Distillation of a Complex Mixture. Part II: Performance Analysis of a Distillation Column Using Exergy
Abstract
:1. Introduction
- Heat transfer due to a finite temperature difference δT
- Mass transfer due to the mixing liquid and vapour streams
- Heat losses through the column wall.
2. Theoretical Considerations
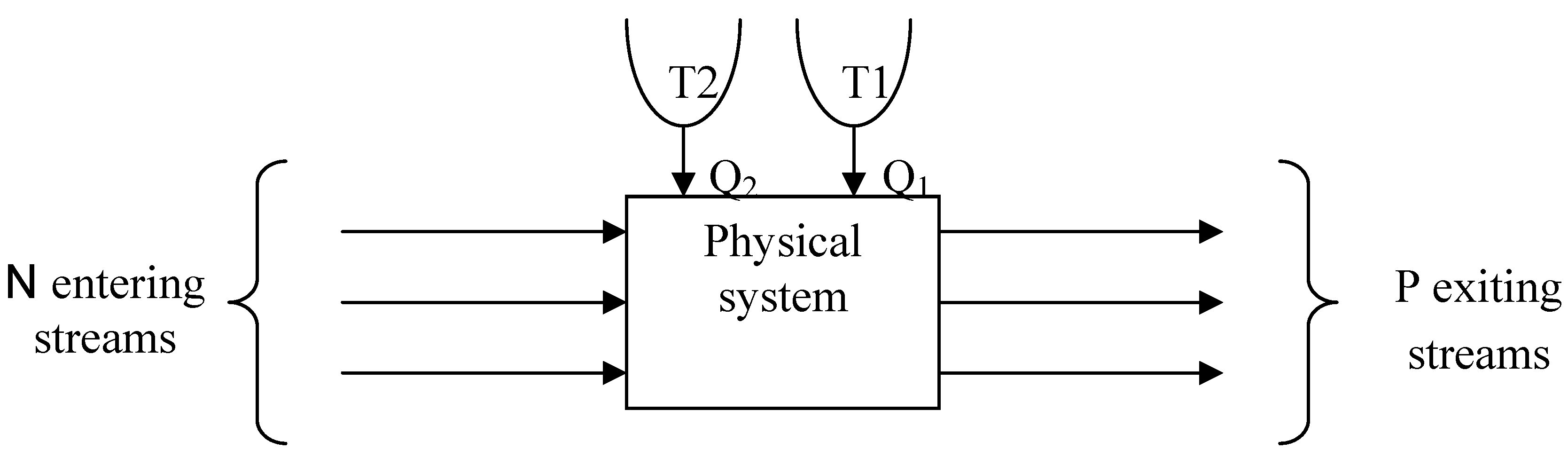
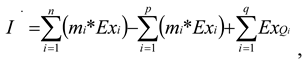
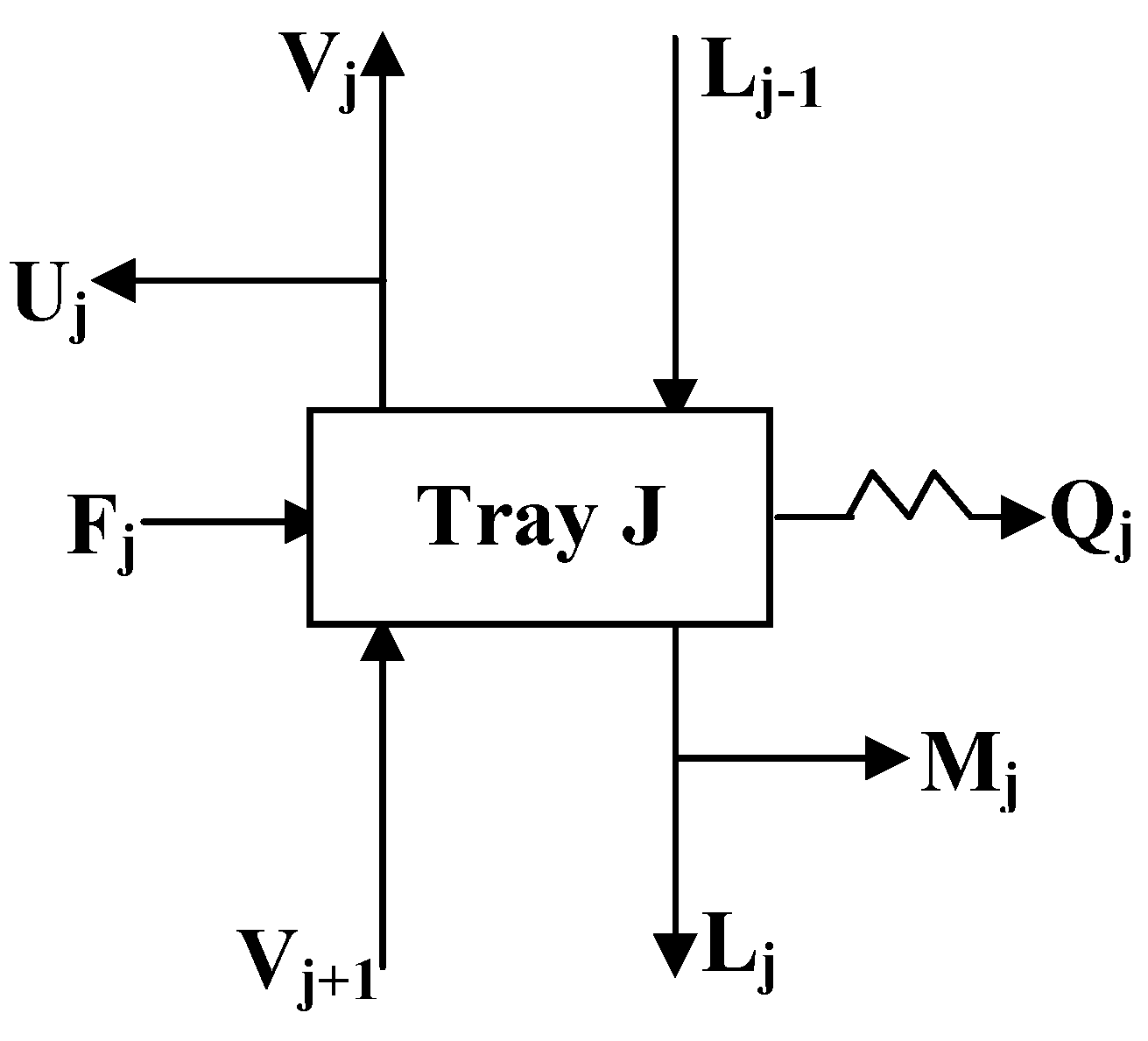
3. Simulation of the Column
- Negligible pressure losses.
- Adiabatic column except at the ends.
- The tray obeys the model of continuously stirred reactor.
- The compositions, the temperatures and the stream flow rates at each tray are determined by the iterative methods of nonlinear algebraic Thomas equations. In order to