Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients
Abstract
:1. Introduction
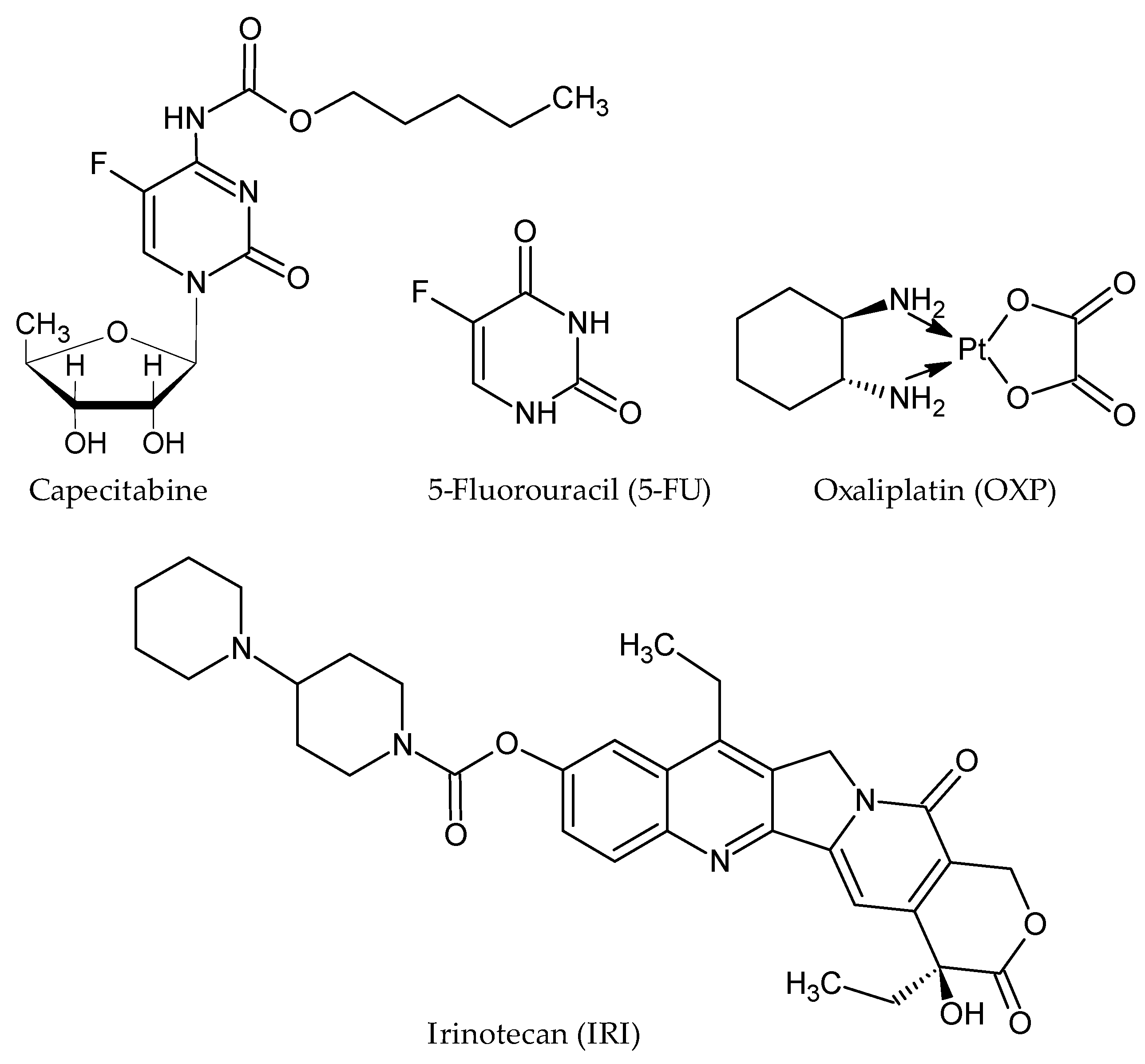
2. Current Pharmacotherapy Available for CRC
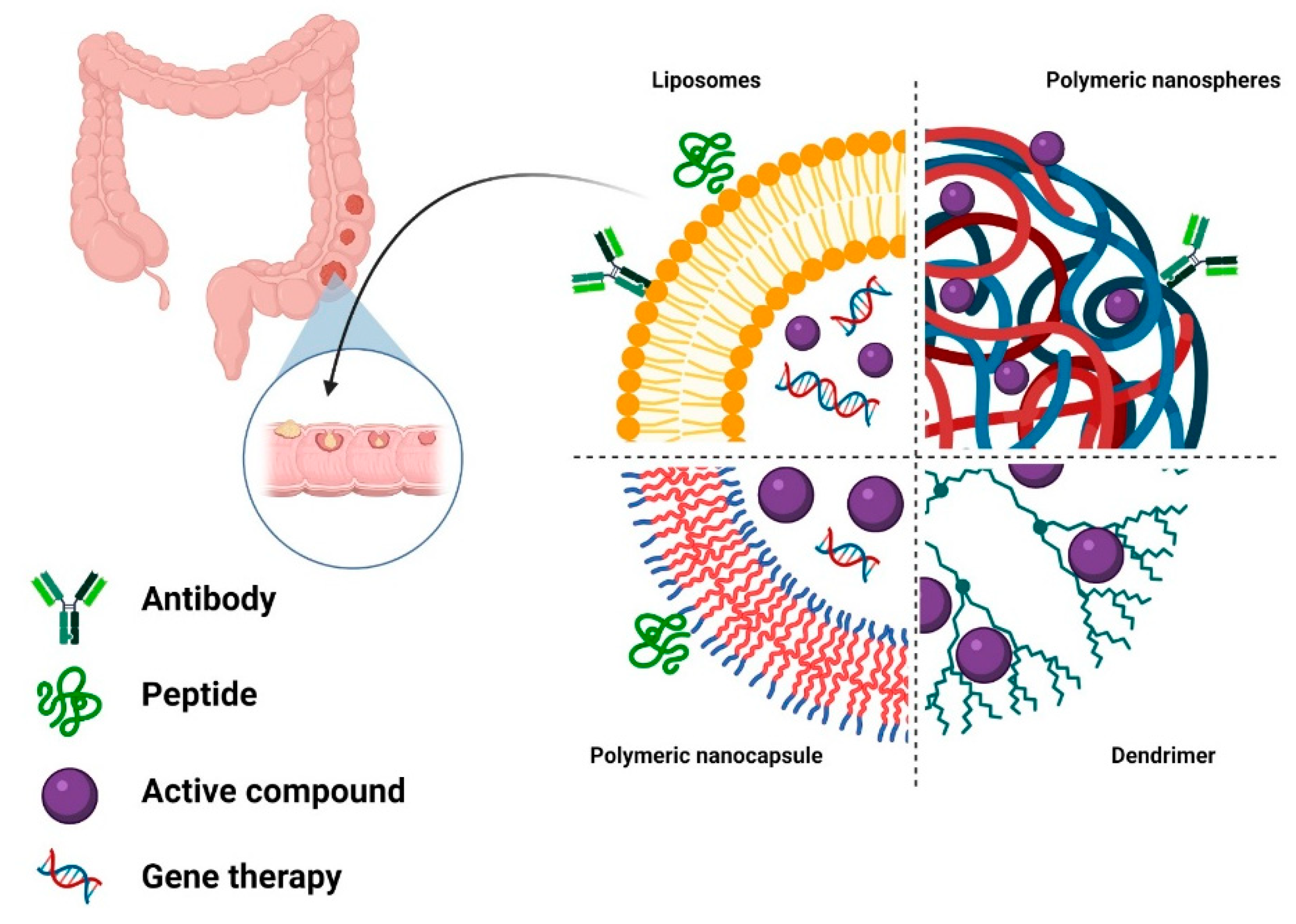
3. Targeting Strategies for Drug-Delivery Systems in CRC
3.1. Passive Targeting
3.2. Active Targeting
4. Organic Nanosized Drug-Delivery Systems for CRC Therapy
4.1. Lipid-Based Drug-Delivery Systems
4.2. Polymer-Based Drug-Delivery Systems
4.3. Hybrid Drug-Delivery Systems
5. Challenges in Drug-Delivery Systems Research
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2020. [Google Scholar] [CrossRef]
- Ferlizza, E.; Solmi, R.; Sgarzi, M.; Ricciardiello, L.; Lauriola, M. The Roadmap of Colorectal Cancer Screening. Cancers 2021, 13, 1101. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A Global View on Cancer Incidence and National Levels of the Human Development Index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Bevan, R.; Rutter, M.D. Colorectal Cancer Screening-Who, How, and When? Clin. Endosc. 2018, 51, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Przeglad Gastroenterol. 2019, 14, 89. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of Metastasis in Colon and Rectal Cancer. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F. Increasing Incidence of Colorectal Cancer in Young Adults in Europe over the Last 25 Years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Wolf, A.M.; Fontham, E.T.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T. Colorectal Cancer Screening for Average-risk Adults: 2018 Guideline Update from the American Cancer Society. Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Quirke, P. What Is the Role for the Circumferential Margin in the Modern Treatment of Rectal Cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef]
- Heald, R.; Ryall, R. Recurrence and Survival after Total Mesorectal Excision for Rectal Cancer. Lancet 1986, 327, 1479–1482. [Google Scholar] [CrossRef]
- Trastulli, S.; Cirocchi, R.; Listorti, C.; Cavaliere, D.; Avenia, N.; Gulla, N.; Giustozzi, G.; Sciannameo, F.; Noya, G.; Boselli, C. Laparoscopic vs Open Resection for Rectal Cancer: A Meta-analysis of Randomized Clinical Trials. Colorectal Dis. 2012, 14, e277–e296. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.J. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Bujko, K.; Nowacki, M.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term Results of a Randomized Trial Comparing Preoperative Short-course Radiotherapy with Preoperative Conventionally Fractionated Chemoradiation for Rectal Cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef]
- Kim, J.H. Chemotherapy for Colorectal Cancer in the Elderly. World J. Gastroenterol. 2015, 21, 5158. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Loprinzi, C.L.; Sargent, D.J.; Thomé, S.D.; Alberts, S.R.; Haller, D.G.; Benedetti, J.; Francini, G.; Shepherd, L.E.; Francois Seitz, J. Pooled Analysis of Fluorouracil-Based Adjuvant Therapy for Stage II and III Colon Cancer: Who Benefits and by How Much? J. Clin. Oncol. 2004, 22, 1797–1806. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F. Improved Overall Survival with Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 1–30. [Google Scholar]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B. Metastatic Patterns and Survival Outcomes in Patients with Stage IV Colon Cancer: A Population-based Analysis. Cancer Med. 2020, 9, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.-J.; Tveit, K.-M.; Gibson, F. A Review of the Evolution of Systemic Chemotherapy in the Management of Colorectal Cancer. Clin. Colorectal Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Thomas, R.; Goldberg, R.M. Colorectal Cancer Chemotherapy. Aliment. Pharmacol. Ther. 2003, 18, 683–692. [Google Scholar] [CrossRef]
- Alcindor, T.; Beauger, N. Oxaliplatin: A Review in the Era of Molecularly Targeted Therapy. Curr. Oncol. 2011, 18, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a Key Chemotherapeutic Drug for Metastatic Colorectal Cancer. World J. Gastroenterol. 2015, 21, 12234. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Kümler, I.; Sørensen, P.G.; Palshof, J.; Høgdall, E.; Skovrider-Ruminski, W.; Theile, S.; Fullerton, A.; Nielsen, P.; Jensen, B.V.; Nielsen, D. Oral Administration of Irinotecan in Patients with Solid Tumors: An Open-Label, Phase I, Dose Escalating Study Evaluating Safety, Tolerability and Pharmacokinetics. Cancer Chemother. Pharmacol. 2019, 83, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.; Chen, M.; Baskaran, R.; Lin, Y.; Day, C.H.; Lin, Y.; Tu, C.; Vijaya Padma, V.; Kuo, W.; Huang, C. Oxaliplatin Resistance in Colorectal Cancer Cells Is Mediated via Activation of ABCG2 to Alleviate ER Stress Induced Apoptosis. J. Cell. Physiol. 2018, 233, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Villalona-Calero, M. Irinotecan: Mechanisms of Tumor Resistance and Novel Strategies for Modulating Its Activity. Ann. Oncol. 2002, 13, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Negrei, C.; Hudita, A.; Ginghina, O.; Galateanu, B.; Voicu, S.N.; Stan, M.; Costache, M.; Fenga, C.; Drakoulis, N.; Tsatsakis, A.M. Colon Cancer Cells Gene Expression Signature as Response to 5-Fluorouracil, Oxaliplatin, and Folinic Acid Treatment. Front. Pharmacol. 2016, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.R.; Hageboutros, A.; Wang, K.; High, L.; Smith, J.B.; Diasio, R.B. Life-Threatening Toxicity in a Dihydropyrimidine Dehydrogenase-Deficient Patient after Treatment with Topical 5-Fluorouracil. Clin. Cancer Res. 1999, 5, 2006–2011. [Google Scholar] [PubMed]
