The Young Stages of the Cannonball Jellyfish (Stomolophus sp. 2) from the Central Gulf of California (Mexico)
Abstract
:1. Introduction
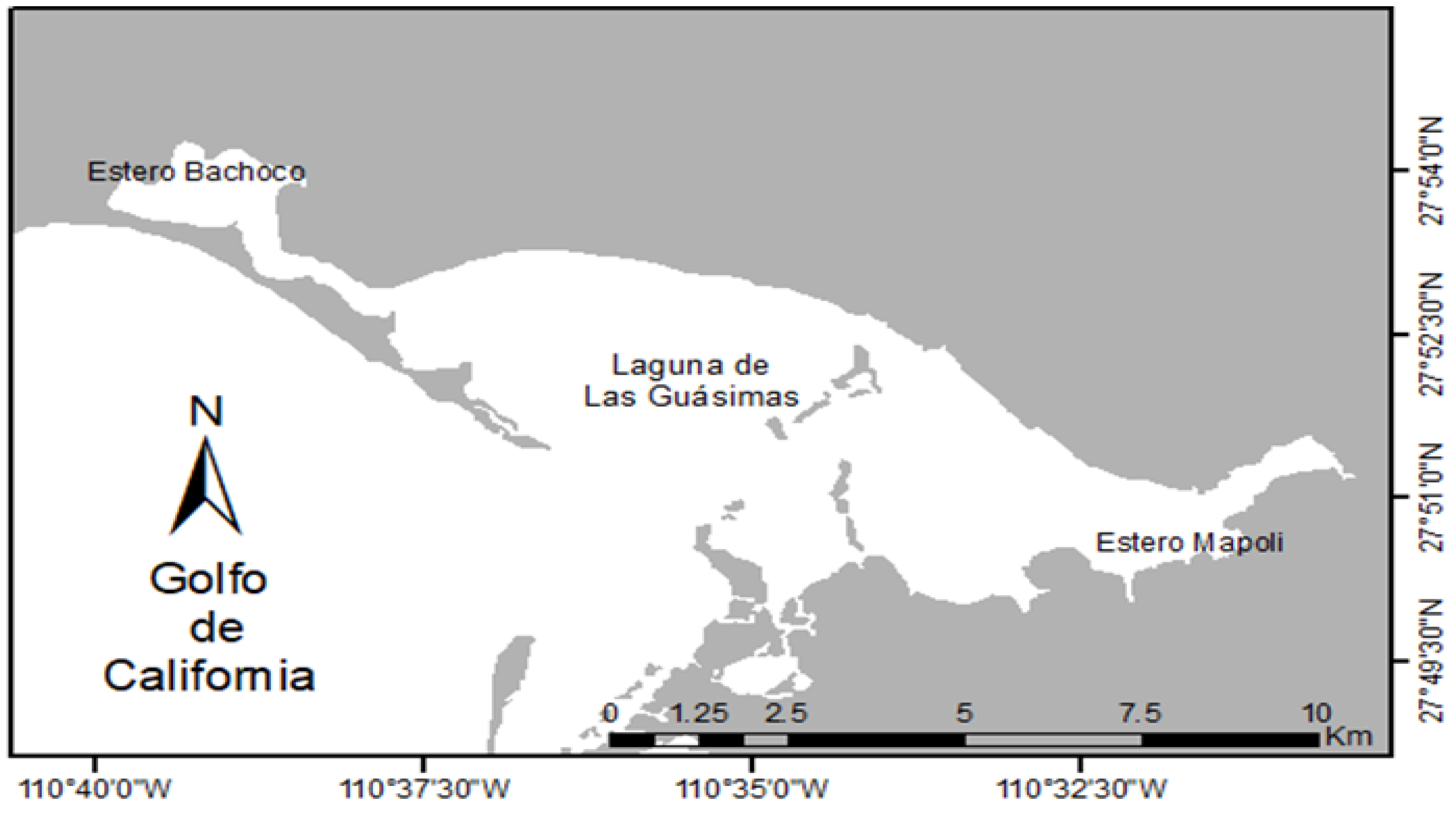
2. Materials and Methods
3. Results
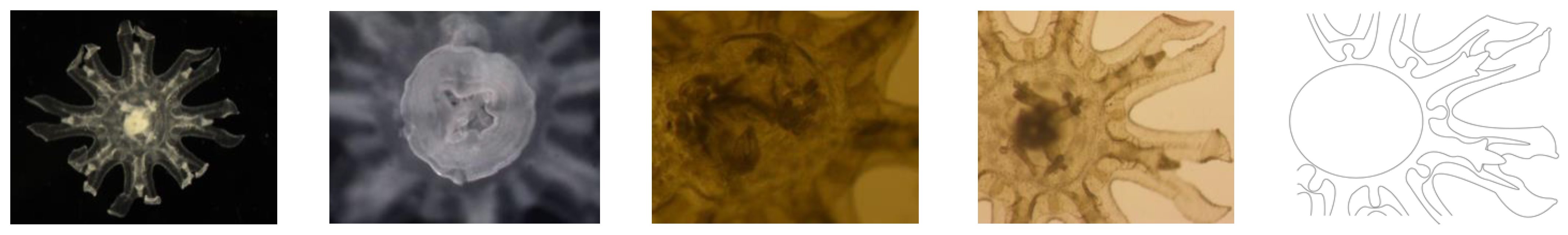
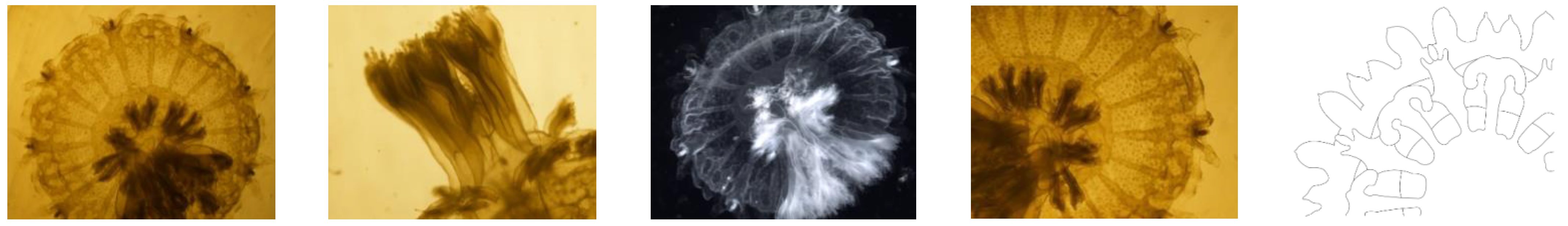
3.1. Features of the Ephyrae of Stomolophus sp. 2
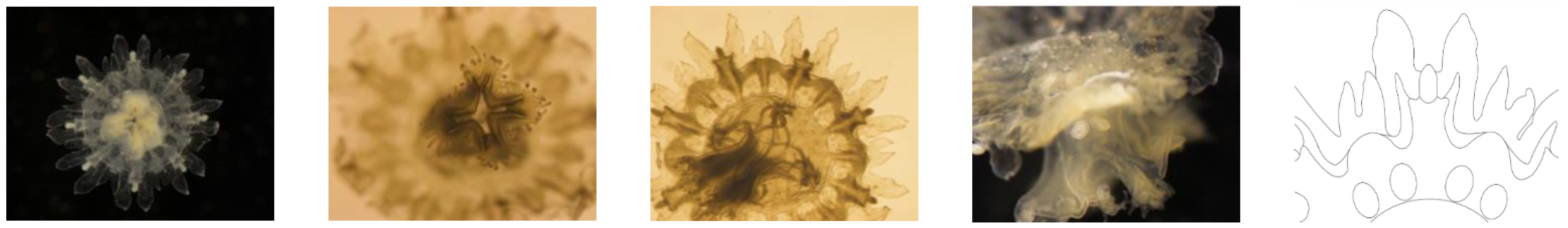
3.2. Different Stages of the Development of Stomolophus sp. 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Brotz, L. Changing Jellyfish Populations: Trends in Large Marine Ecosystems. Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 2011. [Google Scholar] [CrossRef]
- Kim, D.; Seo, J.; Yoon, W.; Suh, Y. Estimating the economic damage caused by jellyfish to fisheries in Korea. Fish. Sci. 2012, 78, 1147–1152. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and Ctenophore Blooms Coincide with Human Proliferations and Environmental Perturbations. Ann. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.P.; Rudloe, J. Potential of utilizing jellyfish as food in Western countries. Trends Food Sci. Technol. 1994, 5, 225–229. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Li, J.; Hsieh, Y.-H.P. Traditional Chinese food technology and cuisine. Asia Pac. J. Clin. Nutr. 2004, 13, 147–155. [Google Scholar] [PubMed]
- Kitamura, M.; Omori, M. Synopsis of edible jellyfishes collected from Southeast Asia, with notes on jellyfish fisheries. Plankton Benthos Res. 2010, 5, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-H.P.; Leong, F.-M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- López-Martínez, J.; Álvarez-Tello, J. The jellyfish fishery in Mexico. Agric. Sci. 2013, 4, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Peggy Hsieh, Y.H.