initialize calculations, it is supposed that the temperature profile is linear along the column with the temperatures at the ends (condenser and reboiler) determined by the method of bubble.
- The balance calculation is completed when convergence criteria are met.
- At this step, the program will calculate the exergy of the various streams after having considered the ambient conditions as the dead state whose temperature is T0 = 298.15 K.
- The last step of the execution consists in calculating the exergetic efficiency by using Equation (15).
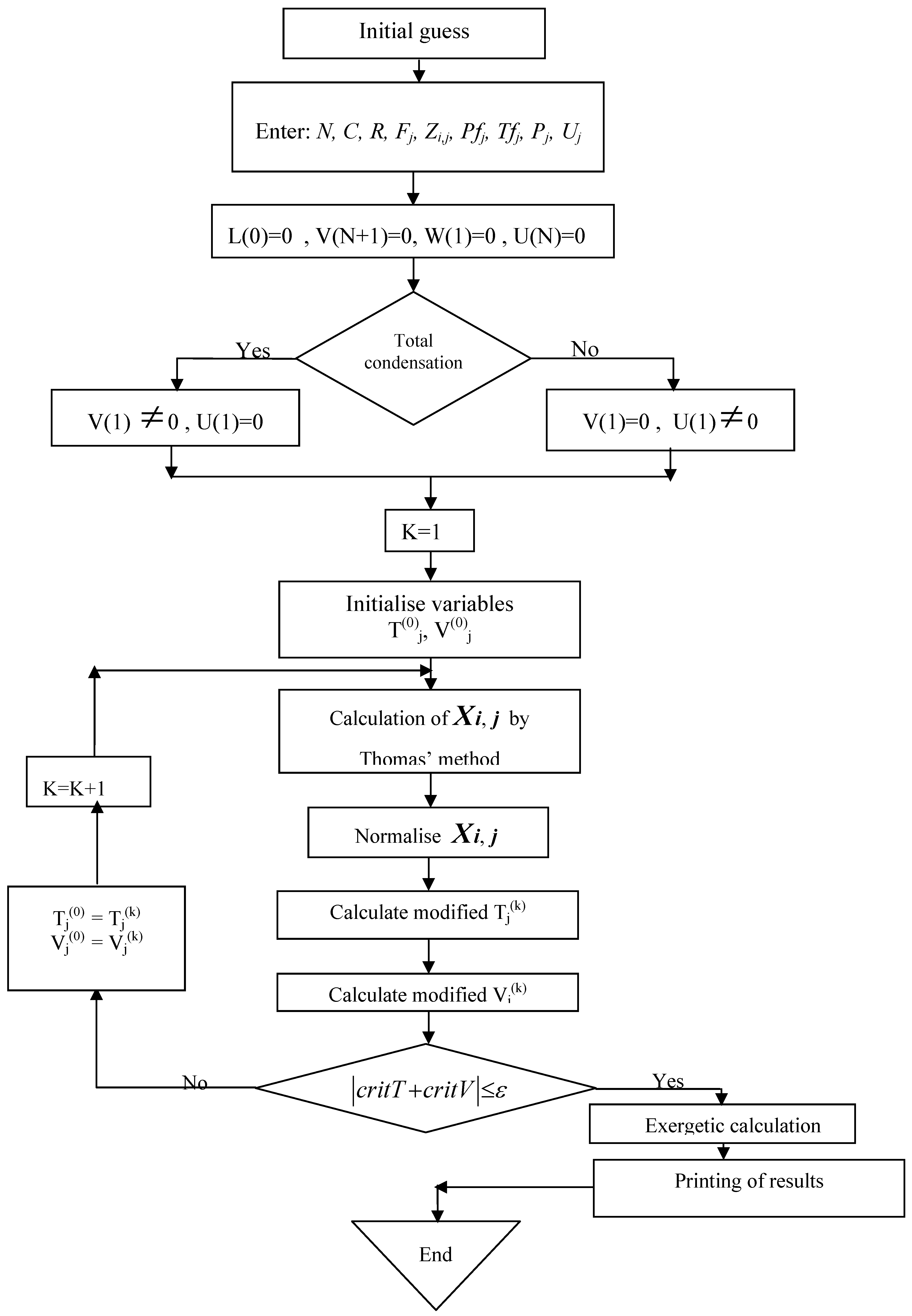
4. Results and Discussion
| Component | C1 | C2 | C3 | Iso-C4 | n-C4 | Iso-C5 | n-C5 | C6+ |
| Composition (% mol. ). | 1.00 | 3.00 | 55.00 | 10.50 | 25.00 | 5.00 | 0.50 | Traces |