- Nikolouzakis, T.K.; Stivaktakis, P.D.; Apalaki, P.; Kalliantasi, K.; Sapsakos, T.M.; Spandidos, D.A.; Tsatsakis, A.; Souglakos, J.; Tsiaoussis, J. Effect of Systemic Treatment on the Micronuclei Frequency in the Peripheral Blood of Patients with Metastatic Colorectal Cancer. Oncol. Lett. 2019, 17, 2703–2712. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Braun, M.S.; Seymour, M.T. Balancing the Efficacy and Toxicity of Chemotherapy in Colorectal Cancer. Ther. Adv. Med. Oncol. 2011, 3, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Tariman, J.D. Changes in Cancer Treatment: Mabs, Mibs, Mids, Nabs, and Nibs. Nurs. Clin. 2017, 52, 65–81. [Google Scholar]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A. FOLFOXIRI plus Bevacizumab versus FOLFIRI plus Bevacizumab as First-Line Treatment of Patients with Metastatic Colorectal Cancer: Updated Overall Survival and Molecular Subgroup Analyses of the Open-Label, Phase 3 TRIBE Study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Osborne, S.; Andre, N.; Waterkamp, D. Bevacizumab plus Capecitabine versus Capecitabine Alone in Elderly Patients with Previously Untreated Metastatic Colorectal Cancer (AVEX): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef]
- Simkens, L.H.; van Tinteren, H.; May, A.; ten Tije, A.J.; Creemers, G.-J.M.; Loosveld, O.J.; de Jongh, F.E.; Erdkamp, F.L.; Erjavec, Z.; van der Torren, A.M. Maintenance Treatment with Capecitabine and Bevacizumab in Metastatic Colorectal Cancer (CAIRO3): A Phase 3 Randomised Controlled Trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A. Ramucirumab versus Placebo in Combination with Second-Line FOLFIRI in Patients with Metastatic Colorectal Carcinoma That Progressed during or after First-Line Therapy with Bevacizumab, Oxaliplatin, and a Fluoropyrimidine (RAISE): A Randomised, Double-Blind, Multicentre, Phase 3 Study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [PubMed]
- Folprecht, G.; Pericay, C.; Saunders, M.P.; Thomas, A.; Lopez, R.L.; Roh, J.; Chistyakov, V.; Höhler, T.; Kim, J.-S.; Hofheinz, R.-D. Oxaliplatin and 5-FU/Folinic Acid (Modified FOLFOX6) with or without Aflibercept in First-Line Treatment of Patients with Metastatic Colorectal Cancer: The AFFIRM Study. Ann. Oncol. 2016, 27, 1273–1279. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; Van Hazel, G.A.; Moiseyenko, V.; Ferry, D. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients with Metastatic Colorectal Cancer Previously Treated with an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of Angiogenesis in Tumor Growth and Metastasis; Elsevier: Amsterdam, The Netherlands, 2002; Volume 29, pp. 15–18. [Google Scholar]
- Seymour, M.T.; Brown, S.R.; Middleton, G.; Maughan, T.; Richman, S.; Gwyther, S.; Lowe, C.; Seligmann, J.F.; Wadsley, J.; Maisey, N. Panitumumab and Irinotecan versus Irinotecan Alone for Patients with KRAS Wild-Type, Fluorouracil-Resistant Advanced Colorectal Cancer (PICCOLO): A Prospectively Stratified Randomised Trial. Lancet Oncol. 2013, 14, 749–759. [Google Scholar] [CrossRef] [Green Version]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J. Randomized, Phase III Trial of Panitumumab with Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chang Chien, C.-R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [Green Version]
- Markman, B.; Javier Ramos, F.; Capdevila, J.; Tabernero, J. EGFR and KRAS in Colorectal Cancer. Adv. Clin. Chem. 2010, 51, 72. [Google Scholar]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar]
- Roskoski, R., Jr. Small Molecule Inhibitors Targeting the EGFR/ErbB Family of Protein-Tyrosine Kinases in Human Cancers. Pharmacol. Res. 2019, 139, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. ErbB Receptors and Cancer. ErbB Recept. Signal. 2017, 3–35. [Google Scholar]
- Hubbard, J.M.; Grothey, A. Progress in Defining First-Line and Maintenance Therapies. Nat. Rev. Clin. Oncol. 2015, 12, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Cremolini, C.; Salvatore, L.; Masi, G.; Sensi, E.; Schirripa, M.; Michelucci, A.; Pfanner, E.; Brunetti, I.; Lupi, C. FOLFOXIRI plus Bevacizumab as First-Line Treatment in BRAF Mutant Metastatic Colorectal Cancer. Eur. J. Cancer 2014, 50, 57–63. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Liu, Z.; Chen, B. Effects of Major Parameters of Nanoparticles on Their Physical and Chemical Properties and Recent Application of Nanodrug Delivery System in Targeted Chemotherapy. Int. J. Nanomed. 2017, 12, 8483. [Google Scholar] [CrossRef] [Green Version]
- Pittella, F.; Zhang, M.; Lee, Y.; Kim, H.J.; Tockary, T.; Osada, K.; Ishii, T.; Miyata, K.; Nishiyama, N.; Kataoka, K. Enhanced Endosomal Escape of SiRNA-Incorporating Hybrid Nanoparticles from Calcium Phosphate and PEG-Block Charge-Conversional Polymer for Efficient Gene Knockdown with Negligible Cytotoxicity. Biomaterials 2011, 32, 3106–3114. [Google Scholar] [CrossRef]
