Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
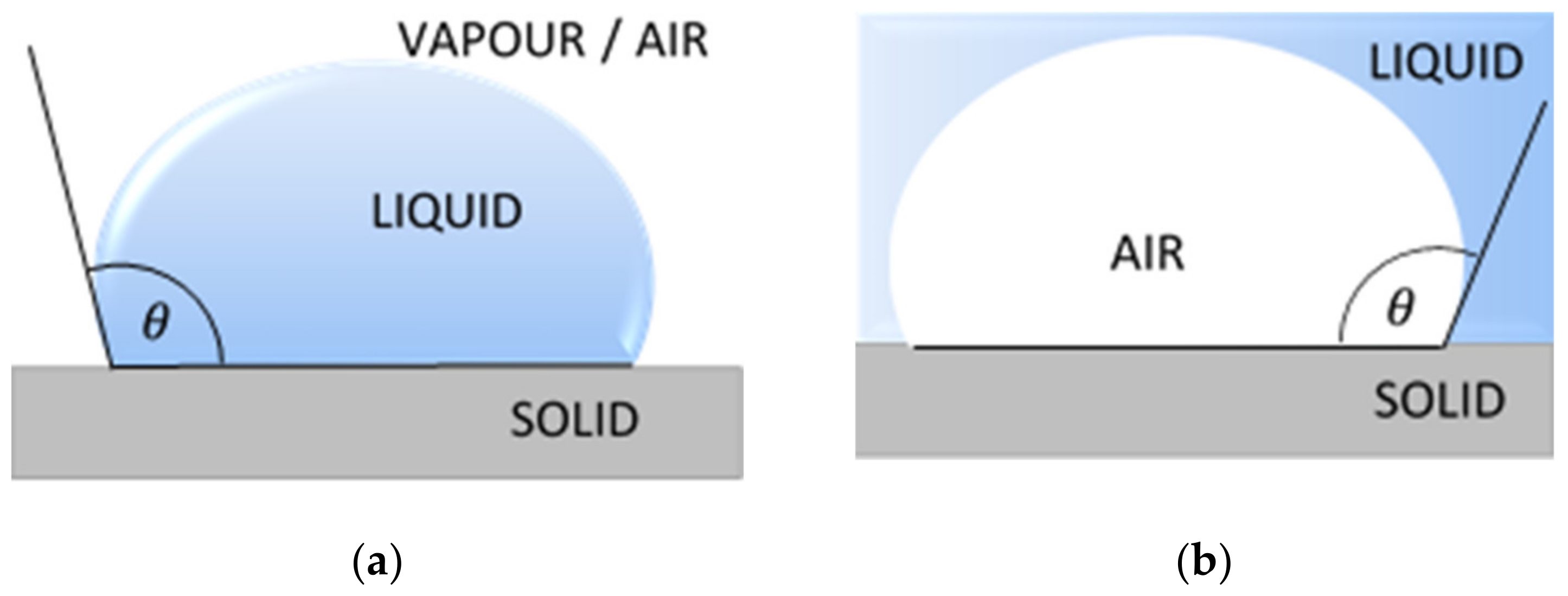
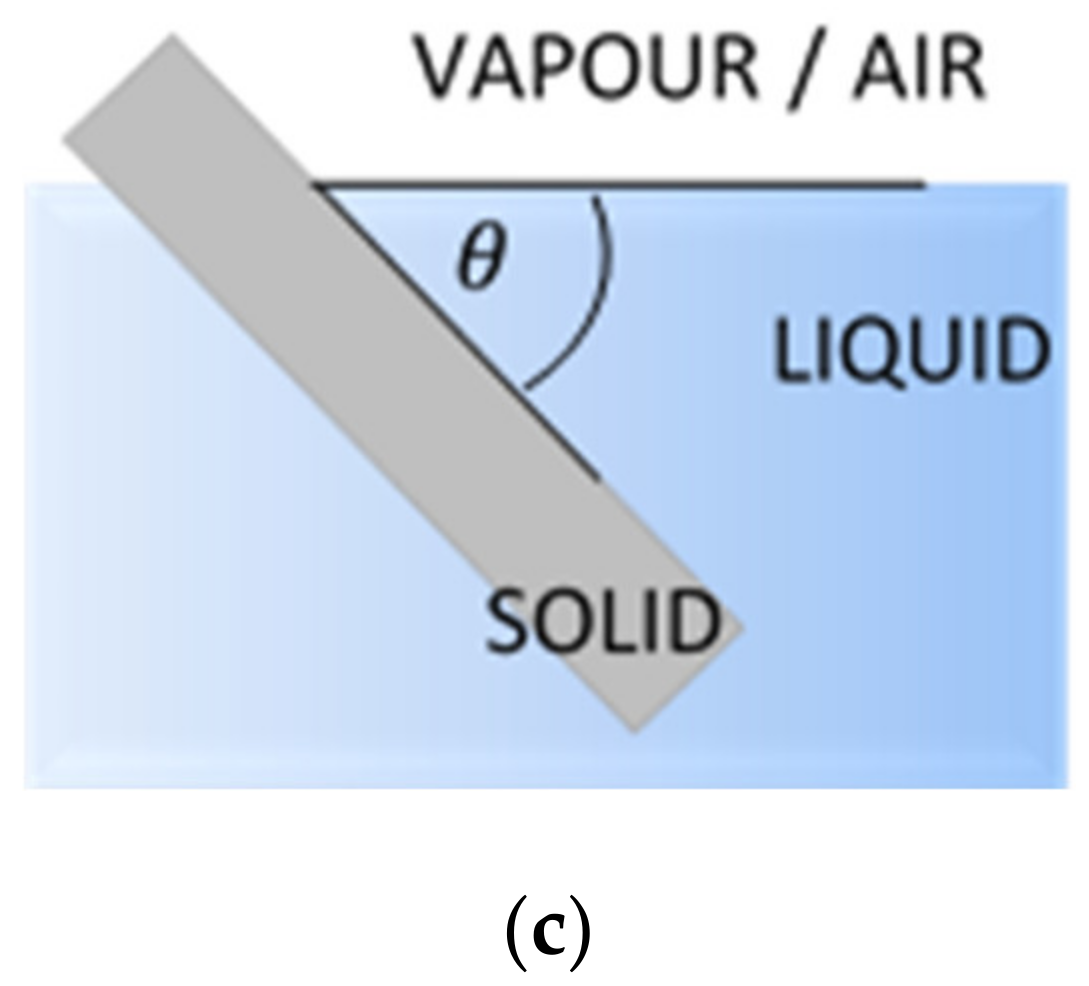
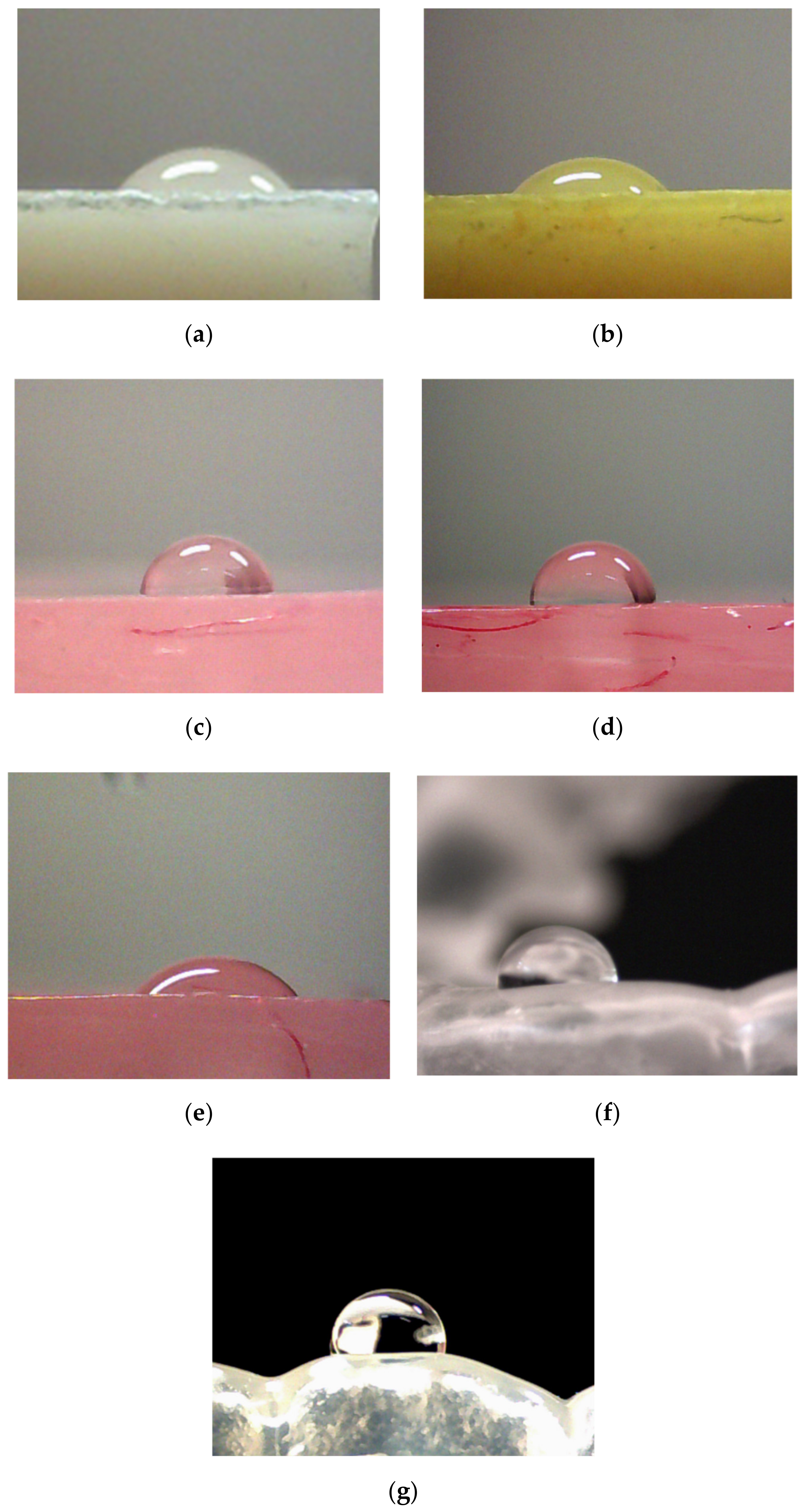
2.2.1. Contact Angle
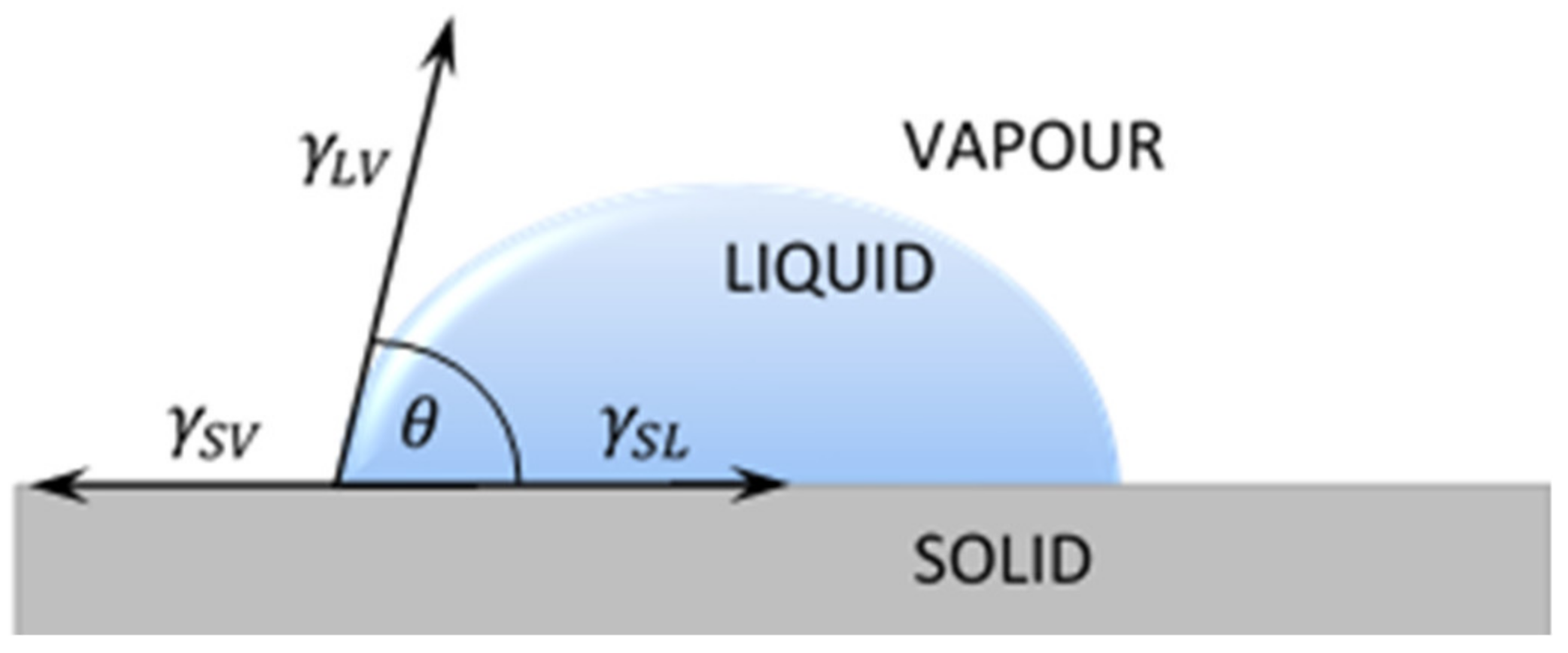
2.2.2. Surface Free Energy Calculation
Owens–Wendt Method
Van Oss–Chaudhury–Good Method
The Zisman’s Method
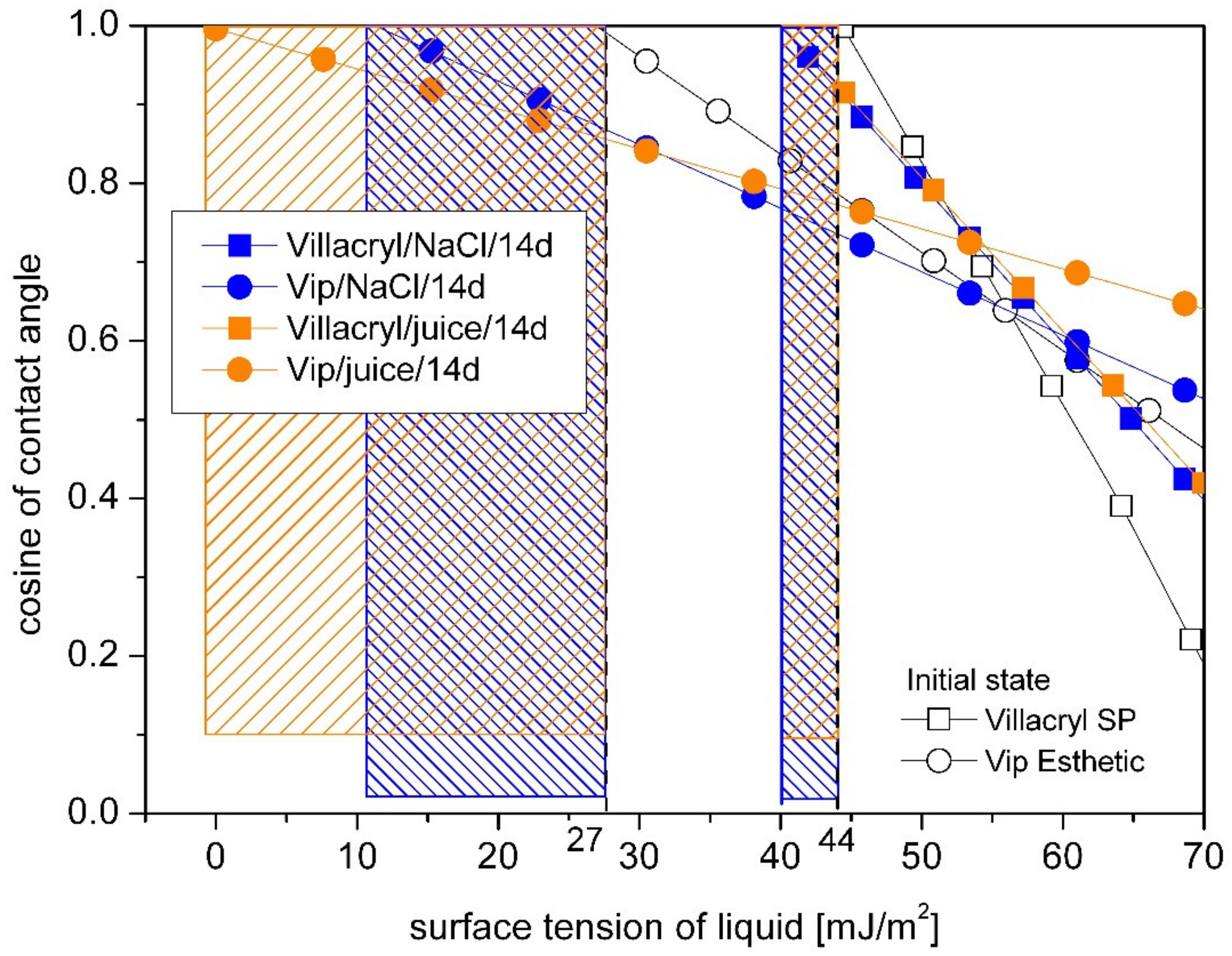
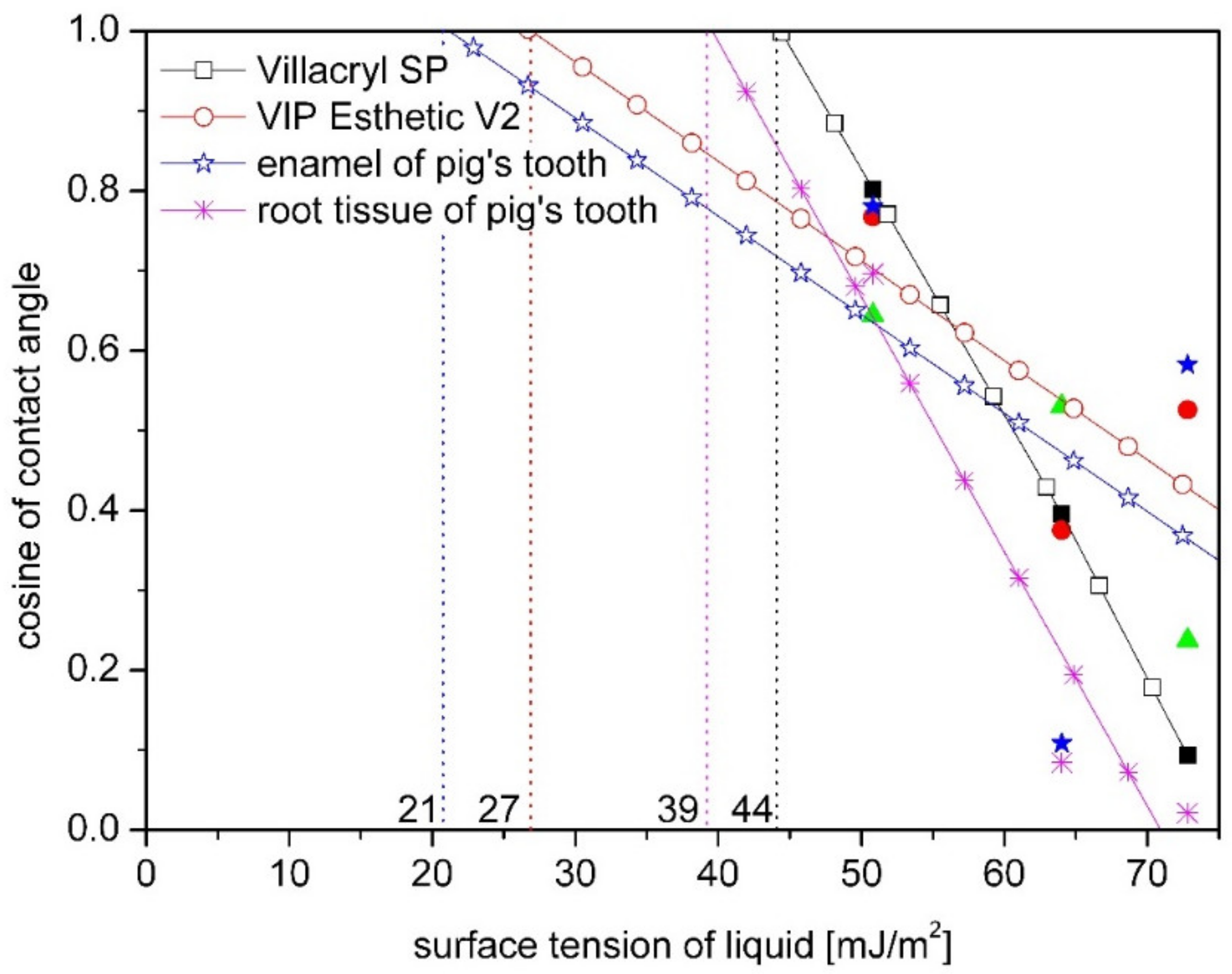
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auschill, T.M.; Arweiler, N.C.; Brecx, M.; Reich, E.; Sculean, A.; Netuschil, L. The effect of dental restorative materials on dental biofilm. Eur. J. Oral. Sci. 2002, 110, 48–53. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. J. Implant Dent. 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Marechal, M.; Busscher, H.; Weerkamp, A.; van Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.R.; Jones, L.W. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chu, P.K. Surface characterization of biomaterials. In Characterization of Biomaterials; Bandyopadhyay, A., Bose, S., Eds.; Elsevier: Cambridge, MA, USA, 2013; pp. 105–174. [Google Scholar]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef]
