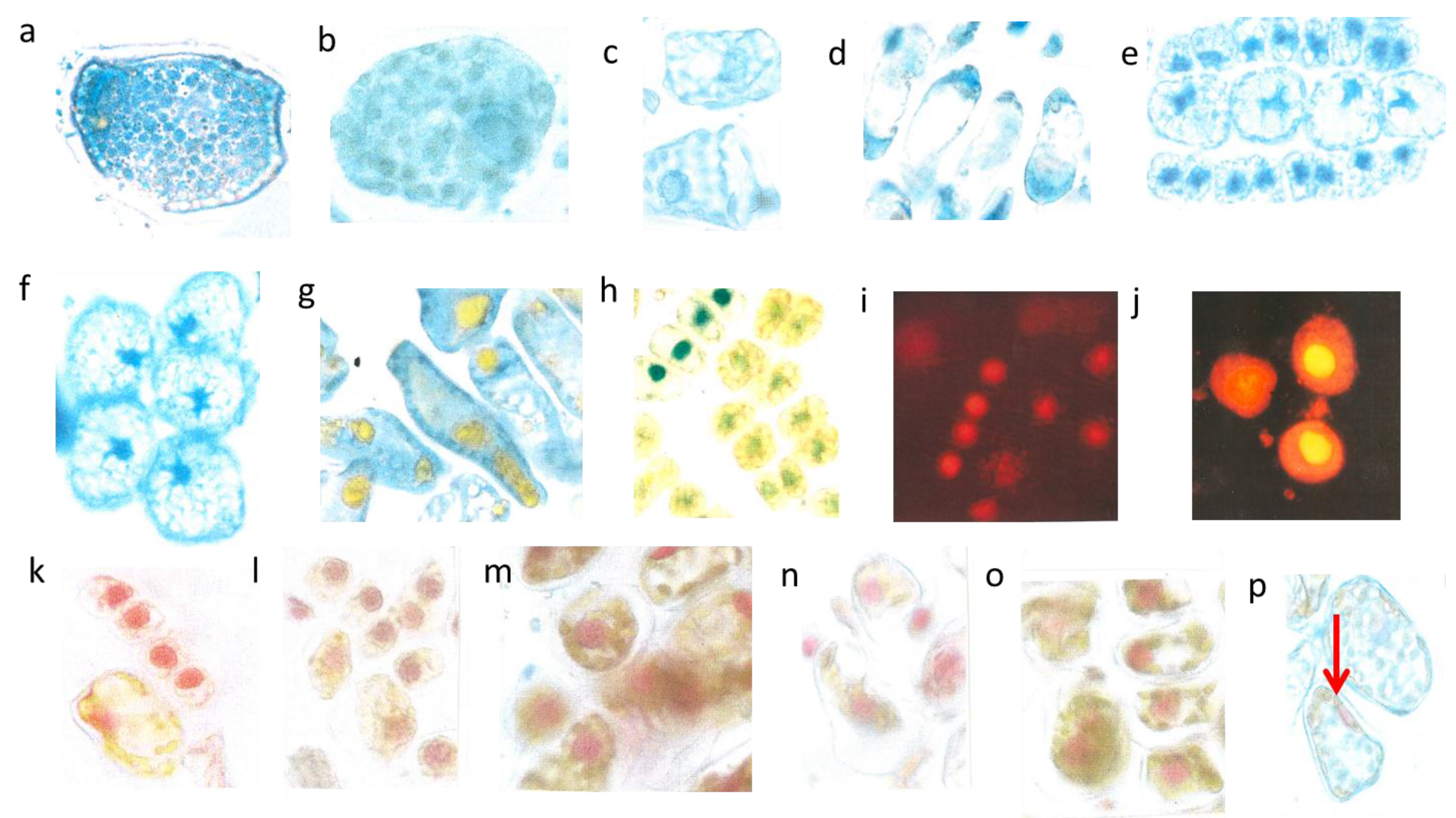
3.1. Abundant Vacuolar Flavanols in the Protective Needle Tissues
The flat uniseriate and tangential elongated epidermis cells were rich in flavanols. This blue staining flavonoid group appeared already when the first tip of the needle arose from the sprouting bud (
Figure 2a–c). Along with cell stretching, the vacuoles were filled more and more with flavanols. Thus, after about three weeks the whole surface of the needle, up to 10 mm in length, was covered with dark blue flavanols. All study trees followed this pattern, irrespective of their size and exposure to light or shady microenvironment.