- Graf, C.; Gao, Q.; Schütz, I.; Noufele, C.N.; Ruan, W.; Posselt, U.; Korotianskiy, E.; Nordmeyer, D.; Rancan, F.; Hadam, S. Surface Functionalization of Silica Nanoparticles Supports Colloidal Stability in Physiological Media and Facilitates Internalization in Cells. Langmuir 2012, 28, 7598–7613. [Google Scholar] [CrossRef]
- Tang, J.; Li, L.; Howard, C.B.; Mahler, S.M.; Huang, L.; Xu, Z.P. Preparation of Optimized Lipid-Coated Calcium Phosphate Nanoparticles for Enhanced in vitro Gene Delivery to Breast Cancer Cells. J. Mater. Chem. B 2015, 3, 6805–6812. [Google Scholar] [CrossRef]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide Nanoparticle Uptake in Human Lung Fibroblasts: Effects of Particle Size, Agglomeration, and Diffusion at Low Concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef]
- Herd, H.; Daum, N.; Jones, A.T.; Huwer, H.; Ghandehari, H.; Lehr, C.-M. Nanoparticle Geometry and Surface Orientation Influence Mode of Cellular Uptake. ACS Nano 2013, 7, 1961–1973. [Google Scholar] [CrossRef] [Green Version]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The Shape of Things to Come: Importance of Design in Nanotechnology for Drug Delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Iftode, A.; Drăghici, G.A.; Macașoi, I.; Marcovici, I.; Coricovac, D.E.; Dragoi, R.; Tischer, A.; Kovatsi, L.; Tsatsakis, A.M.; Cretu, O.; et al. Exposure to Cadmium and Copper Triggers Cytotoxic Effects and Epigenetic Changes in Human Colorectal Carcinoma HT-29 Cells. Exp. Ther. Med. 2021, 21, 100. [Google Scholar] [CrossRef]
- Krasanakis, T.; Nikolouzakis, T.K.; Sgantzos, M.; Mariolis-Sapsakos, T.; Souglakos, J.; Spandidos, D.A.; Tsitsimpikou, C.; Tsatsakis, A.; Tsiaoussis, J. Role of Anabolic Agents in Colorectal Carcinogenesis: Myths and Realities (Review). Oncol. Rep. 2019, 42, 2228–2244. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, N.; Mahipal, A.; Kim, R. Viral Vector Vaccines to Treat Colorectal Cancer. Curr. Colorectal Cancer Rep. 2013, 9, 398–405. [Google Scholar] [CrossRef]
- Cheng, X.J.; Lin, J.C.; Ding, Y.F.; Zhu, L.; Ye, J.; Tu, S.P. Survivin Inhibitor YM155 Suppresses Gastric Cancer Xenograft Growth in Mice without Affecting Normal Tissues. Oncotarget 2016, 7, 7096–7109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basak, S.; Eck, S.; Gutzmer, R.; Smith, A.J.; Birebent, B.; Purev, E.; Staib, L.; Somasundaram, R.; Zaloudik, J.; Li, W.; et al. Colorectal Cancer Vaccines: Antiidiotypic Antibody, Recombinant Protein, and Viral Vector. Ann. N. Y. Acad. Sci. 2000, 910, 237–253. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Lackmann, M.; Achen, M.G.; Stacker, S.A. Vascular Remodeling in Cancer. Oncogene 2014, 33, 3496–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y. Tumor Angiogenesis and Molecular Targets for Therapy. Front. Biosci. Landmark Ed. 2009, 14, 3962–3973. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for Its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, M.S.; Procopio, W.N.; Makonnen, S.; Feldman, M.D.; Yeilding, N.M.; Lee, W.M. Tumor Vessel Development and Maturation Impose Limits on the Effectiveness of Anti-Vascular Therapy. Am. J. Pathol. 2003, 162, 183–193. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.M.; Baluk, P. Significance of Blood Vessel Leakiness in Cancer. Cancers Res. 2002, 62, 5381–5385. [Google Scholar]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of Transport Pathways in Tumor Vessels: Role of Tumor Type and Microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lo, C.; Hsiue, G. Multifunctional Nanomicellar Systems for Delivering Anticancer Drugs. J. Biomed. Mater. Res. A 2014, 102, 2024–2038. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for Imaging and Therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabizon, A.A.; Shmeeda, H.; Zalipsky, S. Pros and Cons of the Liposome Platform in Cancer Drug Targeting. J. Liposome Res. 2006, 16, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.M.; Stewart, P.L.; Steinmetz, N.F. Bioinspired Shielding Strategies for Nanoparticle Drug Delivery Applications. Mol. Pharm. 2018, 15, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D. Schubert, U.S. Poly (Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the “PEG Dilemma” in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The Transferrin Receptor Part I: Biology and Targeting with Cytotoxic Antibodies for the Treatment of Cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Prutki, M.; Poljak-Blazi, M.; Jakopovic, M.; Tomas, D.; Stipancic, I.; Zarkovic, N. Altered Iron Metabolism, Transferrin Receptor 1 and Ferritin in Patients with Colon Cancer. Cancer Lett. 2006, 238, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Shah, Y.M. Intestinal Iron Homeostasis and Colon Tumorigenesis. Nutrients 2013, 5, 2333–2351. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, F.; Matsunaga, N.; Okazaki, H.; Utoguchi, N.; Suzuki, R.; Maruyama, K.; Koyanagi, S.; Ohdo, S. Circadian Rhythm of Transferrin Receptor 1 Gene Expression Controlled by C-Myc in Colon Cancer–Bearing Mice. Cancer Res. 2010, 70, 6238–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardoiwala, M.N.; Kushwaha, A.C.; Dev, A.; Shrimali, N.; Guchhait, P.; Karmakar, S.; Roy Choudhury, S. Hypericin-Loaded Transferrin Nanoparticles Induce PP2A-Regulated BMI1 Degradation in Colorectal Cancer-Specific Chemo-Photodynamic Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3139–3153. [Google Scholar] [CrossRef]