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M.; et al. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish Biol Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Torri, L.; Tuccillo, F.; Bonelli, S.; Piraino, S.; Leone, A. The Attitudes of Italian Consumers towards Jellyfish as Novel Food. Food Qual. Prefer. 2020, 79, 103782. Available online: http://www.sciencedirect.com/science/article/pii/S0950329318310346 (accessed on 27 August 2019). [CrossRef]
- Dong, Z.; Liu, D.; Keesing, J.K. Contrasting Trends in Populations of Rhopilema esculentum and Aurelia aurita in Chinese Waters. In Jellyfish Blooms; Pitt, K., Lucas, C., Eds.; Springer: Dordrecht, Holland, 2014; pp. 207–218. [Google Scholar] [CrossRef]
- Kramp, P.L. Zoogeographical studies on Rhizostomeae. Vidensk. Medd. Fra Dansk Naturh. Foren. 1970, 133, 7–30. [Google Scholar]
- Jarms, G.; Morandini, A.C. World Atlas of Jellyfish; Dölling und Galitz Verlag: Hamburg, Germany, 2019; p. 816. [Google Scholar]
- Mayer, A.G. Medusae of the world III: The Scyphomedusae. Publ. Carnegie Inst. Wash. 1910, 109, 499–735. [Google Scholar]
- Kramp, P.L. A revision of Ernst Haeckel’s determinations of a collection of medusae belonging to the Zoological Museum of Copenhagen. Deep Sea Res. 1955, 3, 149–168. [Google Scholar]
- Kramp, P.L. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. UK 1961, 40, 7–469. [Google Scholar] [CrossRef]
- Uchida, T. Some medusae of the central Pacific. J. Fac. Sci. Hokkaido Univ. Ser. 6 Zool. 1947, 9, 297–319. [Google Scholar]
- Mianzan, H.W.; Cornelius, P.F.S. Cubomedusae and Scyphomedusae. In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys Publishers: Leiden, Holland, 1999; Volume 1, pp. 513–559. [Google Scholar]