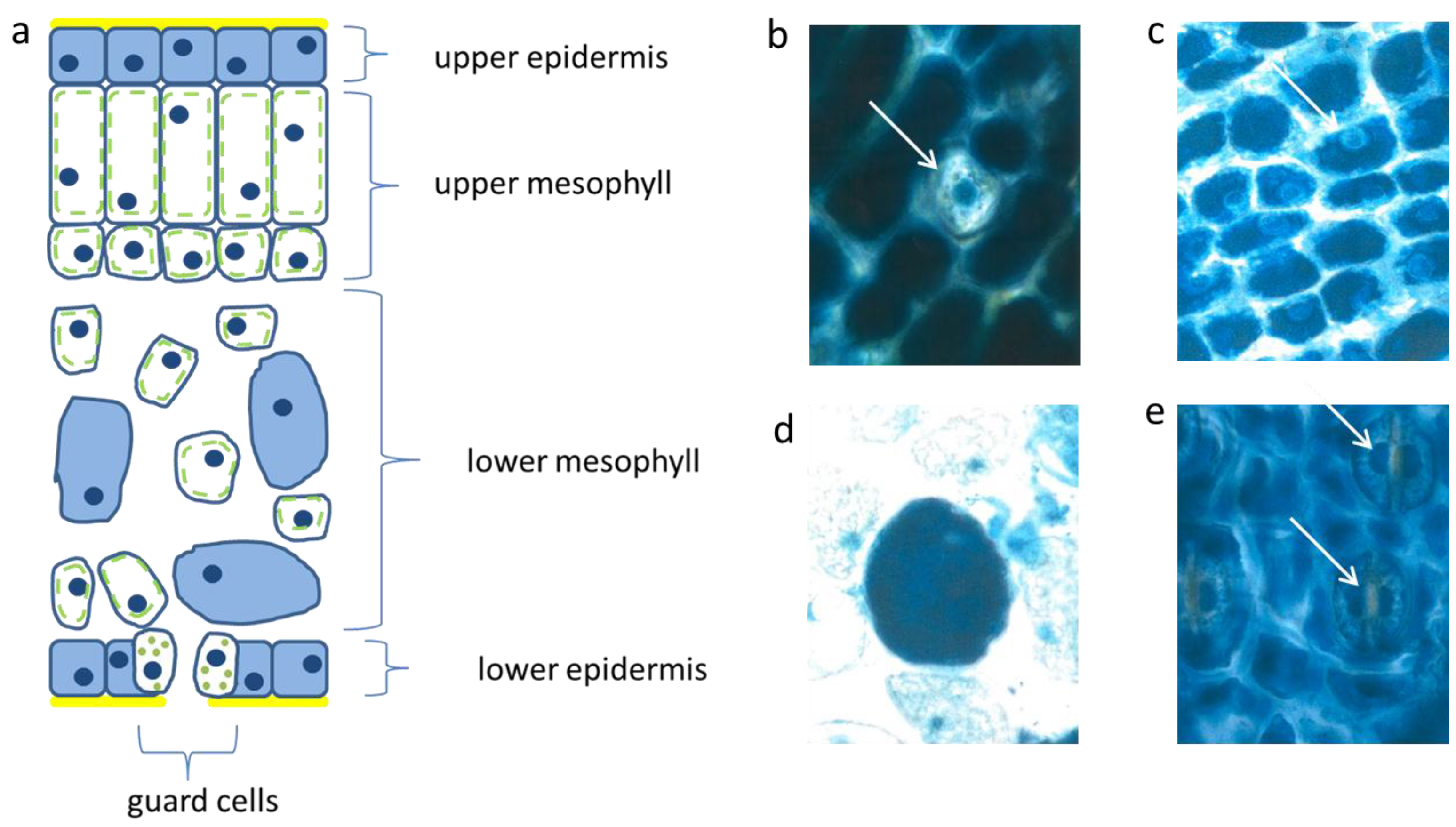
Figure 2.
Schematic picture of a vertical section of a needle (a); upper epidermis (b,c); large mesophyll cell (d); low epidermis (e). Nucl. b 8 µm, c 8 µm, d 7 µm, e 9 µm elongated (white arrows).
Figure 2.
Schematic picture of a vertical section of a needle (a); upper epidermis (b,c); large mesophyll cell (d); low epidermis (e). Nucl. b 8 µm, c 8 µm, d 7 µm, e 9 µm elongated (white arrows).
The palisade layer began its growth with more or less isodiametric cells which were loaded, like the epidermis, with flavanols, but lost them along with elongation. Typically, this cell type was then fairly narrow and elongated in the vertical dimension up to 30 µm. When fully developed, the cells showed an extreme synchrony in shape and size (
Figure 2a). Beneath the upper layer, there is a second palisade-like layer the cells of which show a quadratic cross section.
In the spongy parenchyma the chloroplasts developed rather early together with vacuolar flavanols. So, the beginning of photosynthesis within the needles started readily in the spongy mesophyll. This tissue was richly vacuolarized and in the rounded to ellipsoidal cells the flavanols were more prevalent than the chloroplasts (
Figure 2d). In this respect, there was little variation among the different species and cultivars, except var.
aurea and var.
compacta (
Table 1). As a rule for all species, the intercellular air-space allowing a high gaseous O
2 diffusion between the spongy mesophyll cells was less extended in small, younger needles but became fairly large with increasing needle size and more green chloroplasts.
Table 1.
Percent of cells filled with vacuolar flavanols as collected in mid-summer 2013 from the epidermis and the mesophyll layers. One hundred cross sections per species/varieties were investigated. (SE values of spongy mesophyll cells were calculated). Only in two species the values (b) are different from a (p < 0.05).
Table 1.
Percent of cells filled with vacuolar flavanols as collected in mid-summer 2013 from the epidermis and the mesophyll layers. One hundred cross sections per species/varieties were investigated. (SE values of spongy mesophyll cells were calculated). Only in two species the values (b) are different from a (p < 0.05).
| Tissues | Upper Epidermis | Palisade | Spongy | Lower Epidermis |
|---|
| % | % | % | % |
| Tsuga can. | 100 | 0 | 65 a | 100 |
| Tax. bacc. | 100 | 0 | 68 a | 100 |
| Var. nana | 100 | 0 | 57 a | 100 |
| Var. repens | 100 | 0 | 60 a | 100 |
| Var. aurea | 100 | 0 | 43 b | 100 |
| Var. comp. | 100 | 0 | 81 b | 100 |
The epidermis cells of the lower needle surface were broadly similar in shape and size to those from the upper epidermis (
Figure 2e). However, the overall flavanol density of the lower epidermis was in all species somewhat less intense and more variable. This might causally be linked with the reduced incident light. Especially towards autumn and winter the flavanols were generally somewhat reduced in the lower epidermis on the whole.
Interestingly, also the guard cells contained few small vacuolar flavanol deposits about 1 µm in size and in addition even few chloroplasts. The nuclei of this cell type were generally more variable in the blue colored flavanol expression (
Figure 2e).
3.2. Hot Spells in Summer 2013 and Adaption of the Cells
The two small cells (
Figure 3a, left) correspond to initial stages of the two palisade layers (
Figure 3a, left) which later after stretching develop chloroplasts (
Figure 3a, right). Why were the young palisade cells at first so blue? Each initial cell is very slender in the biophysical structures of the cell wall. Likewise, the fast elongating young epidermis cell, covering the palisade cells, carries the same implication. So, the cytoplasm of these palisade cell needed the flavanols to alleviate the dangerous UV-radiation. The change from the quadratic blue palisade cell type goes hand in hand with stretching and chloroplast formation. This process was finished after about three weeks when the needles were already 10 to 12 mm long. Then, especially the outer cell walls of the epidermis, now about 6–8 µm thick, were effective UV radiation barriers which are physically equipped with close-meshed transverse microfibrils and chemically with flavonoids.
