Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation
Abstract
:1. Introduction
2. Methods
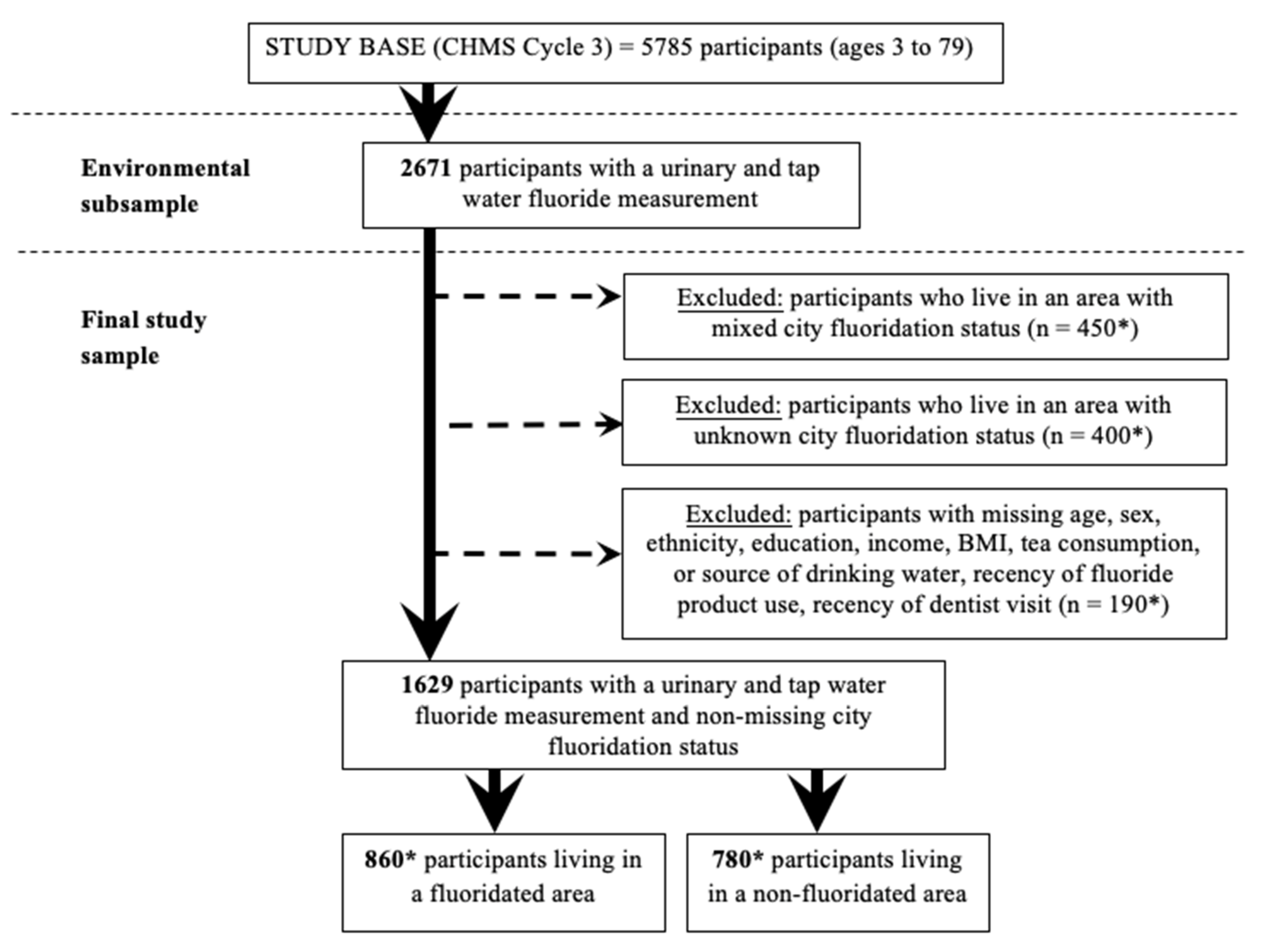
2.1. Data Source and Participants
2.2. Measurement of Water Fluoride Concentration
2.3. Measurement of Urinary Fluoride Concentration
2.4. Drinking Water Habits
2.5. Other Sources of Fluoride Exposure
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Urinary Fluoride Levels by Demographic Characteristics
3.3. Differences in UFSG by Drinking Water Habits
3.4. Differences in UFSG by Dental Product Use
3.5. Differences in UFSG by Tea Drinking Habits
3.6. Predictors of Urinary Fluoride Concentration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Public Health Agency of Canada. The State of Community Water Fluoridation Across Canada. 2017. Available online: https://www.canada.ca/en/services/health/publications/healthyliving/community-water-fluoridation-across-canada-2017.html (accessed on 23 November 2019).