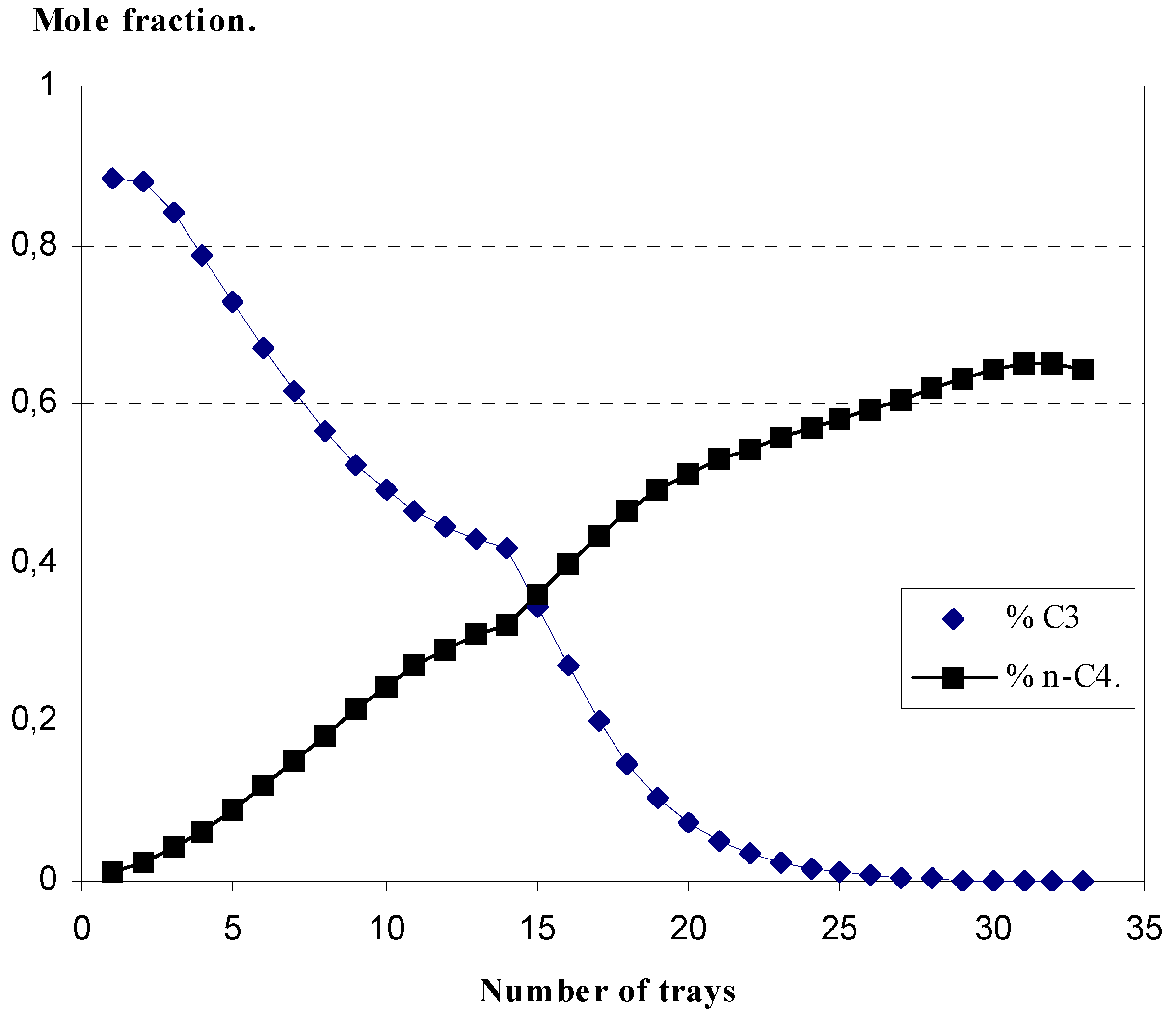
4.1. Profile of key component composition versus tray position
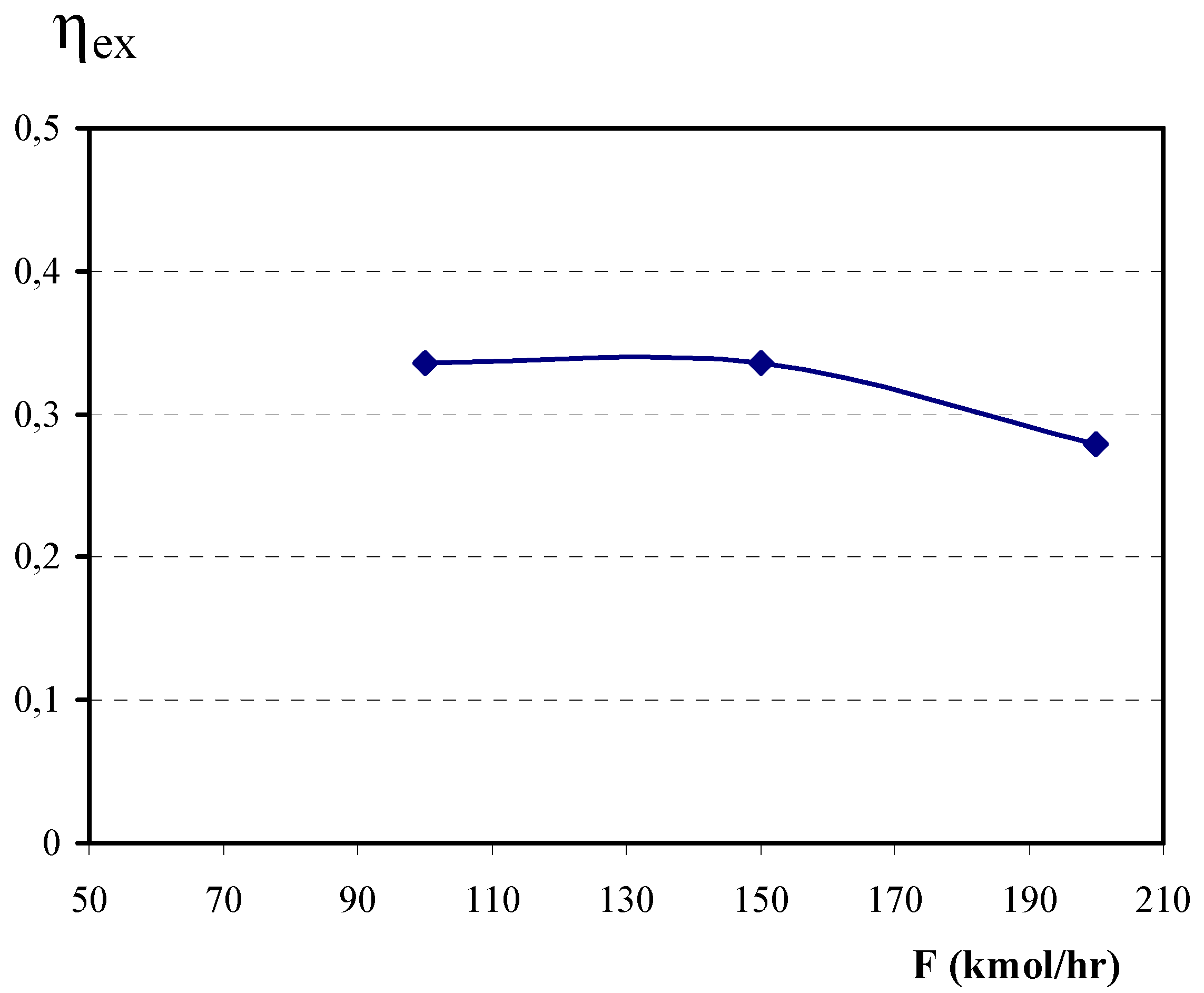
4.2. Influence of the feed rate on the exergetic efficiency.
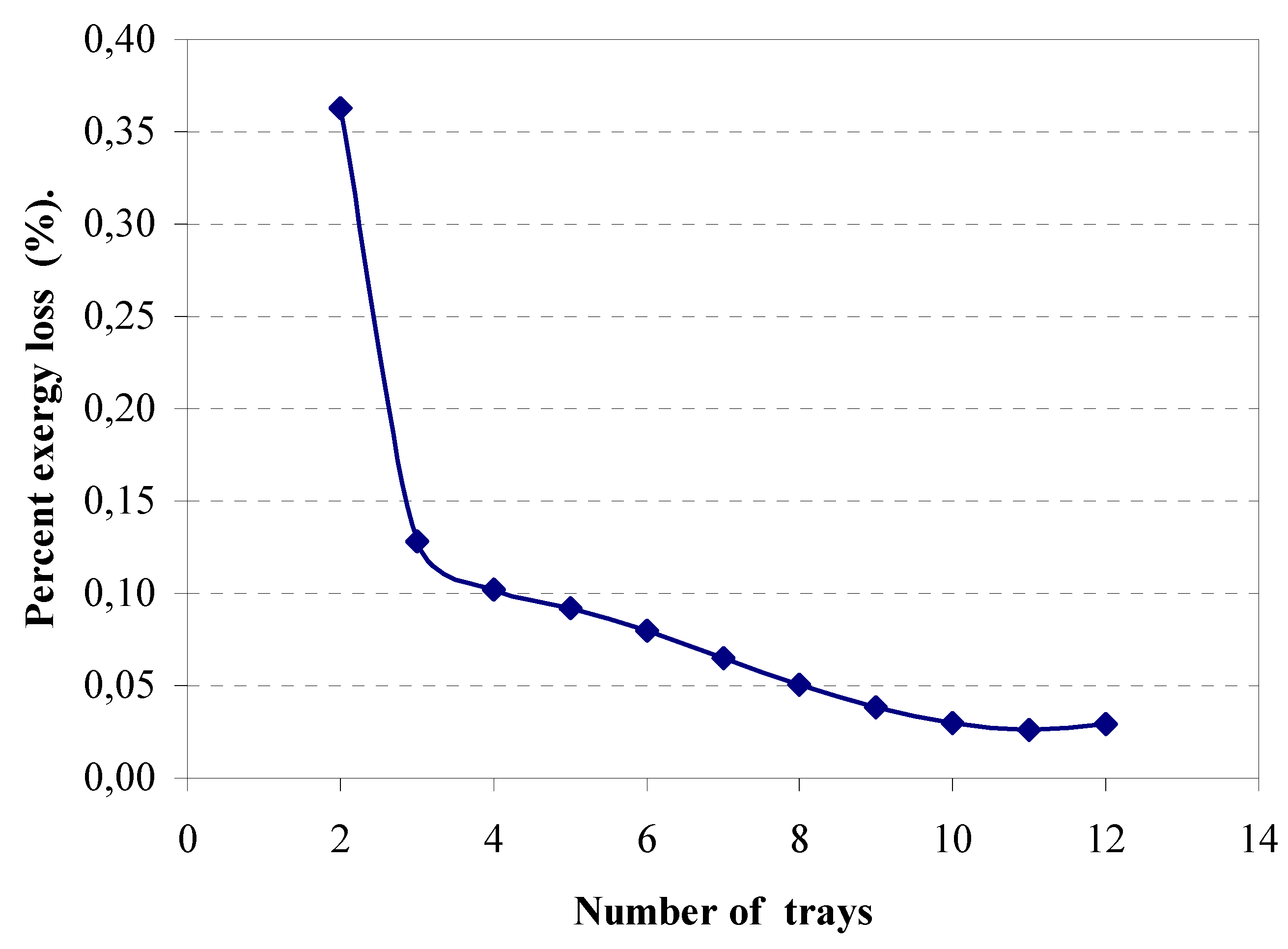
4.3. Distribution of the exergy losses in the column
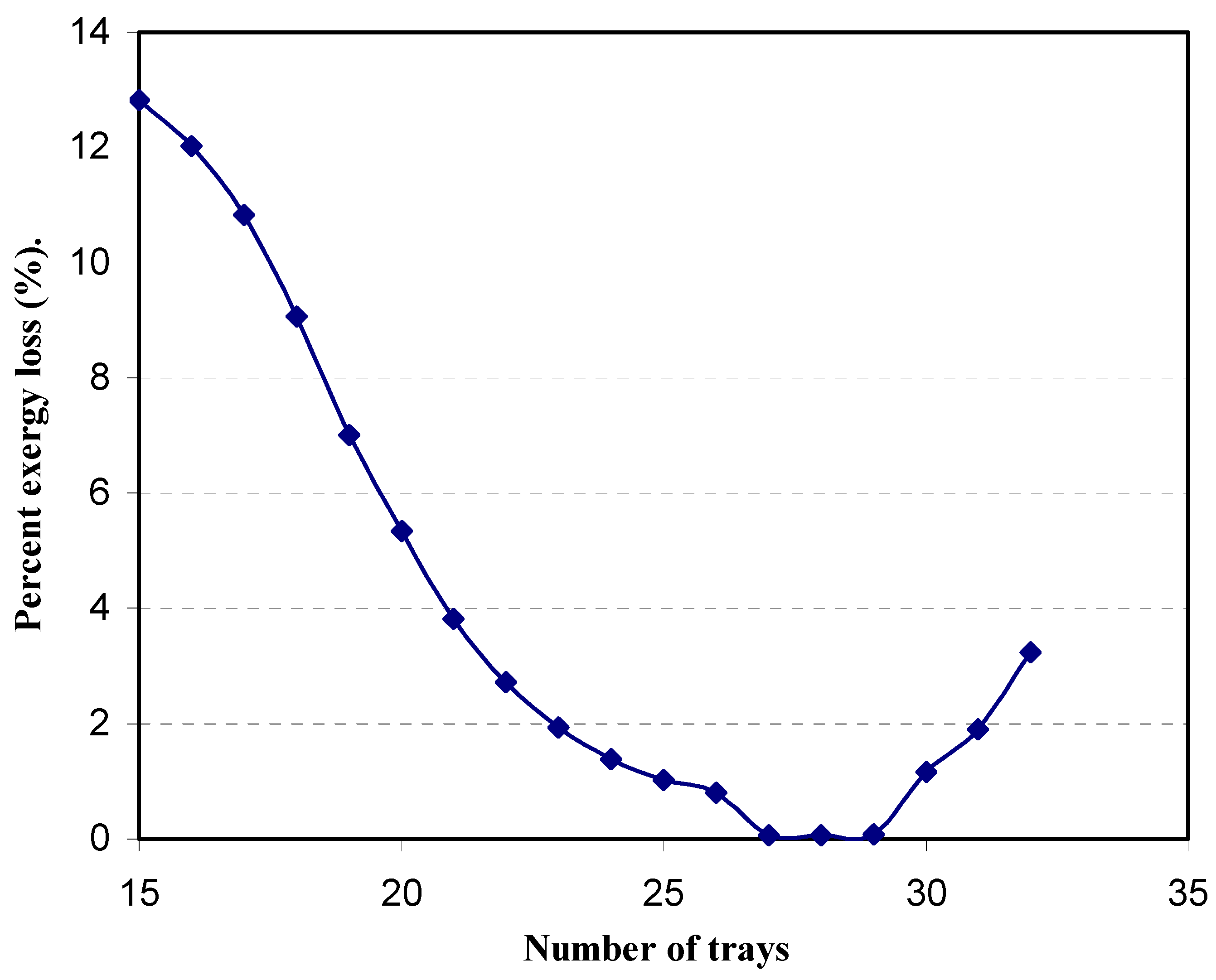
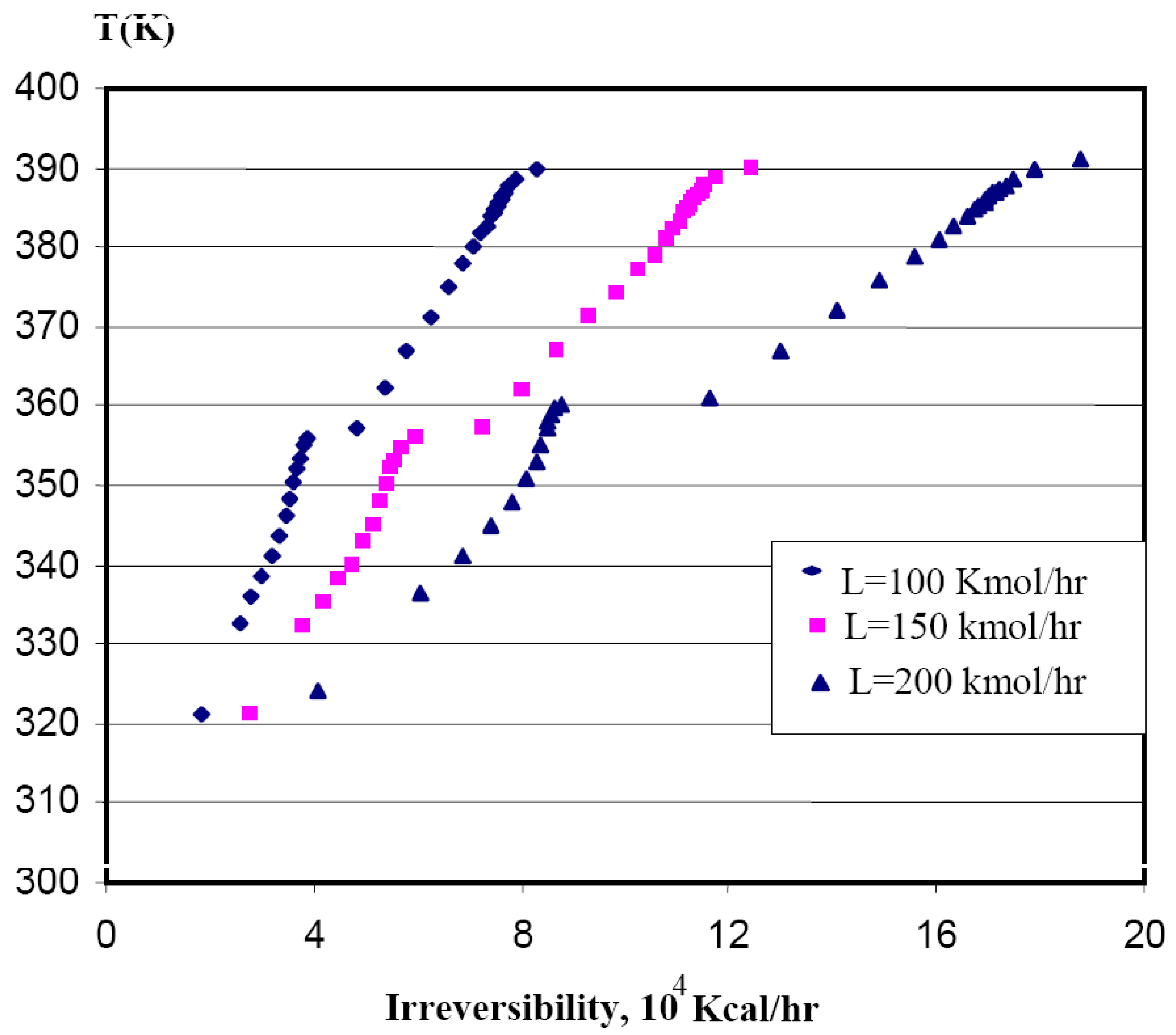
4.4. Profile of the variation of the irreversibilities with tray position
| Section | Operating parameters Exchanged heat and exergy | F=100 kmol/hr D= 0.72 m. | F=150 kmol/hr D=0.87 m. | F=200 kmol/hr D=1.05 m. |
|---|---|---|---|---|
| Rectifying section | Qc (kcal/hr) exchanged heat at the condenser | 6.846 105 | 1.0264 106 | 1.4820 106 |