The expanding palisade cells, as shown by a layer of six whitish lineage cells have nearly lost the flavanols, except those of the nuclei and some tiny residues along the cell walls (
Figure 3b). Furthermore, some small cells of the spongy mesophyll remained already dark blue, like the four enlarged epidermal cells (
Figure 3b).
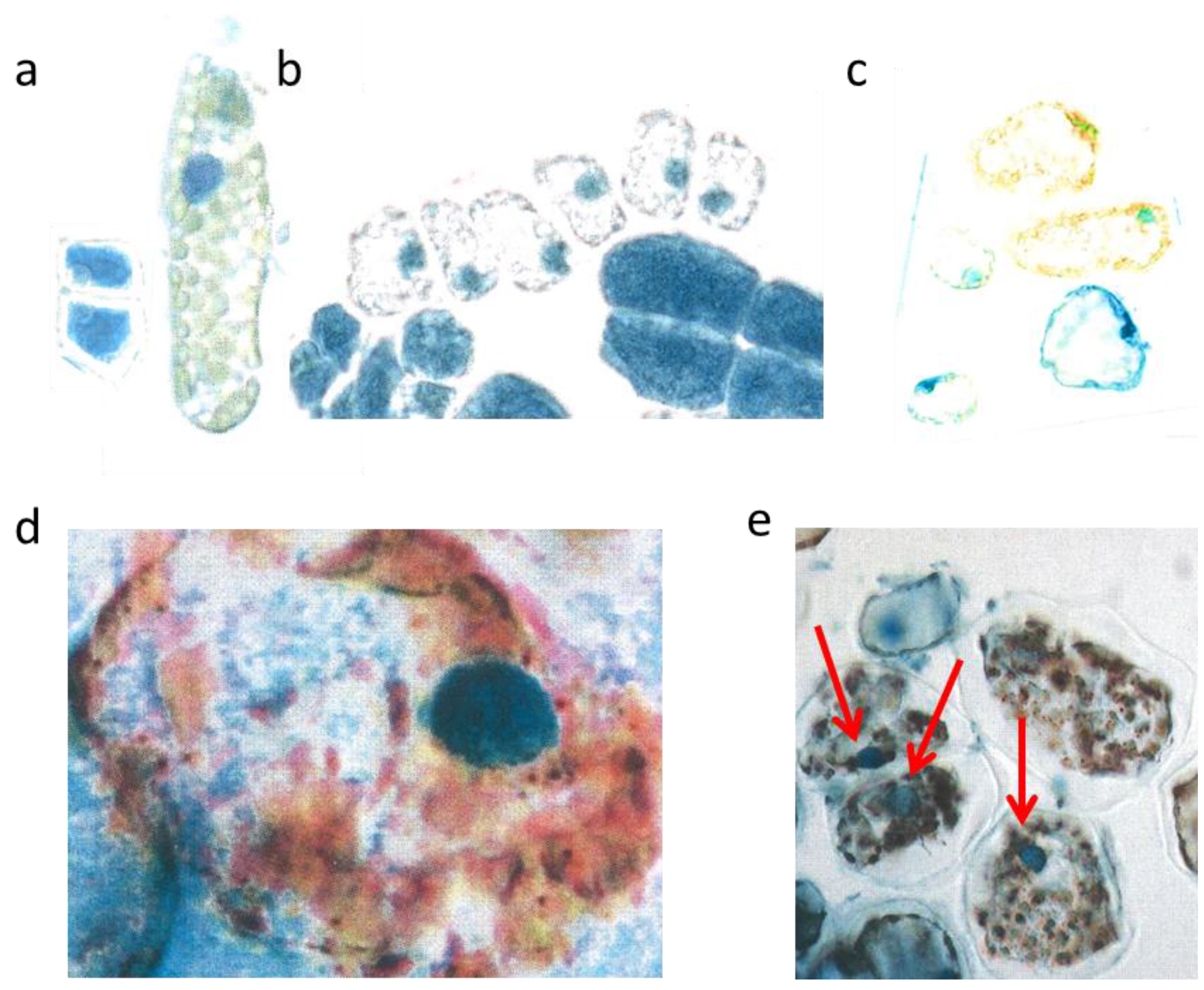
Figure 3.
Mesophyll cells before and after burning. Two very young palisade cells were 15 µm broad (a, left); differentiated mesophyll cell (b, right) nucl. 7 µm; young mesophyll (b nucl. 6 µm); burned cytoplasm (d, nucl. 8 µm); burned chloroplasts (e nucl. 7 µm, red arrows).
Figure 3.
Mesophyll cells before and after burning. Two very young palisade cells were 15 µm broad (a, left); differentiated mesophyll cell (b, right) nucl. 7 µm; young mesophyll (b nucl. 6 µm); burned cytoplasm (d, nucl. 8 µm); burned chloroplasts (e nucl. 7 µm, red arrows).
However, by late May the low water supply 2013 caused first signs of a down-regulation of cellular vitality. Some cells showed an increased yellowing of the plasmalemma with remnants of blue nuclei, and other cells nearby indicated at least a blue hint of too small nuclei (
Figure 3c).