- Lam, C.N.C.; Kim, N.; Hui, D.; Kwok, D.Y.; Hair, M.L.; Neumann, A.W. The effect of liquid properties to contact angle hysteresis. Colloids Surfaces A 2001, 189, 265–278. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am. J. Dent. 2015, 28, 311–320. [Google Scholar]
- Fan, H.; Guo, Z. Bioinspired surfaces with wettability: Biomolecule adhesion behaviors. Biomater. Sci. 2020, 8, 1502–1535. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Ye, Z.; Fischer, N.G.; Skoe, P.E.; Echeverría, C.; Wu, J.; Aparicio, C. Physical-chemical interactions between dental materials surface, salivary pellicle and Streptococcus gordonii. Colloids Surf. B 2020, 190, 110938. [Google Scholar] [CrossRef]
- Cavalcanti, Y.W.; Wilson, M.; Lewis, M.; Williams, D.; Senna, P.M.; Del-Bel-Cury, A.A.; Silva, W.J. Salivary pellicles equalise surfaces’ charges and modulate the virulence of Candida albicans biofilm. Arch. Oral Biol. 2016, 6, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Invest. 2020, 24, 4237–4242. [Google Scholar] [CrossRef]
- Namen, F.M.; Ferrandini, E.; Galan, J. Surface energy and wettability of polymers light-cured by two different systems. J. Appl. Oral Sci. 2011, 19, 517–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, J.G.; Danelon, M.; Pessan, J.P.; Figueiredo, L.R.; Camargo, E.R.; Delbem, A.C.B. Surface free energy of enamel treated with sodium