- Centers for Disease Control and Prevention. Fluoridation Statistics. 2014. Available online: https://www.cdc.gov/fluoridation/statistics/2014stats.htm (accessed on 6 October 2019).
- Powell, N. Ireland Reviews Water Fluoridation. Can. Med. Assoc. J. 2014, 186, E343–E344. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015, 130, 318–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Fluoride. 2010. Available online: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/healthy-living-vie-saine/water-fluoride-fluorure-eau/alt/water-fluoride-fluorure-eau-eng.pdf (accessed on 10 December 2019).
- Jean, K.; Wassef, N.; Gagnon, F.; Valcke, M. A Physiologically-Based Pharmacokinetic Modeling Approach Using Biomonitoring Data in Order to Assess the Contribution of Drinking Water for the Achievement of an Optimal Fluoride Dose for Dental Health in Children. Int. J. Environ. Res. Public Health 2018, 15, 1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency. Fluoride: Relative Source Contribution Analysis. In Health and Ecological Criteria Division Office of Water; United States Environmental Protection Agency: Washington, DC, USA, 2010. [Google Scholar]
- Li, H.R.; Liu, W.Y.; Wang, L.S.; Li, Y.H.; Feng, F.J.; Zhao, Z.; Hou, K.; Wang, G. Fluoride in Drinking Water, Brick Tea Infusion and Human Urine in Two Counties in Inner Mongolia, China. J. Hazard. Mater. 2009, 167, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.; Potter, W.; Limeback, H.; Godfrey, M. Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and Its Implications for Public Health and Water Fluoridation. Int. J. Environ. Res. Public Health 2016, 13, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, A.K.; Kaushik, C.P.; Haritash, A.K.; Singh, B.; Raghuvanshi, S.P.; Kansal, A. Determination of Exposure and Probable Ingestion of Fluoride through Tea, Toothpaste, Tobacco and Pan Masala. J. Hazard. Mater. 2007, 142, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Saravanabhavan, G.; Werry, K.; Walker, M.; Haines, D.; Malowany, M.; Khoury, C. Human Biomonitoring Reference Values for Metals and Trace Elements in Blood and Urine Derived from the Canadian Health Measures Survey 2007–2013. Int. J. Hyg. Environ. Health 2017, 220, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Health Canada. Third Report on Human Biomonitoring of Environmental Chemicals in Canada—Results of the Canadian Health Measures Survey Cycle 3 (2012–2013). 2015. Available online: www.healthcanada.gc.ca/biomonitoring (accessed on 22 October 2019).
- Valcke, M.; Karthikeyan, S.; Walker, M.; Gagné, M.; Copes, R.; St-Amand, A. Regional Variations in Human Chemical Exposures in Canada: A Case Study Using Biomonitoring Data from the Canadian Health Measures Survey for the Provinces of Quebec and Ontario. Int. J. Hyg. Environ. Health 2020, 225, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Till, C.; Cantoral, A.; Lanphear, B.; Martinez-Mier, E.; Ayotte, P.; Wright, R.O.; Tellez-Rojo, M.M.; Malin, A.J. Associations between Urinary, Dietary, and Water Fluoride Concentrations among Children in Mexico and Canada. Toxics 2020, 8, 110. [Google Scholar] [CrossRef]
- Kumar, S.; Lata, S.; Yadav, J.; Yadav, J.P. Relationship between Water, Urine and Serum Fluoride and Fluorosis in School Children of Jhajjar District, Haryana, India. Appl. Water Sci. 2017, 7, 3377–3384. [Google Scholar] [CrossRef] [Green Version]
- Rango, T.; Vengosh, A.; Jeuland, M.; Tekle-Haimanot, R.; Weinthal, E.; Kravchenko, J.; Paul, C.; McCornick, P. Fluoride Exposure from Groundwater as Reflected by Urinary Fluoride and Children’s Dental Fluorosis in the Main Ethiopian Rift Valley. Sci. Total Environ. 2014, 496, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Till, C.; Green, R.; Grundy, J.G.; Hornung, R.; Neufeld, R.; Martínez-Mier, E.A.; Ayotte, P.; Muckle, G.; Lanphear, G. Community Water Fluoridation and Urinary Fluoride Concentrations in a National Sample of Pregnant Women in Canada. Environ. Health Perspect. 2018, 126, 1–13. [Google Scholar] [CrossRef]
- Jain, R.B. Concentrations of Fluoride in Water and Plasma for US Children and Adolescents: Data from NHANES 2013–2014. Environ. Toxicol. Pharmacol. 2017, 50, 20–31. [Google Scholar] [CrossRef]
- Ahmed, I.; Rafique, T.; Hasan, S.K.; Khan, N.; Khan, M.H.; Usmani, T.H. Correlation of Fluoride in Drinking Water with Urine, Blood Plasma, and Serum Fluoride Levels of People Consuming High and Low Fluoride Drinking Water in Pakistan. Fluoride 2012, 45, 384–388. Available online: http://fluoridealert.org/wp-content/uploads/ahmed-2012.pdf (accessed on 2 June 2021).