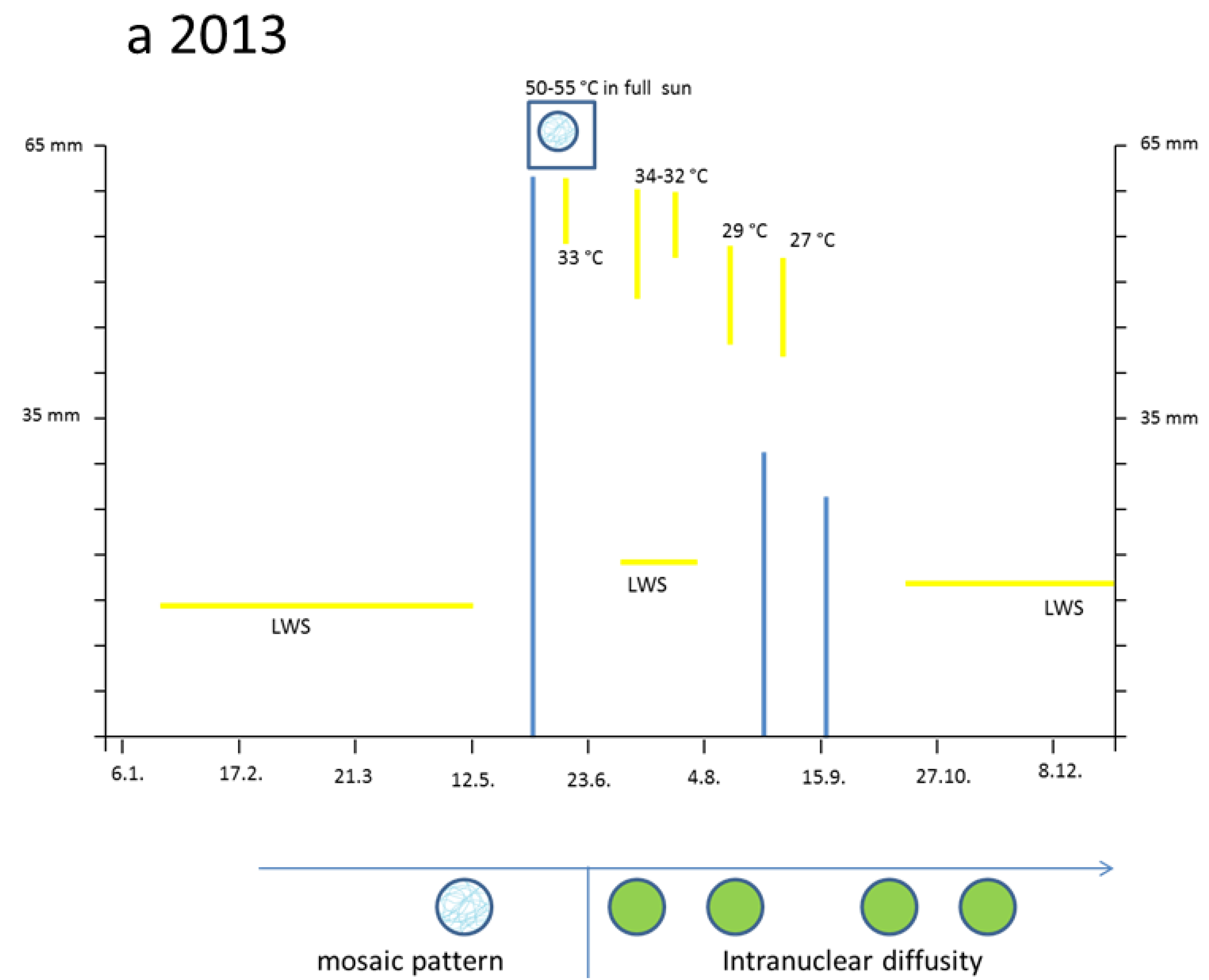
The heat damage in 2013 was highest by 18 June in the free standing shrub of Tax. bacc. At the sun-exposed southern side of the canopy the temperature reached 50 °C from 1:00 p.m. to 5:00 p.m. For about 30 min up to 55 °C was measured. In the shadow, the values increased up to 30 and 32 °C.
Viewed with the naked eye, the needles remained during the following weeks with a green habit. However, by microscopy it became apparent that, in some needles of the very sun-exposed twigs distinct cells from the upper mesophyll layers, there was a brown sunburned cytoplasm (
Figure 3d; the blue shreds in this Figure are from the epidermis). Probably, only distinct small sectors of the flat
Taxus needles were exposed during mid-afternoon for a longer time to a maximal incident solar angle. However spectacularly, the nucleus located amidst the browned cytoplasm of the palisade cell stained the typical normal blue for flavanols pointing out that no damage had occurred (
Figure 3d). This finding provides strong support that flavanols fulfil a crucial role as protective agents against heat stress in
Taxus nuclei.
Also, in the upper epidermis all nuclei showed the blue protective casing. Most of these nuclei were found to be located below blue vacuole of the epidermis. So, the nuclei profit from a double security system, namely their own flavanols and those of the superimposed vacuole. The vertical extension of the epidermal vacuole overlaying the nucleus measured between 20–27 µm which is three to four times that of the nucleus with 7 µm in diameter. If during the staining procedures an epidermal vacuole accidentally was broken out, the blue nucleus within the small rim of cytoplasm became visible (
Figure 2b).
Towards October 2013 roughly about 15% of the
lower mesophyll cells located at the southern periphery of the free standing brush revealed an increasing disintegration and browning of chloroplasts (
Figure 3e). Nevertheless, stable blue and compact nuclei could still be detected within the oxidized plastids.
By and large, the entire sun-exposed Taxus brush looked green as ever over the rest of the year. However, in the following spring, from early March to mid-April 2014, up to 20% of the shoots grown in 2013 and from the southern canopy side turned completely dark brown. If examined microscopically, all cells of the needles were completely oxidized. Obviously, a slowly progressing activity of destructive oxidative systems took place during the wintry rest period.