hexametaphosphate, calcium and phosphate. Arch. Oral Biol. 2018, 90, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Rüttermann, S.; Beikler, T.; Janda, R. Contact angle and surface free energy of experimental resin-based dental restorative materials after chewing simulation. Dent. Mater. 2014, 30, 702–707. [Google Scholar] [CrossRef]
- Brajkovic, D.; Antonijevic, D.; Milovanovic, P.; Kisic, D.; Zelic, K.; Djuric, M.; Rakocevic, Z. Surface characterization of the cement for retention of implant supported dental prostheses: In vitro evaluation of cement roughness and surface free energy. Appl. Surf. Sci. 2014, 311, 131–138. [Google Scholar] [CrossRef]
- Siboni, S.; Volpe, C.D.; Maniglio, D.; Brugnara, M. The solid surface free energy calculation II. The limits of the Zisman and of the “equation-of-state” approaches. J. Colloid. Interf. Sci. 2004, 271, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Jiang, L. Contact angle measurement of natural materials. Colloids Surf. B 2018, 161, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell adhesion to biomaterials: Concept of biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; Intech: London, UK, 2013; pp. 208–240. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshiz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 8, 927–941. [Google Scholar]
- Baier, R.E. The role of surface energy in thrombogenesis. Bull. N.Y. Acad. Med. 1972, 48, 257–272. [Google Scholar]
- Baier, R.E. Correlations of materials surface properties with biological responses. J. Surf. Eng. Mater. Adv. Technol. 2015, 5, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.R.; Ritter, H.; Janda, R. A new approach to influence contact angle and surface free energy of resin-based dental restorative materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef]


| Material | Basic Composition, Application | Manufactures |
|---|---|---|
| Villacryl SP | cold-curing acrylic resin for frameworks, making partial and full prostheses by pouring in hydrocolloids, mask silicones and duplicating silicones; it can also be used for repairs and for indirect relining | Zhermack, Italy |
| Villacryl H Plus | heat-curing acrylic resins, specially formulated for denture bases, removable full or partial prostheses and for the indirect relining of removable prostheses | Zhermack, Italy |
| Villacryl S V4 | self-curing acrylic resin for the repair and indirect relining of removable prostheses (color pink veined) | Zhermack, Italy |
| VertexTH Self-Curing | self -polymerizing cold-curing acrylic material used both for repair and relining of full and partial dentures | Vertex-Dental, The Netherlands |