- Wei, Y.; Gu, X.; Sun, Y.; Meng, F.; Storm, G.; Zhong, Z. Transferrin-Binding Peptide Functionalized Polymersomes Mediate Targeted Doxorubicin Delivery to Colorectal Cancer in Vivo. J. Control. Release 2020, 319, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Kumari, S.; Kondapi, A.K. Evaluation of Antiproliferative Activity, Safety and Biodistribution of Oxaliplatin and 5-Fluorouracil Loaded Lactoferrin Nanoparticles for the Management of Colon Adenocarcinoma: An in vitro and an in Vivo Study. Pharm. Res. 2018, 35, 1–17. [Google Scholar] [CrossRef]
- Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Dorkoosh, F.A.; Handali, S. Transferrin Targeted Liposomal 5-Fluorouracil Induced Apoptosis via Mitochondria Signaling Pathway in Cancer Cells. Life Sci. 2018, 194, 104–110. [Google Scholar] [CrossRef]
- Quici, S.; Casoni, A.; Foschi, F.; Armelao, L.; Bottaro, G.; Seraglia, R.; Bolzati, C.; Salvarese, N.; Carpanese, D.; Rosato, A. Folic Acid-Conjugated Europium Complexes as Luminescent Probes for Selective Targeting of Cancer Cells. J. Med. Chem. 2015, 58, 2003–2014. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in Targeting the Folate Receptor in the Treatment/Imaging of Cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Tiernan, J.; Perry, S.; Verghese, E.; West, N.; Yeluri, S.; Jayne, D.; Hughes, T. Carcinoembryonic Antigen Is the Preferred Biomarker for in Vivo Colorectal Cancer Targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Chang, M.-C.; Huang, C.-Y.; Chiang, Y.-C.; Lin, H.-W.; Chen, C.-A.; Hsieh, C.-Y.; Cheng, W.-F. Serous Ovarian Carcinoma Patients with High Alpha-Folate Receptor Had Reducing Survival and Cytotoxic Chemo-Response. Mol. Oncol. 2012, 6, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Süntar, I.; Yakıncı, Ö.F. Potential risks of phytonutrients associated with high-dose or long-term use. In Phytonutrients in Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–155. [Google Scholar]
- Vlahov, I.R.; Leamon, C.P. Engineering Folate–Drug Conjugates to Target Cancer: From Chemistry to Clinic. Bioconjug. Chem. 2012, 23, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Canevari, S.; Thigpen, T. Targeting the Folate Receptor: Diagnostic and Therapeutic Approaches to Personalize Cancer Treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef]
- Ruman, U.; Buskaran, K.; Pastorin, G.; Masarudin, M.J.; Fakurazi, S.; Hussein, M.Z. Synthesis and Characterization of Chitosan-Based Nanodelivery Systems to Enhance the Anticancer Effect of Sorafenib Drug in Hepatocellular Carcinoma and Colorectal Adenocarcinoma Cells. Nanomaterials 2021, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.C.; Zerillo, L.; Cruz, L.J.; Schomann, T.; Chan, A.B.; de Carvalho, T.G.; Souza, S.V.; de Araújo, A.A.; de Geus-Oei, L.-F.; de Araújo Júnior, R.F. Maximizing the Potency of Oxaliplatin Coated Nanoparticles with Folic Acid for Modulating Tumor Progression in Colorectal Cancer. Mater. Sci. Eng. C 2021, 120, 111678. [Google Scholar] [CrossRef]
- Martín, M.J.; Azcona, P.; Lassalle, V.; Gentili, C. Doxorubicin Delivery by Magnetic Nanotheranostics Enhances the Cell Death in Chemoresistant Colorectal Cancer-Derived Cells. Eur. J. Pharm. Sci. 2021, 158, 105681. [Google Scholar] [CrossRef]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.-H.; Ku, S.K. Folate-Targeted Nanostructured Chitosan/Chondroitin Sulfate Complex Carriers for Enhanced Delivery of Bortezomib to Colorectal Cancer Cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Rajpoot, K.; Jain, S.K. Oral Delivery of PH-Responsive Alginate Microbeads Incorporating Folic Acid-Grafted Solid Lipid Nanoparticles Exhibits Enhanced Targeting Effect against Colorectal Cancer: A Dual-Targeted Approach. Int. J. Biol. Macromol. 2020, 151, 830–844. [Google Scholar] [CrossRef]
- Ansari, L.; Derakhshi, M.; Bagheri, E.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Folate Conjugation Improved Uptake and Targeting of Porous Hydroxyapatite Nanoparticles Containing Epirubicin to Cancer Cells. Pharm. Dev. Technol. 2020, 25, 601–609. [Google Scholar] [CrossRef]
- Kumar, C.S.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted Delivery and Apoptosis Induction of Trans-Resveratrol-Ferulic Acid Loaded Chitosan Coated Folic Acid Conjugate Solid Lipid Nanoparticles in Colon Cancer Cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Xie, L.; Huang, A.; Xue, C.; Gu, Z.; Wang, K.; Zong, S. The Prognostic and Clinical Value of CD44 in Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 309. [Google Scholar] [CrossRef]
- Du, L.; Wang, H.; He, L.; Zhang, J.; Ni, B.; Wang, X.; Jin, H.; Cahuzac, N.; Mehrpour, M.; Lu, Y. CD44 Is of Functional Importance for Colorectal Cancer Stem Cells. Clin. Cancer Res. 2008, 14, 6751–6760. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.; Kim, H.J.; Kim, C.H.; Kim, Y.J.; Lee, J.H.; Kim, H.R. Colon Cancer Stem Cell Markers CD44 and CD133 in Patients with Colorectal Cancer and Synchronous Hepatic Metastases. Int. J. Oncol. 2015, 46, 1582–1588. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Abedi-Gaballu, F.; Abbaspour, S.; Ghasabi, M.; Yekta, R.; Shirjang, S.; Dehghan, G.; Hamblin, M.R.; Baradaran, B. Hyaluronic Acid-decorated Liposomal Nanoparticles for Targeted Delivery of 5-fluorouracil into HT-29 Colorectal Cancer Cells. J. Cell. Physiol. 2020, 235, 6817–6830. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.C.; Krishnan, V.; Salinas, A.K.; Kim, J.; Sun, T.; Ravid, S.; Peng, K.; Wu, D.; Nurunnabi, M.; Nelson, J.A. Hyaluronic a Cid–Doxorubicin Nanoparticles for Targeted Treatment of Colorectal Cancer. Bioeng. Transl. Med. 2021, 6, e10166. [Google Scholar] [CrossRef]