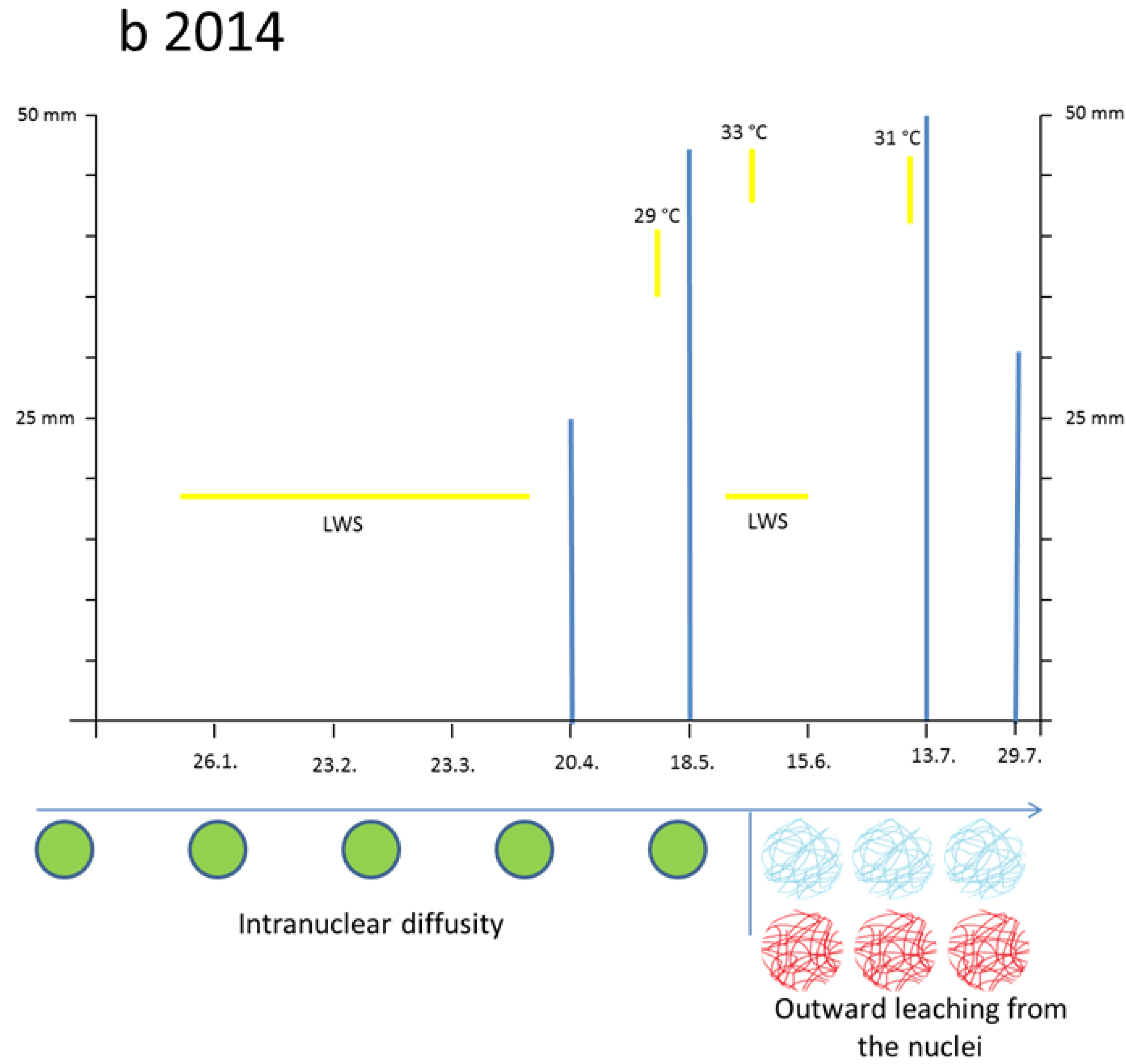
3.3. Leaching Flavanols Outwardly from the Nuclei after the Flood in Late May 2014
The first months of 2014 (January, February, and March) were droughty. During winter time the nuclei of conifers were usually hardly blue, if at all. By late April the nuclei of the newly sprouting needles attained slowly a diffuse bluish-green chromatin. Similarly, also the entire cytoplasm turned greenish as a mixture of yellow and blue. Such a curious expression of flavonoids in very young and sprouting cells was, to our 15 years of experience, quite unusual. In reality, these nuclei should reveal a blue mosaic pattern.
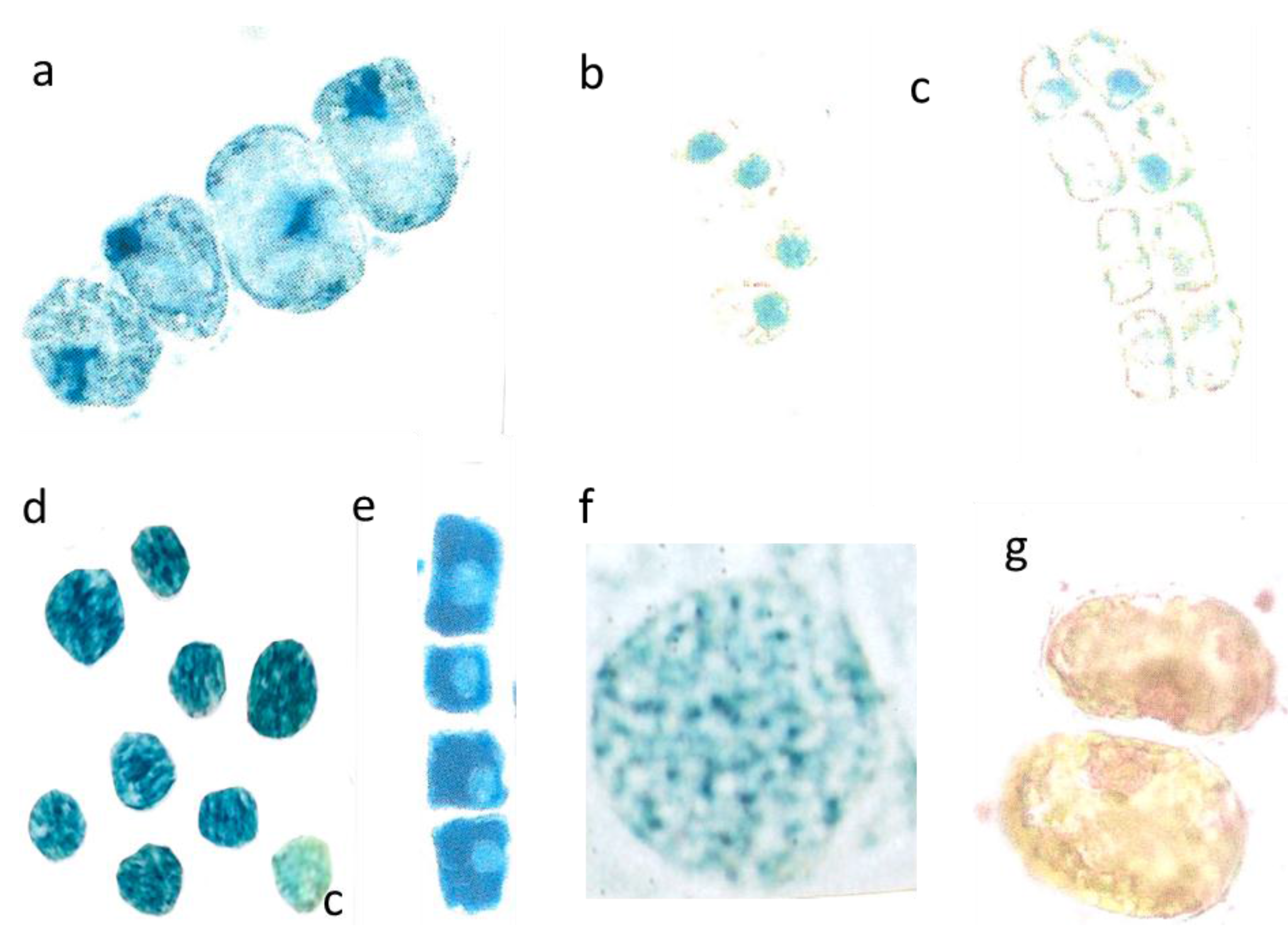
Two or three days after the great flood on 26th May 2014 in a number of rather large spongy mesophyll cells the chloroplasts attained a visible blue indicative of flavanols. Again, this is an extreme and curious feature. An example of
var.
