y.; Athanasou, N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 2003, 32, 1–7. [Google Scholar] [CrossRef]
- Devlin, R.D.; Reddy, S.V.; Savino, R.; Ciliberto, G.; Roodman, G.D. IL-6 mediates the effects of IL-1 or TNF, but not PTHrP or 1,25(OH)2D3, on osteoclast-like cell formation in normal human bone marrow cultures. J. Bone Miner. Res. 1998, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F. Neuronal signaling and the regulation of bone remodeling. Cell. Mol. Life Sci. 2005, 62, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys. 2009, 482, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Nagasawa, T.; Koshihara, Y.; Yamamoto, S.; Togari, A. Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim. Biophys. Acta 2003, 1640, 137–142. [Google Scholar] [CrossRef]
- Sanders, J.L.; Chattopadhyay, N.; Kifor, O.; Yamaguchi, T.; Butters, R.R.; Brown, E.M. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 2000, 141, 4357–4364. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Guise, T.A.; Yin, J.J.; Taylor, S.D.; Kumagai, Y.; Dallas, M.; Boyce, B.F.; Yoneda, T.; Mundy, G.R. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Investig. 1996, 98, 1544–1549. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef]
- Kagiya, T. MicroRNAs and Osteolytic Bone Metastasis: The Roles of MicroRNAs in Tumor-Induced Osteoclast Differentiation. J. Clin. Med. 2015, 4, 1741–1752. [Google Scholar] [CrossRef]
- Chiechi, A.; Waning, D.L.; Stayrook, K.R.; Buijs, J.T.; Guise, T.A.; Mohammad, K.S. Role of TGF-β in breast cancer bone metastases. Adv. Biosci. Biotechnol. 2013, 4, 15–30. [Google Scholar] [CrossRef]
- Kakonen, S.-M.; Selander, K.S.; Chirgwin, J.M.; Yin, J.J.; Burns, S.; Rankin, W.A.; Grubbs, B.G.; Dallas, M.; Cui, Y.; Guise, T.A. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 2002, 277, 24571–24578. [Google Scholar] [CrossRef] [PubMed]
- Buijs, J.T.; Stayrook, K.R.; Guise, T.A. TGF-β in the Bone Microenvironment: Role in Breast Cancer Metastases. Cancer Microenviron. 2011, 4, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Selander, K.; Chirgwin, J.M.; Dallas, M.; Grubbs, B.G.; Wieser, R.; Massagué, J.; Mundy, G.R.; Guise, T.A. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999, 103, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Nyman, J.S.; Alvarez, J.; Chakrabarti, A.; Ayres, A.; Sterling, J.; Edwards, J.; Rana, T.; Johnson, R.; Perrien, D.S.; et al. Anti-transforming growth factor ß antibody treatment rescues bone loss and prevents breast cancer metastasis to bone. PLoS ONE 2011, 6, e27090. [Google Scholar] [CrossRef]
- Ganapathy, V.; Ge, R.; Grazioli, A.; Xie, W.; Banach-Petrosky, W.; Kang, Y.; Lonning, S.; McPherson, J.; Yingling, J.M.; Biswas, S.; et al. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer 2010, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Yan, J.; Lu, X.; Xu, S.; Lerit, D.A.; Kang, Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat. Med. 2009, 15, 960–966. [Google Scholar] [CrossRef]
- Guise, T.A.; Mundy, G.R. Cancer and Bone*. Endocr. Rev. 1998, 19, 18–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guise, T.A.; Chirgwin, J.M. Transforming growth factor-beta in osteolytic breast cancer bone metastases. Clin. Orthop. Relat. Res. 2003, S32–S38. [Google Scholar] [CrossRef]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef]
- Regan, J.N.; Mikesell, C.; Reiken, S.; Xu, H.; Marks, A.R.; Mohammad, K.S.; Guise, T.A.; Waning, D.L. Osteolytic Breast Cancer Causes Skeletal Muscle Weakness in an Immunocompetent Syngeneic Mouse Model. Front. Endocrinol. 2017, 8, 358. [Google Scholar] [CrossRef]
- Regan, J.N.; Trivedi, T.; Guise, T.A.; Waning, D.L. The Role of TGFβ in Bone-Muscle Crosstalk. Curr. Osteoporos. Rep. 2017, 15, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Madel, M.-B.; Ibáñez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef]
- Ibáñez, L.; Abou-Ezzi, G.; Ciucci, T.; Amiot, V.; Belaïd, N.; Obino, D.; Mansour, A.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. Inflammatory Osteoclasts Prime TNFα-Producing CD4+ T Cells and Express CX3 CR1. J. Bone Miner. Res. 2016, 31, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.S.; Kiesel, J.R.; DiPaolo, R.; Pagadala, M.S.; Aurora, R. Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro. PLoS ONE 2012, 7, e38199. [Google Scholar] [CrossRef]
- Kiesel, J.R.; Buchwald, Z.S.; Aurora, R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J. Immunol. 2009, 182, 5477–5487. [Google Scholar] [CrossRef]
- Madel, M.-B.; Ibáñez, L.; Ciucci, T.; Halper, J.; Rouleau, M.; Boutin, A.; Hue, C.; Duroux-Richard, I.; Apparailly, F.; Garchon, H.-J.; et al. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Acharya, C.; Feng, X.; Wen, K.; Zhong, M.; Zhang, L.; Munshi, N.C.; Qiu, L.; Tai, Y.-T.; Anderson, K.C. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: Therapeutic implication. Blood 2016, 128, 1590–1603. [Google Scholar] [CrossRef]