- Banha, T.N.S.; Morandini, A.C.; Rosário, R.P.; Martinelli Filho, J.E. Scyphozoan jellyfish (Cnidaria, Medusozoa) from Amazon coast: Distribution, temporal variation and length–weight relationship. J. Plankton Res. 2020, 42, 767–778. [Google Scholar] [CrossRef]
- Gómez-Daglio, L.; Dawson, M.N. Species richness of jellyfishes (Scyphozoa: Discomedusae) in the Tropical Eastern Pacific: Missed taxa, molecules, and morphology match in a biodiversity hotspot. Invertebr. Syst. 2017, 31, 635–663. [Google Scholar] [CrossRef]
- Getino Mamet, L.N.; Gómez-Daglio, L.; García-De Léon, F.J. High genetic differentiation in the edible cannonball jellyfish (Cnidaria: Scyphozoa: Stomolophus spp.) from the Gulf of California, Mexico. Fish. Res. 2019, 219, 105328. [Google Scholar] [CrossRef]
- Lopez-Martinez, J.; Arzola-Sotelo, E.A.; Nevarez-Martinez, M.O.; Alvarez-Tello, F.J.; Morales-Bojorquez, E. Modeling growth on the cannonball jellyfish Stomolophus meleagris based on a multi-model inference approach. Hydrobiologia 2020, 847, 1399–1422. [Google Scholar] [CrossRef]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.; Parsons, T.; Piraino, S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 2008, 18, 299–310. [Google Scholar] [CrossRef]
- Gibbons, M.; Boero, F.; Brotz, L. We should not assume that fishing jellyfish will solve our jellyfish problem. ICES J. Mar. Sci. 2016, 73, 1012–1018. [Google Scholar] [CrossRef] [Green Version]
- Straehler-Pohl, I.; Jarms, G. Identification key for young ephyrae: A first step for early detection of jellyfish blooms. Hydrobiologia 2010, 645, 3–21. [Google Scholar] [CrossRef]
- Holst, S. Morphology and development of benthic and pelagic life stages of North Sea jellyfish (Scyphozoa, Cnidaria) with special emphasis on the identification of ephyra stages. Mar. Biol. 2012, 159, 2707–2722. [Google Scholar] [CrossRef]