- Qu, C.-Y.; Zhou, M.; Chen, Y.; Chen, M.; Shen, F.; Xu, L.-M. Engineering of Lipid Prodrug-Based, Hyaluronic Acid-Decorated Nanostructured Lipid Carriers Platform for 5-Fluorouracil and Cisplatin Combination Gastric Cancer Therapy. Int. J. Nanomed. 2015, 10, 3911. [Google Scholar]
- Wang, Z.; Zang, A.; Wei, Y.; An, L.; Hong, D.; Shi, Y.; Zhang, J.; Su, S.; Fang, G. Hyaluronic Acid Capped, Irinotecan and Gene Co-Loaded Lipid-Polymer Hybrid Nanocarrier-Based Combination Therapy Platform for Colorectal Cancer. Drug Des. Devel. Ther. 2020, 14, 1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, M.; Cao, N.; Qin, W.; Zhao, M.; Wu, J.; Lin, D. Construction of a Tumor Microenvironment PH-Responsive Cleavable PEGylated Hyaluronic Acid Nano-Drug Delivery System for Colorectal Cancer Treatment. Biomater. Sci. 2020, 8, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Liszbinski, R.B.; Romagnoli, G.G.; Gorgulho, C.M.; Basso, C.R.; Pedrosa, V.A.; Kaneno, R. Anti-EGFR-Coated Gold Nanoparticles in vitro Carry 5-Fluorouracil to Colorectal Cancer Cells. Materials 2020, 13, 375. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S. Anti-EGFR-MAb and 5-Fluorouracil Conjugated Polymeric Nanoparticle for Colorectal Cancer. Recent Pat. Anticancer Drug Discov. 2020. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Wang, L.; Zhou, J.; Tan, Y.; Peng, C.; Yang, P.; Peng, W.; Li, J.; Gu, Q. Cetuximab Functionalization Strategy for Combining Active Targeting and Antimigration Capacities of a Hybrid Composite Nanoplatform Applied to Deliver 5-Fluorouracil: Toward Colorectal Cancer Treatment. Biomater. Sci. 2021, 9, 2279–2294. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Tabarzad, M.; Ranjbari, J.; de la Guardia, M.; Hejazi, M.; Ramezani, M. Aptamers as Smart Ligands for Nano-Carriers Targeting. TrAC Trends Anal. Chem. 2016, 82, 316–327. [Google Scholar] [CrossRef]
- Yao, F.; An, Y.; Li, X.; Li, Z.; Duan, J.; Yang, X.-D. Targeted Therapy of Colon Cancer by Aptamer-Guided Holliday Junctions Loaded with Doxorubicin. Int. J. Nanomed. 2020, 15, 2119. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.G.; Surendran, S.P.; Jeong, Y.Y. Tumor Microenvironment-Stimuli Responsive Nanoparticles for Anticancer Therapy. Front. Mol. Biosci. 2020, 7, 610533. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Yu, L.; Chen, X.; Sun, X.; Wang, L.; Chen, S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J. Cancer 2017, 8, 3430–3440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Priyadarshi, R.; Deeba, F.; Kulshreshtha, A.; Kumar, A.; Agrawal, G.; Gopinath, P.; Negi, Y.S. Redox Responsive Xylan-SS-Curcumin Prodrug Nanoparticles for Dual Drug Delivery in Cancer Therapy. Mater. Sci. Eng. C 2020, 107, 110356. [Google Scholar]
- Juang, V.; Chang, C.; Wang, C.; Wang, H.; Lo, Y. PH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and MicroRNA to Enhance Tumor-Specific Therapy. Small 2019, 15, 1903296. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wei, Y.; Fan, Q.; Sun, H.; Cheng, R.; Zhong, Z.; Deng, C. CRGD-Decorated Biodegradable Polytyrosine Nanoparticles for Robust Encapsulation and Targeted Delivery of Doxorubicin to Colorectal Cancer in Vivo. J. Control. Release 2019, 301, 110–118. [Google Scholar] [CrossRef]
- Gogineni, V.R.; Maddirela, D.R.; Park, W.; Jagtap, J.M.; Parchur, A.K.; Sharma, G.; Ibrahim, E.-S.; Joshi, A.; Larson, A.C.; Kim, D.-H.; et al. Localized and Triggered Release of Oxaliplatin for the Treatment of Colorectal Liver Metastasis. J. Cancer 2020, 11, 6982–6991. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Gao, S.; Shui, S.; Liu, S.; Qu, H.; Liu, C.; Zheng, L. Small Interfering RNA-Loaded Chitosan Hydrochloride/Carboxymethyl Chitosan Nanoparticles for Ultrasound-Triggered Release to Hamper Colorectal Cancer Growth in Vitro. Int. J. Biol. Macromol. 2020, 162, 1303–1310. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Kim, B.S. NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells. Cells 2020, 9, 2122. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Singh, M. Selenium Nanoparticles: Potential in Cancer Gene and Drug Delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Abedini, F.; Hosseinkhani, H.; Ismail, M.; Domb, A.J.; Omar, A.R.; Chong, P.P.; Hong, P.-D.; Yu, D.-S.; Farber, I.-Y. Cationized Dextran Nanoparticle-Encapsulated CXCR4-SiRNA Enhanced Correlation between CXCR4 Expression and Serum Alkaline Phosphatase in a Mouse Model of Colorectal Cancer. Int. J. Nanomed. 2012, 7, 4159. [Google Scholar]
- Marquez, J.; Fernandez-Piñeiro, I.; Araúzo-Bravo, M.J.; Poschmann, G.; Stühler, K.; Khatib, A.; Sanchez, A.; Unda, F.; Ibarretxe, G.; Bernales, I. Targeting Liver Sinusoidal Endothelial Cells with Mi R-20a-loaded Nanoparticles Reduces Murine Colon Cancer Metastasis to the Liver. Int. J. Cancer 2018, 143, 709–719. [Google Scholar] [CrossRef]
- Bangham, A.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–IN27. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., II; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [Green Version]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3. [Google Scholar] [CrossRef]