| IQc (kcal/hr): exergy exchanged at the condenser | 1.850 104 | 2.7730 104 | 4.1100 104 | |
| Itot (kcal/hr): Irreversibilities consumed at the section | 3.848 104 | 5.7650 104 | 8.7430 104 | |
| Stripping section | Qr (kcal/hr): exchanged heat at the reboiler | 7.123 105 | 1.0680 106 | 1.5298 106 |
| IQr (kcal/hr): exergy exchanged at the reboiler | 4.456 103 | 6.8160 103 | 9.8040 104 | |
| Itot (kcal/hr): Irreversibilities consumed at the section | 3.485 104 | 5.2280 104 | 7.1530 104 | |
| Total exergy loss (kcal/hr) | 8.3288 104 | 1.24811 105 | 1.8790 105 | |
| Minimum separation work (kcal/hr) | 4.2069 104 | 6.3163 104 | 7.2796 104 | |
| Exergy consumed (kcal/hr) | 1.2536 105 | 1.8790 105 | 2,.070 105 | |
5. Conclusions
- Temperature pinch δT (= (TN - T1)).
- Thermal power exchanged at the condenser (Qc) and the reboiler (Qr).
Nomenclature
| COP | Coefficient of performance | |
| D | Distillate flow rate | (kmol/hr) |
| Ex | Stream exergy | (kcal/kmol) |
| F | Feed rate | (kmol/hr) |
| H | Stream enthalpy | (kcal/kmol) |
| I | Irreversibilities flux | (kcal/hr) |
| L | Liquid stream flow rate | (kmol/hr) |
| M | Liquid side stream | (kmol/hr) |
| m | Stream molar flow rate | (kmol/hr) |
| Po | Ambient medium pressure | (1 atm.) |
| Qc | Heat rate exchanged at the condenser | (kcal/hr) |
| Qr | Heat rate exchanged at the reboiler | (kcal/hr) |