- Carwile, J.L.; Ahrens, K.A.; Seshasayee, S.M.; Lanphear, B.; Fleisch, A.F. Predictors of Plasma Fluoride Concentrations in Children and Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 9205. [Google Scholar] [CrossRef] [PubMed]
- McLaren, L. Fluoridation Exposure Status Based on Location of Data Collection in the Canadian Health Measures Survey: Is It Valid. J. Can. Dent. Assoc. 2016, 82, 1–7. Available online: https://jcda.ca/g17 (accessed on 25 October 2019).
- Riddell, J.K.; Malin, A.J.; Flora, D.; McCague, H.; Till, C. Association of Water Fluoride and Urinary Fluoride Concentrations with Attention Deficit Hyperactivity Disorder in Canadian Youth. Environ. Int. 2019, 133, 105190. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Whitford, G.M. Fluoride Metabolism. In Fluoride and the Oral Environment; Karger Publishers: Basel, Switzerland, 2011; Volume 22, pp. 20–36. [Google Scholar]
- Aylward, L.L.; Hays, S.M.; Vezina, A.; Deveau, M.; St-Amand, A.; Nong, A. Biomonitoring Equivalents for Interpretation of Urinary Fluoride. Regul. Toxicol. Pharmacol. 2015, 72, 158–167. [Google Scholar] [CrossRef]
- Buzalaf, C.P.; Leite, A.D.L.; Buzalaf, M.A.R. Fluoride Metabolism. In Food and Nutritional Components in Focus No. 6—Fluorine: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- National Research Council. Fluoride in Drinking Water: A Scientific Review of EPA’s Standards; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Day, B.; Langlois, R.; Tremblay, M.; Knoppers, B.M. Canadian Health Measures Survey: Ethical, Legal and Social Issues. Health Rep. 2006, 18, 37–51. Available online: https://www150.statcan.gc.ca/n1/en/pub/82-003-s/2007000/article/10364-eng.pdf?st=Ct6iM5Eh (accessed on 16 October 2019).
- Statistics Canada. Canadian Health Measures Survey—Cycle 3. 2017. Available online: https://www.statcan.gc.ca/eng/statistical-programs/instrument/5071_Q1_V3#a98 (accessed on 14 October 2019).
- Government of Canada. Page 3: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Fluoride. Part II. Science and Technical Considerations. 2011. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-fluoride/page-3-guidelines-canadian-drinking-water-quality-guideline-technical-document-fluoride.html (accessed on 29 October 2019).
- Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Statistics Canada. Canadian Health Measures Survey (CHMS) Data User Guide—Cycle 3; Statistics Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nermell, B.; Lindberg, A.-L.; Rahman, M.; Berglund, M.; Åke Persson, L.; El Arifeen, S.; Vahter, M. Urinary Arsenic Concentration Adjustment Factors and Malnutrition. Environ. Res. 2008, 106, 212–218. [Google Scholar] [CrossRef]
- Moriguchi, J.; Ezaki, T.; Tsukahara, T.; Fukui, Y.; Ukai, H.; Okamoto, S.; Shimbo, S.; Sakurai, H.; Ikeda, M. Decreases in Urine Specific Gravity and Urinary Creatinine in Elderly Women. Int. Arch. Occup. Environ. Health 2005, 78, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Åkesson, A.; Alfvén, T.; Järup, L.; Vahter, M. Creatinine versus Specific Gravity-Adjusted Urinary Cadmium Concentrations. Biomarkers 2005, 10, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Laisalmi, M.; Soikkeli, A.; Kokki, H.; Markkanen, H.; Yli-Hankala, A.; Rosenberg, P.; Lindgren, L. Fluoride Metabolism in Smokers and Non-Smokers Following Enflurane Anaesthesia. Br. J. Anaesth. 2003, 91, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Miles, J. Tolerance and Variance Inflation Factor. In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; p. stat06593. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Report on the Findings of the Oral Health Component of the Canadian Health Measures Survey 2007–2009. 2010. Available online: http://publications.gc.ca/site/eng/369649/publication.html (accessed on 3 December 2019).