- Gonda, A.; Zhao, N.; Shah, J.V.; Calvelli, H.R.; Kantamneni, H.; Francis, N.L.; Ganapathy, V. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med. One 2019, 4, e190021. [Google Scholar] [PubMed] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition Design and Medical Application of Liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A. A Novel 5-Fluorouracil Targeted Delivery to Colon Cancer Using Folic Acid Conjugated Liposomes. Biomed. Pharmacother. 2018, 108, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Zhu, W.-T.; Lin, C.-Y.; Yuan, Z.-W.; Li, Z.-H.; Yan, P.-K. Delivery of Rapamycin by Liposomes Synergistically Enhances the Chemotherapy Effect of 5-Fluorouracil on Colorectal Cancer. Int. J. Nanomed. 2021, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Yang, D.; Zhang, J.; Ma, J.; Cheng, D.; Chen, J.; Deng, L. Novel SN38 Derivative-Based Liposome as Anticancer Prodrug: An in vitro and in Vivo Study. Int. J. Nanomed. 2019, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Pinel, B.; Jabalera, Y.; Ortiz, R.; Cabeza, L.; Jimenez-Lopez, C.; Melguizo, C.; Prados, J. Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In vitro Study for Potential Application in Colon Cancer. Pharmaceutics 2020, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Liposomes: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Xiang, B.; Cao, D.-Y. Preparation of Drug Liposomes by Thin-Film Hydration and Homogenization. In Liposome-Based Drug Delivery Systems; Lu, W.-L., Qi, X.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–11. ISBN 978-3-662-49231-4. [Google Scholar]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. MTOR Signaling in Cancer and MTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef] [Green Version]
- Marquard, F.E.; Jücker, M. PI3K/AKT/MTOR Signaling as a Molecular Target in Head and Neck Cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef]
- Casadó, A.; Sagristá, M.L.; Mora, M. A Novel Microfluidic Liposomal Formulation for the Delivery of the SN-38 Camptothecin: Characterization and in vitro Assessment of Its Cytotoxic Effect on Two Tumor Cell Lines. Int. J. Nanomed. 2018, 13, 5301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, Y.; He, Y.; Wang, H.; Chen, B.; Tu, B.; Zhu, S.; Huang, Y. Anti-PD-L1 Mediating Tumor-Targeted Codelivery of Liposomal Irinotecan/JQ1 for Chemo-Immunotherapy. Acta Pharmacol. Sin. 2020, 1–8. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, X.; Wang, Z.; Zhang, H.; Chen, P.; Zhou, G.; Sun, C.; Gu, N.; Ji, M. Novel Lipophilic SN38 Prodrug Forming Stable Liposomes for Colorectal Carcinoma Therapy. Int. J. Nanomed. 2019, 14, 5201. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-R.; Lee, M.-H.; Li, W.-S.; Wu, H.-C. Liposomal Irinotecan for Treatment of Colorectal Cancer in a Preclinical Model. Cancers 2019, 11, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Üner, M.; Yener, G. Importance of Solid Lipid Nanoparticles (SLN) in Various Administration Routes and Future Perspectives. Int. J. Nanomed. 2007, 2, 289. [Google Scholar]
- López-García, R.; Ganem-Rondero, A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of Smart Solid Lipid Nanoparticles to Enhance the Efficacy of 5-Fluorouracil in the Treatment of Colorectal Cancer. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Schaffazick, S.R.; Pohlmann, A.R.; Dalla-Costa, T.; Guterres, S.S. Freeze-Drying Polymeric Colloidal Suspensions: Nanocapsules, Nanospheres and Nanodispersion. A Comparative Study. Eur. J. Pharm. Biopharm. 2003, 56, 501–505. [Google Scholar] [CrossRef]
- García, M.C. Nano-and microparticles as drug carriers. In Engineering Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–110. [Google Scholar]
- Lu, X.-Y.; Wu, D.-C.; Li, Z.-J.; Chen, G.-Q. Polymer Nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 299–323. [Google Scholar] [PubMed]
- Tan, C.; Arshadi, M.; Lee, M.C.; Godec, M.; Azizi, M.; Yan, B.; Eskandarloo, H.; Deisenroth, T.W.; Darji, R.H.; Pho, T.V. A Robust Aqueous Core–Shell–Shell Coconut-like Nanostructure for Stimuli-Responsive Delivery of Hydrophilic Cargo. ACS Nano 2019, 13, 9016–9027. [Google Scholar] [CrossRef]
- Qian, K.; Wu, J.; Zhang, E.; Zhang, Y.; Fu, A. Biodegradable Double Nanocapsule as a Novel Multifunctional Carrier for Drug Delivery and Cell Imaging. Int. J. Nanomed. 2015, 10, 4149. [Google Scholar]
- Johnston, A.P.; Cortez, C.; Angelatos, A.S.; Caruso, F. Layer-by-Layer Engineered Capsules and Their Applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 203–209. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural Biodegradable Polymers Based Nano-Formulations for Drug Delivery: A Review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st Century: Synthetic Polymers in Drug Delivery Applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Catalin Balaure, P.; Mihai Grumezescu, A. Smart Synthetic Polymer Nanocarriers for Controlled and Site-Specific Drug Delivery. Curr. Top. Med. Chem. 2015, 15, 1424–1490. [Google Scholar] [CrossRef]
- Osorio, M.; Martinez, E.; Naranjo, T.; Castro, C. Recent Advances in Polymer Nanomaterials for Drug Delivery of Adjuvants in Colorectal Cancer Treatment: A Scientific-Technological Analysis and Review. Molecules 2020, 25, 2270. [Google Scholar] [CrossRef]