- Ell, B.; Mercatali, L.; Ibrahim, T.; Campbell, N.; Schwarzenbach, H.; Pantel, K.; Amadori, D.; Kang, Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 2013, 24, 542–556. [Google Scholar] [CrossRef]
- Lee, D.Y.; Deng, Z.; Wang, C.-H.; Yang, B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef]
- O’Day, E.; Lal, A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010, 12, 201. [Google Scholar] [CrossRef]
- Gainford, M.C.; Dranitsaris, G.; Clemons, M. Recent developments in bisphosphonates for patients with metastatic breast cancer. BMJ 2005, 330, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Pavlakis, N. Optimal management of bone metastases in breast cancer patients. Breast Cancer 2011, 3, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Clines, G.A.; Guise, T.A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 2008, 10, e7. [Google Scholar] [CrossRef]
- Barre, P.V.; Padmaja, G.; Rana, S. Tiamongla Stress and Quality of Life in Cancer Patients: Medical and Psychological Intervention. Indian J. Psychol. Med. 2018, 40, 232–238. [Google Scholar] [CrossRef]
- Weber, D.; O’Brien, K. Cancer and Cancer-Related Fatigue and the Interrelationships with Depression, Stress, and Inflammation. J. Evid. Based Complement. Altern. Med. 2017, 22, 502–512. [Google Scholar] [CrossRef]
- Porcerelli, J.H.; Bornstein, R.F.; Porcerelli, D.; Arterbery, V.E. The complex role of personality in cancer treatment: Impact of dependency-detachment on health status, distress, and physician-patient relationship. J. Nerv. Ment. Dis. 2015, 203, 264–268. [Google Scholar] [CrossRef]
- Thornton, L.M.; Andersen, B.L.; Carson, W.E. Immune, endocrine, and behavioral precursors to breast cancer recurrence: A case-control analysis. Cancer Immunol. Immunother. 2008, 57, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Baqutayan, S.M.S. The effect of anxiety on breast cancer patients. Indian J. Psychol. Med. 2012, 34, 119–123. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, H.-Y. Clinical utility of sympathetic blockade in cardiovascular disease management. Expert Rev. Cardiovasc. Ther. 2017, 15, 277–288. [Google Scholar] [CrossRef]
- Fryzek, J.P.; Poulsen, A.H.; Lipworth, L.; Pedersen, L.; Nørgaard, M.; McLaughlin, J.K.; Friis, S. A cohort study of antihypertensive medication use and breast cancer among Danish women. Breast Cancer Res. Treat. 2006, 97, 231–236. [Google Scholar] [CrossRef]
- Li, C.I.; Malone, K.E.; Weiss, N.S.; Boudreau, D.M.; Cushing-Haugen, K.L.; Daling, J.R. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer 2003, 98, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef]
- Spera, G.; Fresco, R.; Fung, H.; Dyck, J.R.B.; Pituskin, E.; Paterson, I.; Mackey, J.R. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: A retrospective analysis of the ROSE/TRIO-012 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1836–1841. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Ciccolini, J.; Carre, M.; Giacometti, S.; Fanciullino, R.; Pouchy, C.; Montero, M.-P.; Serdjebi, C.; Kavallaris, M.; Andre, N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget 2011, 2. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Munzone, E.; Botteri, E.; Rotmensz, N.; Cipolla, C.M.; Zanelotti, A.; Adamoli, L.; Viale, G.; Goldhirsch, A.; Gandini, S. Prognostic effect of beta blockers (BB) in triple-negative breast cancer (TNBC) patients. J. Clin. Oncol. 2013, 31, 1061. [Google Scholar] [CrossRef]
- Modi, N.D.; Tan, J.Q.E.; Rowland, A.; Koczwara, B.; Kichenadasse, G.; McKinnon, R.A.; Wiese, M.D.; Sorich, M.J.; Hopkins, A.M. The Influence of Pre-Existing Beta-Blockers Use on Survival Outcomes in HER2 Positive Advanced Breast Cancer: Pooled Analysis of Clinical Trial Data. Front. Oncol. 2020, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madel, M.-B.; Elefteriou, F. Mechanisms Supporting the Use of Beta-Blockers for the Management of Breast Cancer Bone Metastasis. Cancers 2021, 13, 2887. https://doi.org/10.3390/cancers13122887
Madel M-B, Elefteriou F. Mechanisms Supporting the Use of Beta-Blockers for the Management of Breast Cancer Bone Metastasis. Cancers. 2021; 13(12):2887. https://doi.org/10.3390/cancers13122887
Chicago/Turabian StyleMadel, Maria-Bernadette, and Florent Elefteriou. 2021. "Mechanisms Supporting the Use of Beta-Blockers for the Management of Breast Cancer Bone Metastasis" Cancers 13, no. 12: 2887. https://doi.org/10.3390/cancers13122887
APA StyleMadel, M.-B., & Elefteriou, F. (2021). Mechanisms Supporting the Use of Beta-Blockers for the Management of Breast Cancer Bone Metastasis. Cancers, 13(12), 2887. https://doi.org/10.3390/cancers13122887