- Calder, D.R. Life history of the cannonball jellyfish, Stomolophus meleagris L. Agassiz, 1860 (Scyphozoa, Rhizostomida). Biol. Bull. 1982, 162, 149–162. [Google Scholar] [CrossRef]
- Gómez-Aguirre, S. Larva éfira y diferenciación de Stomolophus meleagris (Scyphozoa Rhizostomeae) en plancton de lagunas costeras de Tabasco, México. An. Inst. Biol. Univ. Nac. Autón. México Ser. Zool. 1991, 62, 383–389. [Google Scholar]
- Stiasny, G. Papers from Dr. Th. Mortensen’s Pacific Expedition 1914-16. XII. Zur Kenntnis der Entwicklung von Stomolophus meleagris L. Agassiz. Vidensk Medd Fra Dansk Naturh Foren 1922, 73, 499–511. [Google Scholar]
- Burrola-Sánchez, M.S.; López-Martínez, J.; Padilla-Arredondo, G.; Urias-Laborin, D.; Padilla-Serrato, J.G. Influencia de los procesos costeros sobre la distribución de la medusa bola de cañón Stomolophus melegris (Agassiz, 1860) en el Golfo de California. In La Variabilidad Ambiental y Las Pesquerías De México; López-Martínez, J., Ed.; Comisión Nacional de Acuacultura y Pesca: Mazatlán, México, 2008; pp. 156–177. [Google Scholar]
- López-Martínez, J.; Rodríguez-Romero, J. Primer registro de la asociación del jurelillo negro Hemicaranx zelotes Gilbert (Pisces: Carangidae) con la medusa bala de cañón Stomolophus meleagris Agassiz (Scyphozoa: Rhizostomatidae) en Bahía de Kino, Golfo de California. Hidrobiológica 2008, 18, 173–176. [Google Scholar]
- Nevárez-López, C.A.; Váldez-Holguín, J.E.; Hernández-Saavedra, N.Y. Caracterización Genética de los fenotipos de la medusa “Bola de (Stomolophus meleagris, L. AGASSIZ 1862) en las Guásimas, Sonora. In Biotecnología Marina; XII Congreso Nacional de Biotecnología y Bioingeniería y VII Simposio Internacional de Producción de Alcoholes y Levaduras: Acapulco, Guerrero, México, 2009. [Google Scholar]