| Flexite T-Val | polyolefin thermoplastic for flexible partial dentures | Flexite Company, USA |
| Vip Esthetic | BIS-GMA- light curing resin and inorganic filler particles od 0.05–1.5 μm, suitable for all cavities | Olident, Poland |
| Dental retainer | Duram®+—polyethylene terephthalate glycol-modified (PET-G) used for dental retainers | SCHEU_DENTAL GmHb, Germany |
| Material | Environment | Temperature [°C] | Time |
|---|---|---|---|
| Villacryl SP | 0.9% NaCl / Orange juice (pH 3.5) | 37 | 14 days |
| Villacryl H Plus |
| 22/37 | 24 h/14 days |
| 22/37 | 24 h/14 days | |
| Vertex TH Self-Curing | 0.9% NaCl | 37 | 21 days |
| Flexite T-Val | 0.9% NaCl | 37 | 21 days |
| Vip Esthetic | 0.9% NaCl / Orange juice (pH 3.5) | 37 | 14 days |
| Dental retainer | water (pH 4.9)/orange juice (pH 3.5)/coffee (4.8 g + 100 mL water) pH 4.9)/0.9% NaCl | 37 | 60 days |
| Swine molar | 0.9% NaCl | 37 | 24 h |
| Material | Contact Angle (°) | |
|---|---|---|
| Initial State | After Incubation | |
| Swine tooth enamel | 50.2 ± 17.6 | 54.4 ± 17.0 |
| Swine root tissue | 62.1 ± 7.7 | 88.8 ± 9.3 |
| Villacryl SP | 84.72 ± 3.79 | 65.48 ± 3.43 in 0.9 NaCl |
| 65.01 ± 3.37 in orange juice | ||