- Luss, A.L.; Kulikov, P.P.; Romme, S.B.; Andersen, C.L.; Pennisi, C.P.; Docea, A.O.; Kuskov, A.N.; Velonia, K.; Mezhuev, Y.O.; Shtilman, M.I. Nanosized Carriers Based on Amphiphilic Poly-N-Vinyl-2-Pyrrolidone for Intranuclear Drug Delivery. Nanomedicine 2018, 13, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Piñón-Castillo, H.A.; Martínez-Chamarro, R.; Reyes-Martínez, R.; Salinas-Vera, Y.M.; Manjarrez-Nevárez, L.A.; Muñoz-Castellanos, L.N.; López-Camarillo, C.; Orrantia-Borunda, E. Palladium Nanoparticles Functionalized with PVP-Quercetin Inhibits Cell Proliferation and Activates Apoptosis in Colorectal Cancer Cells. Appl. Sci. 2021, 11, 1988. [Google Scholar] [CrossRef]
- Radu, I.C.; Hudita, A.; Zaharia, C.; Galateanu, B.; Iovu, H.; Tanasa, E.; Georgiana Nitu, S.; Ginghina, O.; Negrei, C.; Tsatsakis, A. Poly (3-Hydroxybutyrate-CO-3-Hydroxyvalerate) PHBHV Biocompatible Nanocarriers for 5-FU Delivery Targeting Colorectal Cancer. Drug Deliv. 2019, 26, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Saremy, S.; Dorkoosh, F.A. Co-Delivery of 5-Fluorouracil and Oxaliplatin in Novel Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate Acid)/Poly (Lactic-Co-Glycolic Acid) Nanoparticles for Colon Cancer Therapy. Int. J. Biol. Macromol. 2019, 124, 1299–1311. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, H.; Zhuang, Y.; Sun, X.; Gu, N. Combined Therapeutic Effects of 131I-Labeled and 5Fu-Loaded Multifunctional Nanoparticles in Colorectal Cancer. Int. J. Nanomed. 2020, 15, 2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced Antitumor Efficacy in Colon Cancer Using EGF Functionalized PLGA Nanoparticles Loaded with 5-Fluorouracil and Perfluorocarbon. BMC Cancer 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shad, P.M.; Karizi, S.Z.; Javan, R.S.; Mirzaie, A.; Noorbazargan, H.; Akbarzadeh, I.; Rezaie, H. Folate Conjugated Hyaluronic Acid Coated Alginate Nanogels Encapsulated Oxaliplatin Enhance Antitumor and Apoptosis Efficacy on Colorectal Cancer Cells (HT29 Cell Line). Toxicology 2020, 65, 104756. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Qiu, Y.; Ding, M.; Zhang, Y. MiRNA-204-5p and Oxaliplatin-Loaded Silica Nanoparticles for Enhanced Tumor Suppression Effect in CD44-Overexpressed Colon Adenocarcinoma. Int. J. Pharm. 2019, 566, 585–593. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, X.; Chen, Y.; Lee, R.J.; Wang, J.; Yao, J.; Zhang, Y.; Zhang, C.; Wang, K.; Yu, B. Anticancer Activity of Polymeric Nanoparticles Containing Linoleic Acid-SN38 (LA-SN38) Conjugate in a Murine Model of Colorectal Cancer. Colloids Surf. B Biointerfaces 2019, 181, 822–829. [Google Scholar] [CrossRef]
- Salmanpour, M.; Yousefi, G.; Samani, S.M.; Mohammadi, S.; Anbardar, M.H.; Tamaddon, A. Nanoparticulate Delivery of Irinotecan Active Metabolite (SN38) in Murine Colorectal Carcinoma through Conjugation to Poly (2-Ethyl 2-Oxazoline)-b-Poly (L-Glutamic Acid) Double Hydrophilic Copolymer. Eur. J. Pharm. Sci. 2019, 136, 104941. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Alibolandi, M.; Taghdisi, S.M.; Ramezani, P.; Shamili, F.H.; Farzad, S.A.; Abnous, K.; Ramezani, M. Smart AS1411-Aptamer Conjugated Pegylated PAMAM Dendrimer for the Superior Delivery of Camptothecin to Colon Adenocarcinoma in vitro and in Vivo. Int. J. Pharm. 2017, 519, 352–364. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-Assembled Lipid− Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, L. Lipid–Polymer Hybrid Nanoparticles: Synthesis, Characterization and Applications. Nano Life 2010, 1, 163–173. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-Polymer Hybrid Nanoparticles: Synthesis Strategies and Biomedical Applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–Lecithin–PEG Core–Shell Nanoparticles for Controlled Drug Delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Kadletz, L.; Heiduschka, G.; Domayer, J.; Schmid, R.; Enzenhofer, E.; Thurnher, D. Evaluation of Spheroid Head and Neck Squamous Cell Carcinoma Cell Models in Comparison to Monolayer Cultures. Oncol. Lett. 2015, 10, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures–a Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. AMS 2018, 14, 910. [Google Scholar] [CrossRef]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In vitro Three-dimensional (3D) Models in Cancer Research: An Update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Liu, X.; Raju, P. In vitro Cancer Model for Drug Testing. In Comprehensive Biotechnology. Vol 5: Medical Biotechnology and Healthcare, 2nd ed.; Moo-Young, M., Ed.; Academic Press: London, UK, 2011; pp. 543–549. ISBN 9780080885049. [Google Scholar]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular Tumor Spheroids: An Underestimated Tool Is Catching up Again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Goodman, T.T.; Ng, C.P.; Pun, S.H. 3-D Tissue Culture Systems for the Evaluation and Optimization of Nanoparticle-Based Drug Carriers. Bioconjug. Chem. 2008, 19, 1951–1959. [Google Scholar] [CrossRef] [Green Version]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; Affram, K.; Bulumko, E.; Agyare, E. Evaluation of In-Vitro Cytotoxic Effect of 5-FU Loaded-Chitosan Nanoparticles against Spheroid Models. J. Nat. Sci. 2018, 4, e535. [Google Scholar]