- Helte, E.; Donat Vargas, C.; Kippler, M.; Wolk, A.; Michaëlsson, K.; Åkesson, A. Fluoride in Drinking Water, Diet, and Urine in Relation to Bone Mineral Density and Fracture Incidence in Postmenopausal Women. Environ. Health Perspect. 2021, 129, EHP7404. [Google Scholar] [CrossRef]
- Trautner, K.; Siebert, G. An Experimental Study of Bio-Availability of Fluoride from Dietary Sources in Man. Arch. Oral Biol. 1986, 31, 223–228. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Sharma, D.K.; Damodaran, T. Fluoride in the Environment and Its Metabolism in Humans. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2011; Volume 211, pp. 121–142. [Google Scholar]
- World Health Organization. Guideline for Drinking Water Quality Health Criteria and Other Supporting Information, 2nd ed.; World Health Organization: Geneva, Switzerland, 1997; Volume 2. [Google Scholar]
- Whitford, G.M. The Metabolism and Toxicity of Fluoride, 2nd ed.; Karger: Basel, Switzerland, 1996. [Google Scholar]
- Itai, K.; Onoda, T.; Nohara, M.; Ohsawa, M.; Tanno, K.; Sato, T.; Kuribayashi, T.; Okayama, A. Serum Ionic Fluoride Concentrations Are Related to Renal Function and Menopause Status but Not to Age in a Japanese General Population. Clin. Chim. Acta 2010, 411, 263–266. [Google Scholar] [CrossRef]
- Mostafaei, F.; McNeill, F.E.; Chettle, D.R.; Wainman, B.C.; Pidruczny, A.E.; Prestwich, W.V. Measurements of Fluorine in Contemporary Urban Canadians: A Comparison of the Levels Found in Human Bone Using in Vivo and Ex Vivo Neutron Activation Analysis. Physiol. Meas. 2015, 36, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of Fluoride Concentration and Daily Intake by Human from Tea and Herbal Infusions. Food Chem. Toxicol. 2008, 46, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Shieh, A.; Greendale, G.A.; Cauley, J.A.; Karvonen-Gutierrez, C.; Crandall, C.J.; Karlamangla, A.S. Estradiol and Follicle-Stimulating Hormone as Predictors of Onset of Menopause Transition-Related Bone Loss in Pre- and Perimenopausal Women. J. Bone Min. Res. 2019, 34, 2246–2253. [Google Scholar] [CrossRef]
- Koç, E.; Karademir, B.; Soomro, N.; Uzun, F. The Effects, both separate and interactive, of smoking and tea consumption on urinary fluoride levels. Fluoride 2018, 51, 84–96. [Google Scholar]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Fluoride Contents in Tea and Soil from Tea Plantations and the Release of Fluoride into Tea Liquor during Infusion. Environ. Pollut. 1999, 104, 197–205. [Google Scholar] [CrossRef]
- Krishnankutty, N.; Storgaard Jensen, T.; Kjær, J.; Jørgensen, J.S.; Nielsen, F.; Grandjean, P. Public-Health Risks from Tea Drinking: Fluoride Exposure. Scand. J. Public Health 2021, 1–7. [Google Scholar] [CrossRef]
- Ibiyemi, O.; Zohoori, F.V.; Valentine, R.A.; Maguire, A. Fluoride Intake and Urinary Fluoride Excretion in 4- and 8-Year-Old Children Living in Urban and Rural Areas of Southwest Nigeria. Community Dent. Oral Epidemiol. 2018, 46, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Thornton-Evans, G.; Junger, M.L.; Lin, M.; Wei, L.; Espinoza, L.; Beltran-Aguilar, E. Use of Toothpaste and Toothbrushing Patterns Among Children and Adolescents—United States, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 87–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Hoyos, F.; Barbería, E.; García-Camba, P.; Varela, M. Renal Fluoride Excretion in Children Following Topical Application of Fluoride Varnish. Eur. J. Paediatr. Dent. 2012, 13, 280–284. [Google Scholar]
- Khandare, A.; Rasaputra, K.; Meshram, I.; Rao, S. Effects of smoking, use of aluminium utensils, and tamarind consumption on fluorosis in a fluorotic village of Andhra Pradesh, India. Fluoride 2010, 43, 128–133. [Google Scholar]
- Environmental Protection Agency. Update for Chapter 3 of the Exposure Factors Handbook: Ingestion of Water and Other Select Liquids; Environmental Protection Agency: Washington, DC, USA, 2019; EPA/600/R-18/259F.