- Carvalho-Saucedo, L.; García-Domínguez, F.; Rodriguez-Jaramillo, C.; López-Martínez, J. Variación lipídica en los ovocitos de la medusa Stomolophus meleagris (Scyphozoa: Rhizostomeae), durante el desarrollo gonádico, en la laguna Las Guásimas, Sonora, México. Rev. Biol. Trop. 2010, 58, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Tello, J.; López-Martínez, J.; Lluch-Cota, D. Trophic spectrum and feeding pattern of cannonball jellyfish Stomolophus meleagris (Agassiz, 1862) from central Gulf of California. J. Mar. Biol. Assoc. UK 2015, 6, 1217–1227. [Google Scholar] [CrossRef]
- Straehler-Pohl, I.; Widmer, C.L.; Morandini, A.C. Characterizations of juvenile stages of some semaeostome Scyphozoa (Cnidaria), with recognition of a new family (Phacellophoridae). Zootaxa 2011, 2741, 1–37. [Google Scholar] [CrossRef]
- Russell, F.S. The Medusae of the British Isles II, Pelagic Scyphozoa with a Supplement to the First Volume on Hydromedusae; Cambridge University Press: Cambridge, UK, 1970; pp. 1–284. [Google Scholar]
- Fu, Z.; Shibata, M.; Makake, R.; Ikeda, H.; Uye, S. Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita. Mar. Ecol. Prog. Ser. 2014, 510, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Chiaverano, L.M.; Graham, W.M. Morphological plasticity in Aurelia polyps, with subsequent effects on asexual fecundity and morphology of young medusae. Mar. Ecol. Prog. Ser. 2017, 582, 79–92. [Google Scholar] [CrossRef]
- Morandini, A.C.; Silveira, F.L.; Jarms, G. The life cycle of Chrysaora lactea Eschscholtz, 1829 (Cnidaria, Scyphozoa) with notes on the scyphistoma stage of three other species. Hydrobiologia 2004, 530/531, 347–354. [Google Scholar] [CrossRef]