| S | Stream entropy | (kcal/kmol) |
| Tref | Reference temperature | (K) |
| To | Ambient medium temperature | (273.15 K) |
| U | Vapor side stream | (298.15K) |
| V | Stream flow rate | (kmol/hr) |
| W | Applied mechanical work | (kcal/kmol) |
| Subscripts | ||
| i | Component i in the mixture | |
| j | Tray number | |
| Greek Symbols | ||
| Φ | Carnot’s coefficient | |
| ηex | Exergetic efficiency |
References and Notes
- Okamura, H.; Naitoh, J.; Nanba, T.; Matoba, M.; Nishioka, M.; Anzai, S.; Shimoyama, I.; Fukui, K.; Miura, H.; Nakagawa, H.; Nakagawa, K.; Kinoshita, T.; Bandyopadhyay, S.; Malik, R.K.; Shenoy, U.V. Invariant rectifying-stripping curves for targeting minimum energy and feed location in distillation. Comp. Chem. Eng. 1999, 23, 1109–1124. [Google Scholar]
- Bandyopadhyay, S. Effect of feed on optimal thermodynamic performance of a distillation column. Chem. Eng. J. 2002, 88, 175–186. [Google Scholar] [CrossRef]
- Benedict, M.; Webb, G.B.; Rubin, L.C. An Empirical Equation for Thermodynamic Properties of Light Hydrocarbons and Their Mixtures I. Methane, Ethane, Propane and n-Butane. J. Chem. Phys. 1940, 8, 334–345. [Google Scholar]
- Bouchekima; et al. The Performance of Capilary Film Solar Still Installed in South Algeria. In Presented at Conference on Desalination Strategies in South Mediterranean Countries, Jerba, Tunisie, 11.-13. September 2000.