- Karak, T.; Bhagat, R.M. Trace Elements in Tea Leaves, Made Tea and Tea Infusion: A Review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- IOM. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Bashash, M.; Thomas, D.; Hu, H.; Angeles Martinez-Mier, E.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef] [PubMed]
- Malin, A.J.; Lesseur, C.; Busgang, S.A.; Curtin, P.; Wright, R.O.; Sanders, A.P. Fluoride Exposure and Kidney and Liver Function among Adolescents in the United States: NHANES, 2013–2016. Environ. Int. 2019, 132, 105012. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.L.; Zhang, Y.; Sun, G.; Bellinger, D.C.; Wang, K.; Yang, X.J.; Li, J.S.; Zheng, Q.; Fu, Y.; Grandjean, P. Association of Lifetime Exposure to Fluoride and Cognitive Functions in Chinese Children: A Pilot Study. Neurotoxicol. Teratol. 2015, 47, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malin, A.J.; Riddell, J.; McCague, H.; Till, C. Fluoride Exposure and Thyroid Function among Adults Living in Canada: Effect Modification by Iodine Status. Environ. Int. 2018, 121, 667–674. [Google Scholar] [CrossRef]
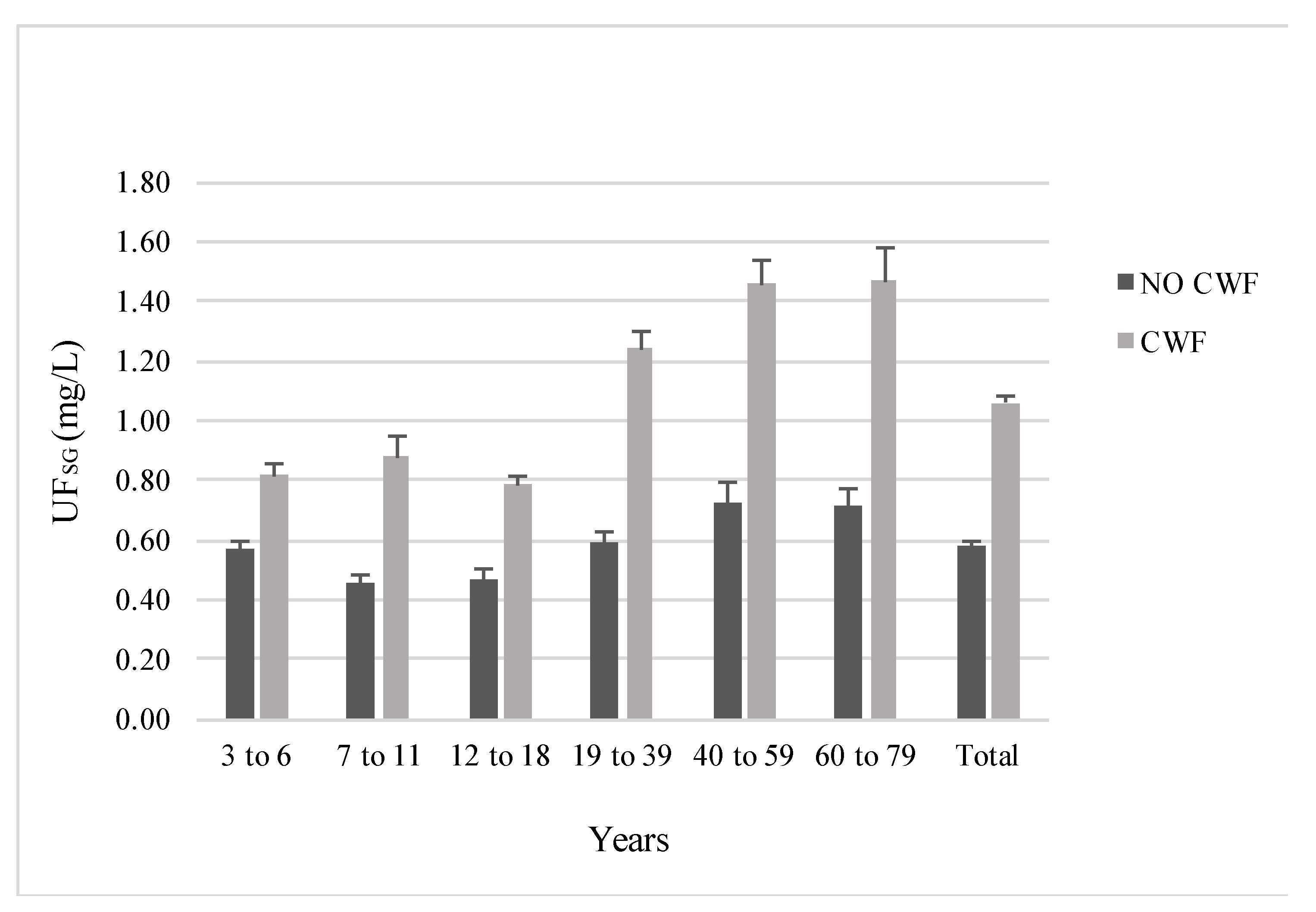
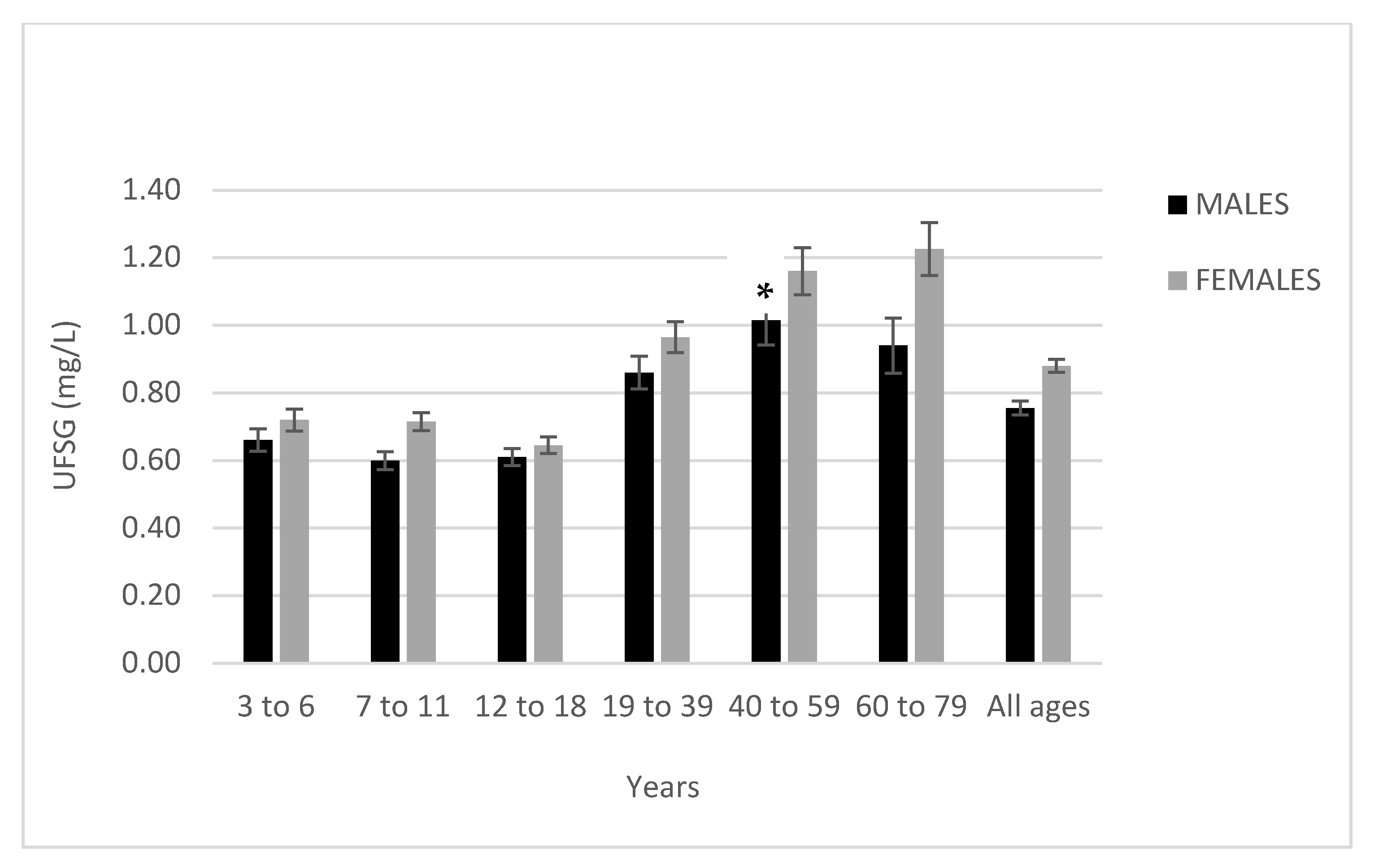
| Age Group and Sex | Fluoridated (N = 860) | Non-Fluoridated (N = 780) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 5th, 95th Percentile | SD | Median | n | Mean | 5th, 95th Percentile | SD | Median | p | |
| Age 3 to 6 | 180 | 0.82 | 0.27, 1.68 | 0.59 | 0.70 | 185 | 0.57 | 0.18, 1.29 | 0.41 | 0.46 | <0.001 |