| Vip Esthetic | 58.8 ± 4.1 | 58.4 ± 3.6 in 0.9 NaCl |
| 49.1 ± 5.7 in orange juice | ||
| Villacryl H Plus | 77.6 ± 6.0 | 85.3 ± 5.0 in Corega, 24 h |
| 69.1 ± 4.5 in Prokudent, 24 h | ||
| 81.3 ± 6.9 in Corega, 14 days | ||
| 60.9 ± 4.7 in Prokudent, 14 days | ||
| Vertex Self Curing | 76.4 ± 2.8 | 71.2 ± 0.6 |
| Flexite T-Val | 75.0 ± 3.7 | 69.9 ± 2.2 |
| Dental retainer | 81.9 ± 1.8 | 99.6 ± 4.5 in 0.9% NaCl |
| 95.6 ± 4.3 in water | ||
| 83.6 ± 6.3 in orange juice | ||
| 85.4 ± 2.9 in coffee | ||
| Material | Owens–Wendt | Van Oss–Chaudhury–Good | ||||||
|---|---|---|---|---|---|---|---|---|
| Pig tooth/ enamel * | 55.57 ± 8.70 | 40.26 ± 3.94 | 15.31 ± 8.92 | 74.88 ± 6.79 | 40.26 ± 3.94 | 34.62 ± 3.75 | 4.97 ± 4.96 | 60.27 ± 5.87 |
| Pig tooth/ root * | 38.06 ± 5.55 | 36.50 ± 4.88 | 1.56 ± 1.45 | 40.11 ± 8.49 | 36.50 ± 4.88 | 3.61 ± 0.61 | 0.53 ± 0.02 | 6.22 ± 0.22 |
| Villacryl SP | 43.09 ± 3.79 | 41.22 ± 3.15 | 1.87 ± 0.52 | 42.83 ± 10.21 | 41.22 ± 13.15 | 1.61 ± 0.77 | 0.37 ± 0.11 | 1.76 ± 0.85 |
| Villacryl SP * | 49.90 ± 1.07 | 42.58 ± 1.12 | 7.32 ± 0.05 | 44.02 ± 9.88 | 42.58 ± 11.12 | 1.44 ± 0.82 | 0.04 ± 0.01 | 16.02 ± 3.66 |
| Villacryl SP ** | 49.96 ± 0.77 | 40.10 ± 1.22 | 9.87 ± 0.05 | 40.62 ± 11.72 | 40.10 ± 10.22 | 0.53 ± 0.02 | 0.01 ± 0.01 | 19.52 ± 4.95 |