- Tchoryk, A.; Taresco, V.; Argent, R.H.; Ashford, M.; Gellert, P.R.; Stolnik, S.; Grabowska, A.; Garnett, M.C. Penetration and Uptake of Nanoparticles in 3D Tumor Spheroids. Bioconjug. Chem. 2019, 30, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; Feijão, T.; Gonçalves, A.; Shahbazi, M.-A.; Liu, Z.; Barrias, C.; Oliveira, M.J.; Granja, P.; Santos, H.A.; Sarmento, B. Colorectal Cancer Triple Co-Culture Spheroid Model to Assess the Biocompatibility and Anticancer Properties of Polymeric Nanoparticles. J. Control. Release 2020, 323, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Cekanova, M.; Rathore, K. Animal Models and Therapeutic Molecular Targets of Cancer: Utility and Limitations. Drug Des. Devel. Ther. 2014, 8, 1911–1921. [Google Scholar] [CrossRef] [Green Version]
- Corpet, D.E.; Pierre, F. How Good Are Rodent Models of Carcinogenesis in Predicting Efficacy in Humans? A Systematic Review and Meta-Analysis of Colon Chemoprevention in Rats, Mice and Men. Eur. J. Cancer 2005, 41, 1911–1922. [Google Scholar] [CrossRef]
- Cabeza, L.; Perazzoli, G.; Mesas, C.; Jiménez-Luna, C.; Prados, J.; Rama, A.R.; Melguizo, C. Nanoparticles in Colorectal Cancer Therapy: Latest In Vivo Assays, Clinical Trials, and Patents. AAPS PharmSciTech 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Miller, C.R.; Williams, C.R.; Buchsbaum, D.J.; Gillespie, G.Y. Intratumoral 5-Fluorouracil Produced by Cytosine Deaminase/5-Fluorocytosine Gene Therapy Is Effective for Experimental Human Glioblastomas. Cancer Res. 2002, 62, 773–780. [Google Scholar] [PubMed]
- Meng, H.; Leong, W.; Leong, K.W.; Chen, C.; Zhao, Y. Walking the Line: The Fate of Nanomaterials at Biological Barriers. Biomaterials 2018, 174, 41–53. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef]


| Stimuli | Nanosystem Description | Biological Investigation Highlights | Ref. |
|---|---|---|---|
| Redox-responsive | Xylan-SS-curcumin nanoparticles loaded with 5-FU prodrug (5-FU-stearic acid) | Low hemolytic activity. Higher cytotoxicity than free drugs on HT-29 and HCT-115 cells. | [131] |
| pH-responsive | O’-methyl polyethylene glycol (omPEG) IRI liposomes and omPEG miR-200 solid lipid nanoparticle, both functionalized with mitochondria-targeting peptide K (RFKH) | Potent inductor of apoptosis that modulates effects of β-catenin/Multidrug Resistance (MDR)/apoptosis/Epithelial to Mesenchymal Transition (EMT) signaling pathways. In vivo superior tumor growth inhibition and low cytotoxicity on non-cancerous cells. | [132] |
| Enzyme-responsive | Doxorubicin c-RGD polytyrosine nanoparticles | Efficiently internalized by αvβ5 overexpressing HCT-116 colorectal cancer cells and highly cytotoxic. Improve survival rate of tumor-bearing mice by efficient tumor growth inhibition compared with free DOX or DOX liposomal formulation. | [133] |
| Magnetic-responsive | Hybrid liposome-magnetic nanoparticles loaded with Cy5.5 dye and oxaliplatin | Magnetic field stimulation enhanced cytotoxicity of nanoparticles in CC-531 adenocarcinoma cell cultures and directed the selective delivery of oxaliplatin at high concentrations in the targeted tissue. | [134] |
| Ultrasound-responsive | Anti-β-catenin small interfering RNA-loaded chitosan hydrochloride/carboxymethyl chitosan nanoparticle | Efficiently internalized by HT-29 tumor cells and successfully suppress in vitro expression of β-catenin. | [135] |
| Light-responsive | Polythiophene nanoparticles | Exert no cytotoxicity on colon carcinoma CT-26 cells in the range of 25–250 µg/mL concentration, while NIR laser-triggered photothermal treatment in nanoparticle pretreated CT-26 cell cultures triggers reduction of cell viability and apoptosis. | [136] |
| Lipid Composition/Synthesis Method | Drug Cargo | Active Targeting | Ref. |
|---|---|---|---|
| DOTAP:DOPE:DSPE-PEG2000/thin layer film hydration method | 5-FU | HA for CD44 receptor targeting | [118] |
| PC:CHOL:DSPE/thin layer film hydration method | 5-FU | Transferrin for transferrin receptor (TFR) targeting | [100] |
| DPPC:CHOL:DSPE-PEG2000/thin layer film hydration method | 5-FU | Folate for folate receptor (FR) targeting | [147] |
| PC:DSPE-PEG2000/ethanol injection method | rapamycin | Not applicable (NA) | [148] |
| PC-98T:DSPE-PEG2000:CHOL/thin layer film hydration method | SN38 | HA | [149] |
| PC:DSPE-PEG2000/thin layer film hydration method | OXP | NA | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginghină, O.; Hudiță, A.; Zaharia, C.; Tsatsakis, A.; Mezhuev, Y.; Costache, M.; Gălățeanu, B. Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients. Materials 2021, 14, 2440. https://doi.org/10.3390/ma14092440
Ginghină O, Hudiță A, Zaharia C, Tsatsakis A, Mezhuev Y, Costache M, Gălățeanu B. Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients. Materials. 2021; 14(9):2440. https://doi.org/10.3390/ma14092440
Chicago/Turabian StyleGinghină, Octav, Ariana Hudiță, Cătălin Zaharia, Aristidis Tsatsakis, Yaroslav Mezhuev, Marieta Costache, and Bianca Gălățeanu. 2021. "Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients" Materials 14, no. 9: 2440. https://doi.org/10.3390/ma14092440
APA StyleGinghină, O., Hudiță, A., Zaharia, C., Tsatsakis, A., Mezhuev, Y., Costache, M., & Gălățeanu, B. (2021). Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients. Materials, 14(9), 2440. https://doi.org/10.3390/ma14092440