| Male | 93 | 0.80 | 0.27, 1.58 | 0.57 | 0.70 | 86 | 0.52 | 0.16, 1.16 | 0.32 | 0.46 | |
| Females | 87 | 0.83 | 0.27, 1.71 | 0.61 | 0.72 | 99 | 0.61 | 0.21, 1.43 | 0.47 | 0.46 | |
| Age 7 to 11 | 145 | 0.88 | 0.40, 1.52 | 0.78 | 0.72 | 123 | 0.46 | 0.18, 1.10 | 0.28 | 0.38 | <0.001 |
| Male | 77 | 0.77 | 0.40, 1.27 | 0.32 | 0.72 | 57 | 0.43 | 0.18, 1.20 | 0.27 | 0.38 | |
| Female | 68 | 1.00 | 0.40, 2.09 | 1.07 | 0.75 | 66 | 0.43 | 0.18, 1.01 | 0.25 | 0.38 | |
| Age 12 to 18 | 165 | 0.79 | 0.34, 1.52 | 0.37 | 0.70 | 140 | 0.47 | 0.21, 0.90 | 0.34 | 0.40 | <0.001 |
| Male | 79 | 0.79 | 0.34, 1.50 | 0.35 | 0.72 | 67 | 0.43 | 0.18, 0.87 | 0.27 | 0.38 | |
| Female | 86 | 0.78 | 0.36, 1.60 | 0.38 | 0.67 | 73 | 0.51 | 0.23, 0.93 | 0.44 | 0.57 | |
| Age 19 to 39 | 139 | 1.24 | 0.44, 2.47 | 0.78 | 1.05 | 119 | 0.59 | 0.23, 1.22 | 0.38 | 0.51 | <0.001 |
| Male | 64 | 1.22 | 0.42, 2.47 | 0.78 | 1.05 | 58 | 0.50 | 0.23, 1.08 | 0.28 | 0.44 | |
| Female | 75 | 1.25 | 0.49, 2.66 | 0.77 | 1.05 | 61 | 0.68 | 0.27, 1.22 | 0.44 | 0.57 | |
| Age 40 to 59 | 104 | 1.46 | 0.48, 3.61 | 0.86 | 1.27 | 102 | 0.73 | 0.21, 2.09 | 0.66 | 0.50 | <0.001 |
| Male | 42 | 1.38 | 0.55, 3.42 | 0.84 | 1.16 | 55 | 0.65 | 0.19, 1.71 | 0.62 | 0.49 | |
| Female | 62 | 1.51 | 0.42, 3.61 | 0.88 | 1.34 | 47 | 0.81 | 0.21, 2.28 | 0.70 | 0.59 | |
| Age 60 to 79 | 121 | 1.47 | 0.42, 3.80 | 1.24 | 1.06 | 106 | 0.72 | 0.23, 2.09 | 0.64 | 0.54 | <0.001 |
| Male | 58 | 1.37 | 0.36, 4.37 | 1.39 | 1.00 | 49 | 0.51 | 0.21, 1.03 | 0.33 | 0.44 | |