| Vip Esthetic | 52.79 ± 3.82 | 39.66 ± 2.39 | 13.13 ± 3.18 | 45.43 ± 16.92 | 39.66 ± 2.39 | 5.77 ± 0.53 | 0.26 ± 0.09 | 31.77 ± 7.46 |
| Vip Esthetic * | 51.15 ± 7.48 | 36.47 ± 0.15 | 14.68 ± 1.50 | 41.80 ± 8.39 | 36.47 ± 9.25 | 5.33 ± 2.54 | 0.45 ± 0.12 | 23.29 ± 3.63 |
| Vip Esthetic ** | 54.65 ± 8.15 | 36.74 ± 0.26 | 17.91 ± 4.10 | 42.92 ± 10.02 | 36.74 ± 9.26 | 6.18 ± 1.83 | 0.33 ± 0.03 | 29.31 ± 4.23 |
| Villacryl S V4 | 43.55 ± 2.07 | 42.08 ± 1.32 | 1.47 ± 0.75 | - | - | - | - | - |
| Villacryl H Plus | 48.07 ± 3.02 | 44.77 ± 1.18 | 3.31 ± 0.62 | 47.05 ± 3.91 | 44.77 ± 1.18 | 2.28 ± 0.11 | 0.15 ± 0.05 | 8.75 ± 0.63 |
| Villacryl H Plus (Corega 22°C/24 h) | 42.20 ± 1.55 | 40.91 ± 0.83 | 1.92 ± 0.88 | 46.04 ± 3.42 | 40.91 ± 0.83 | 5.77 ± 0.11 | 0.97 ± 0.07 | 8.65 ± 0.01 |
| Villacryl H Plus (Corega37C/14d) | 45.98 ± 3.43 | 43.58 ± 1.82 | 2.40 ± 1.73 | 46.44 ± 4.63 | 43.58 ± 2.06 | 2.85 ± 0.78 | 0.28 ± 0.08 | 7.38 ± 0.47 |
| Villacryl H Plus (Prokudent22°C/24 h) | 49.14 ± 2.90 | 41.71 ± 1.45 | 7.43 ± 1.89 | 53.21 ± 10.38 | 41.71 ± 1.45 | 11.51 ± 0.37 | 2.03 ± 0.60 | 24.74 ± 9.15 |
| Villacryl H Plus (Prokudent37°C/14 d) | 53.10 ± 3.42 | 41.95 ± 1.27 | 11.15 ± 2.82 | 50.70 ± 10.09 | 41.95 ± 1.27 | 8.85 ± 0.58 | 0.64 ± 0.04 | 30.51 ± 1.51 |
| Vertex Self Curing | 44.79 ± 1.52 | 40.11 ± 0.79 | 4.69 ± 0.97 | - | - | - | - | - |