repens showed clear cut images of blue stained chloroplasts (
Figure 4a). A further example showed
var.
nana (
Figure 4b) with completely diffused blue staining chloroplasts. Nuclei were hardly seen. Such an unusual reaction of chloroplasts might be linked with higher flavanol synthesis during the first days of rehydration. (Stimulation of flavanol synthesis by cytokinin localized in the roots is shown in
Figure 5d).
In
Tax.
bacc. many nuclei became water-soaked and overly turgid so that the nuclear diameter increased by about 2 µm (
Table 2).Then, the flavanols began to leach out from the nuclei towards the margins of the cells. This phenomenon was clearly valid for all three trees of
Tax.
bacc. (
Figure 4c,d). It is important to note that all study trees showed leaching of flavanols in many cells all over the entire canopies. Significant differences between light-exposed and shaded twigs or dwarf and vigorous trees were not evident. In var.
repens (
Figure 4e) the nuclear flavanols leached outwardly between the starch grains of the cytoplasm. The shapes of the nuclei were deformed in the four large lineage cells. As a rule for all study trees, the more advanced the development of a cell the more pronounced was leaching. The leaching effect as shown in var.
compacta produced star-shaped nuclei by flux of flavanols between the adjacent starch grains (
Figure 4f), but most of the flavanols were displaced towards the cell walls. Some cells of var.
aurea showed an overall spreading of flavanols whereas the yellow phenols were strictly confined to vacuoles (
Figure 4g). Var.
aurea had a pronounced tendency to synthesize the yellow flavonoid molecules. Overall, the epidermal cell walls and to some extent the cytoplasm of the terminal needle sectors displayed a fairly yellow color (
Figure 4h). As ever, the pale green, rudimentary nuclei indicate a mixture of yellow and blue. By contrast, the recently formed lineage with the four young cells still fastened to each other was equipped with fairly compact, rounded, and prominent dark blue nuclei. As already mentioned above, such a compactness of recently formed young nuclei is readily unusual for a young lineage. Really, the nuclei should be in a fine-granular mosaic state. Obviously, the presence of too intense yellow flavonoids in the cytoplasm is not compatible with blue nuclei.
Figure 4.
Outward leaching of flavanols and DNA from cells and nuclei. Var repens with flavanols covering the chloroplasts; longer diameter 42 µm (a); Var. nana with very diffused flavanol leaching of the choroplasts, longer diameter 42 µm (b). Mesophyll cells of T. bacc. (nucl.7 µm) (c,d); var. repens (rounded nucl 7 µm (e); var. compacta (nucl. about 7 µm (f); totally disappeared nuclei of var. aurea (g). Lineage cell with four compacted nuclei (nucl. 7 µm) (h). T. bacc. indicates the DNA of 7 non-leaching nuclei (7 µm) and three leaching ones 9 to 10 µm (i). Two nuclei have lost most of the DNA (yellow nucleus 11 µm) (j). Var. aurea with compact (7µm) non-leaching and a diffuse nucleus of a yellow mesophyll cell (k). Same symptoms in var. repens, compact and nana (l,m,n). The compacted nuclei measure 7 µm. The species Tsuga can. is equal in the patterning of leaching. Non leaching nuclei measure 7 µm (o,p).
Figure 4.
Outward leaching of flavanols and DNA from cells and nuclei. Var repens with flavanols covering the chloroplasts; longer diameter 42 µm (a); Var. nana with very diffused flavanol leaching of the choroplasts, longer diameter 42 µm (b). Mesophyll cells of T. bacc. (nucl.7 µm) (c,d); var. repens (rounded nucl 7 µm (e); var. compacta (nucl. about 7 µm (f); totally disappeared nuclei of var. aurea (g). Lineage cell with four compacted nuclei (nucl. 7 µm) (h). T. bacc. indicates the DNA of 7 non-leaching nuclei (7 µm) and three leaching ones 9 to 10 µm (i). Two nuclei have lost most of the DNA (yellow nucleus 11 µm) (j). Var. aurea with compact (7µm) non-leaching and a diffuse nucleus of a yellow mesophyll cell (k). Same symptoms in var. repens, compact and nana (l,m,n). The compacted nuclei measure 7 µm. The species Tsuga can. is equal in the patterning of leaching. Non leaching nuclei measure 7 µm (o,p).
In foregoing years (2001–2012), the size of conifer nuclei, if grown under normal climate conditions, proved to be very constant (
Table 2). However, from day 1 to day 7 after the flood 2014, many nuclei of all species suffered from increased water uptake resulting in a larger diameter up to 10 or 12 µm. (
Table 2). In
Tax.
bacc., about 25% of the nuclei from the summer sprouts (S-flush 2014) emerging in late July reached only 5 µm in diameter (not shown in
Table 2).
Figure 5.