| Female | 63 | 1.56 | 0.46, 3.80 | 1.09 | 1.14 | 57 | 0.89 | 0.27, 2.66 | 0.78 | 0.65 | |
| Total | 854 | 1.06 | 0.36,2.47 | 0.83 | 0.84 | 775 | 0.58 | 0.19, 1.43 | 0.47 | 0.46 | <0.001 |
| Male | 413 | 1.00 | 0.34,2.09 | 0.78 | 0.80 | 372 | 0.51 | 0.18, 1.08 | 0.37 | 0.43 | |
| Female | 441 | 1.12 | 0.38,2.66 | 0.86 | 0.86 | 403 | 0.64 | 0.21, 1.60 | 0.53 | 0.49 | |
| Age Group | Used a Fluoridated Product Less Than 6 h before Sample Collection | Used a Fluoridated Product 6 or More Hours before Sample Collection | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | SD | Median | n | Mean (95% CI) | SD | Median | ||
| Age 3 to 6 | 94 | 0.81 (0.72, 0.93) | 0.62 | 0.72 | 144 | 0.62 (0.56, 0.67) | 0.39 | 0.51 | <0.001 |
| Age 7 to 11 | 88 | 0.64 (0.58, 0.69) | 0.32 | 0.57 | 129 | 0.68 (0.55, 0.69) | 0.67 | 0.53 | 0.78 |
| Age 12 to 18 | 136 | 0.65 (0.55, 0.64) | 0.34 | 0.58 | 110 | 0.64 (0.54, 0.65) | 0.37 | 0.55 | 0.97 |
| Age 19 to 39 | 139 | 1.01 (0.83, 0.99) | 0.70 | 0.84 | 86 | 0.83 (0.66, 0.84) | 0.58 | 0.67 | 0.01 |
| Age 40 to 59 | 93 | 1.27 (0.96, 1.18) | 0.86 | 1.10 | 61 | 0.93 (0.73, 1.00) | 0.85 | 0.67 | 0.02 |
| Age 60 to 79 | 103 | 1.29 (1.02, 1.40) | 1.29 | 0.91 | 53 | 1.07 (0.81, 1.14) | 0.85 | 0.84 | 0.06 |
| Total | 653 | 0.94 | 0.79 | 0.72 | 583 | 0.74 | 0.61 | 0.59 | <0.001 |
| Predictor | B | 95% CI | p |
|---|---|---|---|
| Water fluoride (mg/L) | 0.48 | 0.25, 0.71 | <0.01 |
| CWF status (ref: non-fluoridated area) | 0.39 | 0.24, 0.53 | <0.01 |
| Age | 0.03 | 0.01, 0.04 | <0.01 |