| Vertex Self Curing * | 43.81 ± 0.64 | 36.81 ± 1.61 | 8.00 ± 0.32 | - | - | - | - | - |
| Flexite T-Val | 46.56 ± 2.53 | 40.84 ± 1.00 | 5.72 ± 2.36 | - | - | - | - | - |
| Flexite T-Val * | 41.76 ± 1.30 | 31.7 ± 1.61 | 10.09 ± 1.49 | - | - | - | - | - |
| Medium | ||||||
|---|---|---|---|---|---|---|
| (mJ/m2) | ||||||
| Initial State | After Incubation | |||||
| dry/ orange juice | 33.7 ± 2.7 | 28.1 ± 3.4 | 5.6 ± 1.1 | 38.9 ± 2.3 | 35.9 ± 1.9 | 3.1 ± 0.9 |
| dry/ coffee | 32.21 | 25.05 | 7.17 | 41.1 ± 1.8 | 39.0 ± 1.3 | 2.0 ± 0.6 |
| dry/0.9% NaCl | 32.67 | 27.88 | 4.76 | 29.4 ± 1.7 | 28.8 ± 1.6 | 0.5 ± 0.1 |
| dry/ water | 32.25 | 26.68 | 5.57 | 28.7 ± 0.9 | 27.2 ± 1.3 | 1.4 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. https://doi.org/10.3390/ma14112716
Liber-Kneć A, Łagan S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials. 2021; 14(11):2716. https://doi.org/10.3390/ma14112716
Chicago/Turabian StyleLiber-Kneć, Aneta, and Sylwia Łagan. 2021. "Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy" Materials 14, no. 11: 2716. https://doi.org/10.3390/ma14112716
APA StyleLiber-Kneć, A., & Łagan, S. (2021). Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials, 14(11), 2716. https://doi.org/10.3390/ma14112716