- Cornelissen, R.L.; Hirs, G.G.; Kotas, T.J. An exergy analysis of an oil distillation process, Proceedings of the Second Law Analysis of Energy Systems: towards the 21-st century; Ed. Sciubba et Moran: Rome, 1995; pp. 417–429.
- Douani, M. Contribution à l’Etude du Couplage Séchoir-Pompe à Chaleur à Absorption Fonctionnant avec le Système Eau-Triéthylène Glycol. Ph.D. Thesis, I.N.P., Toulouse, October 1989. [Google Scholar]
- Fonyo, Z. Thermodynamic Analysis of rectification. I. Reversible model of rectification. Int. Chem. Eng. 1974, 14, 18. [Google Scholar]
- Halvorsen, I.J. Minimum Energy Requirement in Complex Distillation Arrangement. Dr. Ing. Thesis, Norwegian University of Science and Technology, May 2001; pp. 170–208. [Google Scholar]
- Kaiser, V.; Gourlia, J.P. The ideal column concept: Applying exergy to distillation. Chem. Eng. (N.Y.) 1985, 92, 45–53. [Google Scholar]
- Kaiser, V. Energy optimization. Chem. Eng. 1981, 88, 62–72. [Google Scholar]
- Keenan, J.H. Availability and irreversibility in thermodynamics. Brit. J. Appl. Phys. 1951, 2, 183–192. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Plant Analysis, 1st Ed. ed; Brendon Ltd.: Tiptree, Essex, 1985. [Google Scholar]
- Lee, K.L.; Morabito, M.; Wood, R.M. Refinery Heat Integration Using Pinch Technology. Hydrocarbon Process 1989, 68, 49–56. [Google Scholar]
- Nakaiwa, M.; Huang, K.; Owa, M.; Akiya, T.; Nakane, T.; Sato, M.; Takamatsu, T.; Yoshitome, H. Potential energy savings in ideal heat-integrated distillation column. Appl. Therm. Engng. 1998, 18, 1077–1087. [Google Scholar] [CrossRef]
- Petlyuk, F.B.; Platonov, V.M.; Slavinskii, D.M. Thermodynamically optimal method for separating multicomponent mixtures. Int. Chem. Eng. 1965, 5, 555–561. [Google Scholar]
- Ratkje, S.K.; Sauar, E.; Hansen, E.M.; Lien, K.M.; Hafskjoldt, B. Analysis of entropy production rates for design of distillation columns. Ind. Eng. Chem. Res. 1995, 34, 3001–3007. [Google Scholar] [CrossRef]
- Rivero, R. Exergy simulation and optimization of adiabatic and diabatic binary distillation. Energy 2001, 26, 561–593. [Google Scholar] [CrossRef]
- Sorin, M.V.; Brodyansky, V.; Paris, J. Thermodynamic Evaluation of a Distillation A.E.S., Thermodynamics and the Design Analysis and Improvement of Energy Systems. ASME 1994, 33, 125–134. [Google Scholar]
- Starling, K.E. Proc. NGPA Ann. Conv 1970, 49, 9.
- Szargut, J.; et al. Exergy Analysis of Thermal and Metallurgical Process, 1st Ed. ed; Springer Verlag.
- Tondeur, D.; Kvaalen, E. Equipartition of entropy production, an optimality criterion for transfer and separation processes. Ind. Eng. Chem. Res. 1987, 26, 50–56. [Google Scholar] [CrossRef]
- Vogler, T.C.; Weissman, W. Thermodynamic availability analysis for maximizing a system’s efficiency. Chem. Eng. Progress 1988, 84, 35–42. [Google Scholar]
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mustapha, D.; Sabria, T.; Fatima, O. Distillation of a Complex Mixture. Part II: Performance Analysis of a Distillation Column Using Exergy. Entropy 2007, 9, 137-151. https://doi.org/10.3390/e9030137
Mustapha D, Sabria T, Fatima O. Distillation of a Complex Mixture. Part II: Performance Analysis of a Distillation Column Using Exergy. Entropy. 2007; 9(3):137-151. https://doi.org/10.3390/e9030137
Chicago/Turabian StyleMustapha, Douani, Terkhi Sabria, and Ouadjenia Fatima. 2007. "Distillation of a Complex Mixture. Part II: Performance Analysis of a Distillation Column Using Exergy" Entropy 9, no. 3: 137-151. https://doi.org/10.3390/e9030137
APA StyleMustapha, D., Sabria, T., & Fatima, O. (2007). Distillation of a Complex Mixture. Part II: Performance Analysis of a Distillation Column Using Exergy. Entropy, 9(3), 137-151. https://doi.org/10.3390/e9030137