Detopping T. bacc. in July. Irregular size of cells and nuclei within a lineage (nucleus about 7 µm in diameter) (a); Nuclei with only 5 µm in diameter (b); Total disappearance of nuclei in five cells (nucleus 6 µm in diameter) (c); Application of cytokinin yields extremely dark blue nuclei (c = control is pale blue) (d); Dark blue lineage with pale nuclei (nucleus. 5 µm in diameter) (e); Normal shaped (8 µm in diameter) and activated nucleus sampled in 2012 (f); Pale reddish, diffuse DNA in chaotic, partially yellow cytoplasm (g).
Figure 5.
Detopping T. bacc. in July. Irregular size of cells and nuclei within a lineage (nucleus about 7 µm in diameter) (a); Nuclei with only 5 µm in diameter (b); Total disappearance of nuclei in five cells (nucleus 6 µm in diameter) (c); Application of cytokinin yields extremely dark blue nuclei (c = control is pale blue) (d); Dark blue lineage with pale nuclei (nucleus. 5 µm in diameter) (e); Normal shaped (8 µm in diameter) and activated nucleus sampled in 2012 (f); Pale reddish, diffuse DNA in chaotic, partially yellow cytoplasm (g).
Table 2.
Range of nuclear size (µm in diameter) from the needles sampled under normal growth conditions during previous years 2001–2012. Further sampling was done after the flood in July 2014. Different letters indicate significant differences between normal and flood; (t-test, p ≤ 0.05). Number of nuclei larger than 8 µm in diameter after the flood are given in %.
Table 2.
Range of nuclear size (µm in diameter) from the needles sampled under normal growth conditions during previous years 2001–2012. Further sampling was done after the flood in July 2014. Different letters indicate significant differences between normal and flood; (t-test, p ≤ 0.05). Number of nuclei larger than 8 µm in diameter after the flood are given in %.
| T. bacc. | Var. aurea | Var. nana | Var. rep. | Var. comp. | Tsuga |
|---|
| Normal | 7–8 a | 7–8 a | 7–8 a | 7–8 a | 6–7 a | 7–8 a |
| Flood | 7–12 b | 7–10 a | 7–9 a | 7–9 a | 6–9 a | 7–12 a |
| >8 µm (%) | 42 | 12 | 17 | 21 | 9 | 15 |
3.4. Outward Leaching of DNA from the Nuclei after the Flood in Late May 2014
In all trees of the present study, the nuclear DNA from many mesophyll cells diffused away. The needles were grown in 2013 and 2014. A nuclear size surpassing 8 µm in diameter was a sensitive index of higher leaching susceptibility. Concrete data were shown for
Tax.
bacc. and
Tsuga (
Table 3).
Table 3.
Percent of cells with still well developed, non-leaching nuclei after the flooding stress as recorded in August-September 2014. Each average value for flavanols and DNA is the mean of 40 observed needles (the four cultivars of Taxus varied approximately similar to Tax. bacc). The difference between epidermal and mesophyll layers is indicated by different letters within the same column (t test, p ≤ 0.05).
Table 3.
Percent of cells with still well developed, non-leaching nuclei after the flooding stress as recorded in August-September 2014. Each average value for flavanols and DNA is the mean of 40 observed needles (the four cultivars of Taxus varied approximately similar to Tax. bacc). The difference between epidermal and mesophyll layers is indicated by different letters within the same column (t test, p ≤ 0.05).
| Compound | Flavanol | DNA |
|---|
| Tax. bacc. | Tsuga | Tax. bacc. | Tsuga |
|---|
| Upper epidermis | 100 a | 100 a | 100 a | 100 a |
| Upper mesophyll | 85 b | 86 b | 84 b | 82 b |
| Lower mesophyll | 83 b | 88 b | 83 b | 85 b |
| Lower epidermis | 100 a | 100 a | 100 a | 100 a |
To begin with
Tax.
bacc., only one or two days after the loss of flavanols also DNA was found to leach out from the nuclei. Young four-celled lineages showed still intact nuclei and revealed a bright red fluorescence (
Figure 4i). The diameter of the four intact nuclei was 7 µm each. However, three somewhat older single cells showed diffuse pale red nuclei about 10 µm in diameter (
Figure 4i). Also in
Tax.
bacc., the outward leaching of DNA from the nuclei into the cytoplasm was more precisely demonstrated by the red and yellowish flavonoid fluorescence (
Figure 4j).The two more yellowish nuclei had already lost most of the DNA.
In var.
aurea (
Figure 4k), the four obviously intact lineage nuclei stained a correct and clear-cut rosy red for DNAs, but the adjacent single and enlarged cell with a yellowish cytoplasm yielded a diffuse pale reddish nucleus. In the following example from var.
repens, the red tint of nuclear DNA appeared to be somewhat intermixed with a pale yellow and three nuclei were already diffused (
Figure 4l). In the needles of var.
comp. (
Figure 4m) cell clusters were found with a mixture of reddish and brownish colors. Finally, var.
nana showed large differences from cell to cell regarding diffusing DNA (
Figure 4n).
Leaching symptoms of DNA in enlarged cells with a yellow cytoplasm were also observed in
Tsuga, in contrast to the four young intact lineage cells located nearby (
Figure 4o). Sometimes the chloroplasts of
Tsuga turned to blue colors of flavanols (
Figure 4p). Suggestively, they were leached out from the nuclei. In one of the two cells a rather pale reddish DNA was pressed towards the cell wall. In the second cell there was no more any reddish tint of DNA.
To sum up, in all study trees about 15% of the mesophyll cells were affected by leaching in contrast to the upper and lower epidermis. This is exemplarily shown for
Tax.
bacc. and
Tsuga (
Table 3).
3.5. Breakdown of Cell Cycling at the Start of the Summer Flush 2014
Detopping of Tax. bacc. in July is a common practice to stimulate the branchiness and density of the bushes growing in a garden. Then, the newly emerging summer flushes normally reached about 5 to 10 cm in length. However, detopping in July 2014 resulted in disturbed mitosis even during the first cell divisions. The young coppices started off slowly and the mitotic cells showed a series of structural failures. Finally, the shoots tapered off stunting at 4–6 mm in length. The cells, if too small and lacking DNA synthesis in S-phase did not go on to divide after about five days.