| Age2 | −0.02 | −0.03, −0.01 | <0.01 |
| Sex (ref: male) | 0.12 | 0.03, 0.20 | 0.01 |
| BMI | −0.02 | −0.02, −0.01 | <0.01 |
| Tea consumption (ref: none) | 0.13 | 0.03, 0.22 | 0.01 |
| Smoking allowed in the home (ref: yes) | −0.25 | −0.41, −0.09 | <0.01 |
| Ethnicity (ref: white) | −0.07 | −0.17, 0.04 | 0.20 |
| Household education (ref: less than bachelor’s degree) | 0.05 | −0.05, 0.14 | 0.35 |
| Income (per $100,000 CND) | −0.04 | −0.10, 0.02 | 0.17 |
| Primary source of drinking water (ref: tap) | −0.01 | −0.18, 0.16 | 0.87 |
| Last fluoride treatment at dentist (ref: < 3 months ago) | −0.03 | −0.15, 0.08 | 0.58 |
| Time since use of a fluoride-containing dental product (ref: < 6 h ago) | −0.09 | −0.18, 0.00 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riddell, J.K.; Malin, A.J.; McCague, H.; Flora, D.B.; Till, C. Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation. Int. J. Environ. Res. Public Health 2021, 18, 6203. https://doi.org/10.3390/ijerph18126203
Riddell JK, Malin AJ, McCague H, Flora DB, Till C. Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation. International Journal of Environmental Research and Public Health. 2021; 18(12):6203. https://doi.org/10.3390/ijerph18126203
Chicago/Turabian StyleRiddell, Julia K., Ashley J. Malin, Hugh McCague, David B. Flora, and Christine Till. 2021. "Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation" International Journal of Environmental Research and Public Health 18, no. 12: 6203. https://doi.org/10.3390/ijerph18126203