- Widmer, C.L. Life cycle of Chrysaora fuscescens (Cnidaria: Scyphozoa) and a key to sympatric ephyrae. Pac. Sci. 2008, 62, 71–82. [Google Scholar] [CrossRef] [Green Version]
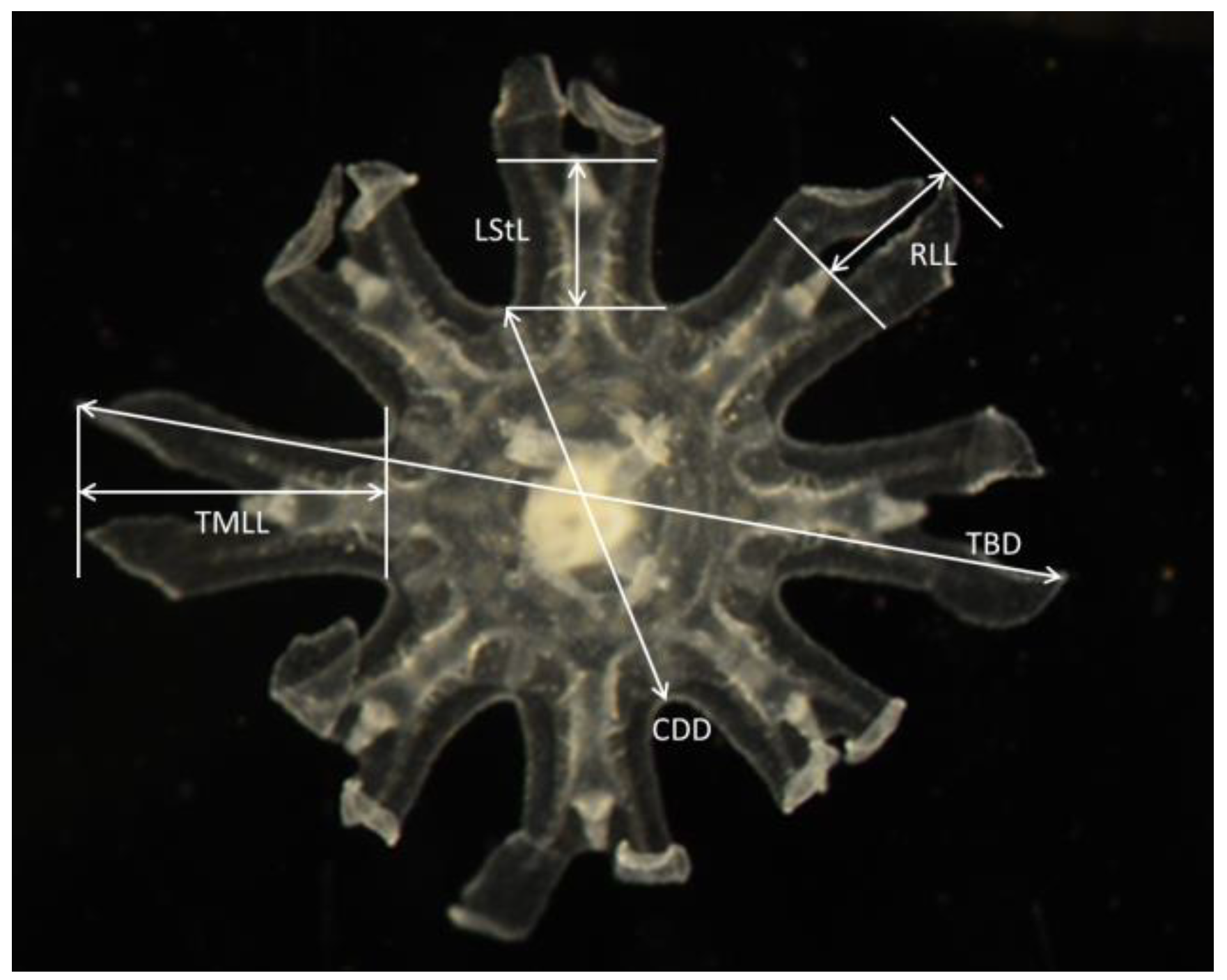
| Specimen | CDD/TBD × 100 | TMLL/TBD × 100 | RLL/TMLL × 100 | LStL/TMLL × 100 |
|---|---|---|---|---|
| 1 | 41.66 | 29.58 | 60.46 | 50.55 |
| 2 | 48.41 | 29.30 | 51.93 | 52.80 |
| 3 | 37.71 | 33.72 | 54.70 | 49.91 |
| 4 | 54.29 | 26.87 | 53.59 | 57.13 |
| 5 | 53.54 | 29.67 | 50.33 | 46.63 |
| 6 | 44.78 | 26.45 | 65.66 | 68.67 |
| 7 | 45.05 | 32.57 | 53.52 | 57.78 |
| 8 | 48.01 | 30.56 | 51.57 | 48.43 |
| 9 | 39.46 | 29.84 | 47.18 | 42.91 |
| 10 | 48.75 | 27.87 | 62.19 | 48.06 |
| 11 | 41.58 | 32.42 | 57.94 | 47.84 |
| 12 | 49.19 | 30.98 | 55.57 | 46.78 |
| 13 | 42.32 | 28.39 | 52.66 | 45.94 |
| 14 | 44.24 | 28.61 | 57.89 | 42.83 |
| 15 | 38.31 | 32.18 | 54.68 | 43.53 |
| 16 | 45.68 | 27.98 | 57.20 | 49.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Salinas, L.C.; López-Martínez, J.; Morandini, A.C. The Young Stages of the Cannonball Jellyfish (Stomolophus sp. 2) from the Central Gulf of California (Mexico). Diversity 2021, 13, 229. https://doi.org/10.3390/d13060229
Gómez-Salinas LC, López-Martínez J, Morandini AC. The Young Stages of the Cannonball Jellyfish (Stomolophus sp. 2) from the Central Gulf of California (Mexico). Diversity. 2021; 13(6):229. https://doi.org/10.3390/d13060229
Chicago/Turabian StyleGómez-Salinas, Laura Cristina, Juana López-Martínez, and André Carrara Morandini. 2021. "The Young Stages of the Cannonball Jellyfish (Stomolophus sp. 2) from the Central Gulf of California (Mexico)" Diversity 13, no. 6: 229. https://doi.org/10.3390/d13060229