The four newly formed lineage cells from
Tax.
bacc. (
Figure 5a) were not correctly synchronized in size and shape. An imbalance of mutual signalling is evident. Normally, conifer nuclei are strictly spheroid. However, the mal-shaped and diffuse nuclei, instead of being located in the central cell position were attached to the cell walls as a sign of silencing. Further division is then not possible. The many diffuse blue patchy flavanols all over the four cells should be enclosed in well-defined vacuoles. There is a severe lack of internal cell organization.
In a further example, the nuclei of four very small lineage cells were still spheroid, but only 5 µm in diameter instead of 7 µm and embedded in a diffuse yellow cytoplasm (
Figure 5b). In two closely parallel located lineages (
Figure 5c) with four cells each, five or six of the eight nuclei failed to produce the obligatory blue staining flavanols. In one case there is only a residual blue of the nucleus. Such a drastic stop in developing nuclear flavanols of a cell lineage was never observed in our previous long-term investigations since 2000.
When cytokinin (0.8 mM in water) was added to such needles with very poorly staining nuclei, then the nuclear flavanols were densely colored. The non-treated control nucleus (co) is only pale blue
(Figure 5d). In a further four-celled lineage, the addition of cytokinin resulted overall in a dark blue cytoplasm but in rather pale blue nuclei as a very curious response (
Figure 5e). To give an example of a perfect nucleus with its granulated structures of euchromatin and heterochromatin, it is necessary to pick up an active nucleus of previous investigations in 2012 (
Figure 5f). Also nuclear DNA molecules of the summer sprouts showed all over the cell as a diffuse red staining mixed with yellow leaching flavonoids (
Figure 5g).
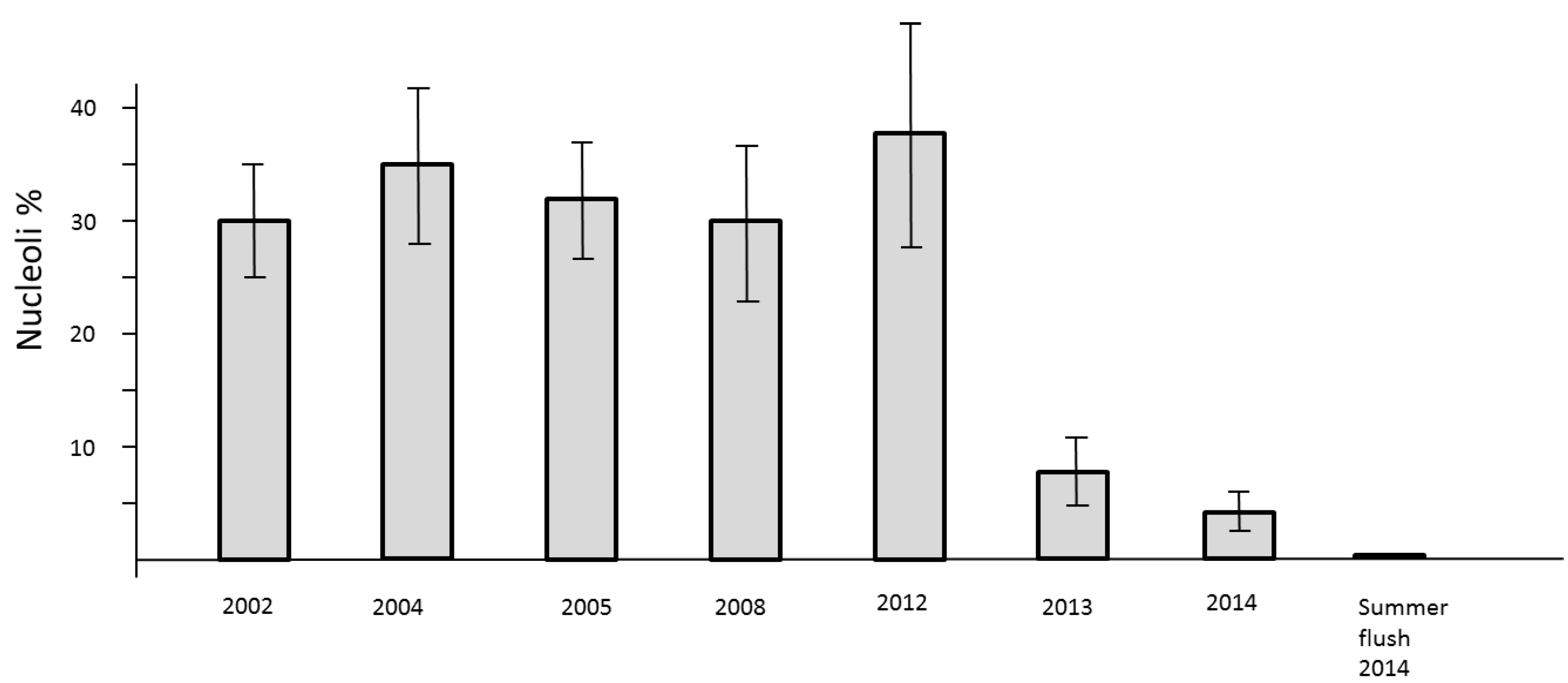
The loss of mitotic activity is well known to be linked with a down-regulation of transcription. In the nuclei such a process is structurally clearly evidenced by the drastic reduction of clearly visible nucleoli. Usually, the nuclei of all study trees are about 2 µm in diameter. They may be less than 1 µm in size and therefore difficult to detect. Consequently, a low level of nucleolar activity und thus decline of RNA synthesis slows down the processes of cell cycling but the formation of new cells is not stopped. This phenomenon was observed in springtime 2013 and 2014. A definite stop of cell division was recognized during the summer-flush 2014 (
Figure 6).
Figure 6.
Number of active nucleoli per nuclei in mitotic cell clusters of needles in previous years 2002, 2004, 2005, 2008, and 2012 as compared with 2013–2014.
Figure 6.
Number of active nucleoli per nuclei in mitotic cell clusters of needles in previous years 2002, 2004, 2005, 2008, and 2012 as compared with 2013–2014.