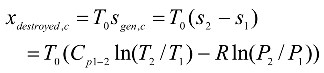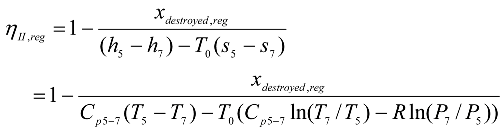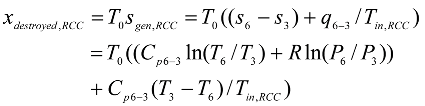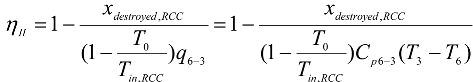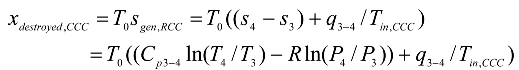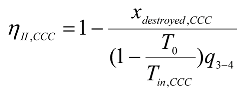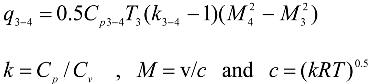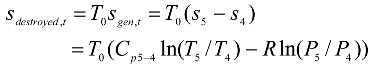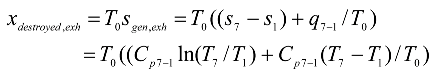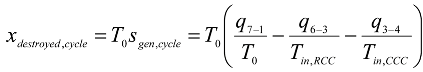Introduction
Brayton cycle has been utilized extensively in applications. Goktun and Yavus [
1], examined the thermal efficiency of a regenerative Brayton cycle with isothermal heat addition. They found that the effect of regenerative heat transfer on the performance of a modified cycle is positive for low pressure ratios and negative for higher pressure ratios. Pure modified Brayton cycles are better than regenerative modified Brayton cycles for pressure ratios beyond
rp of 12:1. Therefore, the use of regeneration to improve the thermal efficiency of Brayton cycles with isothermal heat addition should be carefully checked [
1]. Erbay et al [
2], used the maximum power density method to determine the optimal operating conditions of the regenerative Brayton gas turbine engine with isothermal heat addition. They found that the low efficiency problem noted for the regenerative Brayton cycles has been eliminated by the isothermal heat addition. Tyagi et al [
3], carried out an ecological function optimization of an irreversible regenerative Brayton cycle with isothermal heat addition along with real processes using the concept of finite time thermodynamics. They indicated that the ecological is an increasing function of the components efficiencies, the effectiveness and the temperature on the isothermal-, sink- and regenerative –side heat exchangers while it is found to be a decreasing function of the working fluid heat capacitance rate and the temperature and effectiveness on the isobaric –side heat exchanger. Kaushik et al [
4], studied the effect of various parameters on the performance of the Brayton heat engine and showed that there is a significant improvement in the performance of Brayton cycle (above 15 %) with isothermal heat addition over the conventional cycle. Wang et al [
5], explained that there exists an optimal total pressure ratio that leads to a double-maximum power output. An optimal cycle working range, i.e. high power output and high efficiency, can be obtained according to the characteristic of the maximum power versus its corresponding efficiency. Many authors in the recent years carried out the exergy analysis of various thermal systems. Zheng et al [
6], demonstrated the effect of various parameters on the exergy performance for a Braysson cycle and conclude that the specific work and exergy efficiency of the Braysson cycle are larger than those of Brayton cycle under the same conditions. Rosen and D’ncer [
7], brought into view that the potential usefulness of exergy analysis in addressing and solving energy-related sustainable development and environmental problems is substantial, and that exergy is a confluence of energy, environment and sustainable development. An exergy-costing method was developed by assigning a single unit cost to a specific exergy, regardless of the type of exergy stream and state of the stream. This methodology provides some information that is related to the actual production process within the system, Kim et al [
8]. The effect of various parameters, such as gas turbine pressure ratio, turbine inlet temperature and others, on the exergy performance of a gas-turbine combined-cycle power plant was presented by Ertesvag et al [
9]. Very important conclusion about exergetics is found in the study of Mei Gong and Goran Wall [
10], exergy is a useful concept since it is a link between the physical and engineering world and the surrounding environment. Exergy also expresses the true efficiency of engineering systems; therefore, it is strongly recommended that exergy is used in the design of engineering systems. The second law of thermodynamics may well turn out to be the central scientific truth of the twenty-first century. Nothing disappears and every thing disperses, so exergy is a suitable and necessary concept in the development of a sustainable society Wall [
11].
The exergy destroyed and the second law efficiency form an important performance characteristic of Brayton cycles and, in practice, has a major impact on operating costs. In this paper, the exergy destroyed and the second law efficiency of a regenerative Brayton cycle with isothermal heat addition has been derived and evaluated with a detailed numerical example and results.
Theoretical model
Reheating in gas turbine engines limits the extent to which an isothermal heat addition is approached. In a simple heat addition process, the temperature of a compressible gas flowing with subsonic velocity through a frictionless constant area duct increases along the duct. Decreasing the duct area with the same flowing conditions, results in decreasing the temperature along the duct. From the theoretical point of view and based on the nature of these two types of flow, simple heat addition (Raleigh flow) and simple area change (isentropic conditions) may be combined to yield an isothermal process Vecchiarelli et al [
12]. This idealized process consists of a compressible gas with subsonic velocity flowing through a frictionless converging duct while heated such that, every infinitesimal decrease in temperature due to the area reduction is exactly compensated by simple heat addition. From the first law of thermodynamics point of view, the kinetic energy of the flowing gas (and hence, its Mach number) should increase during this isothermal heat addition process. Gas turbine engines using air as a working fluid form one of the appropriate applications of the idealized isothermal heat addition process.
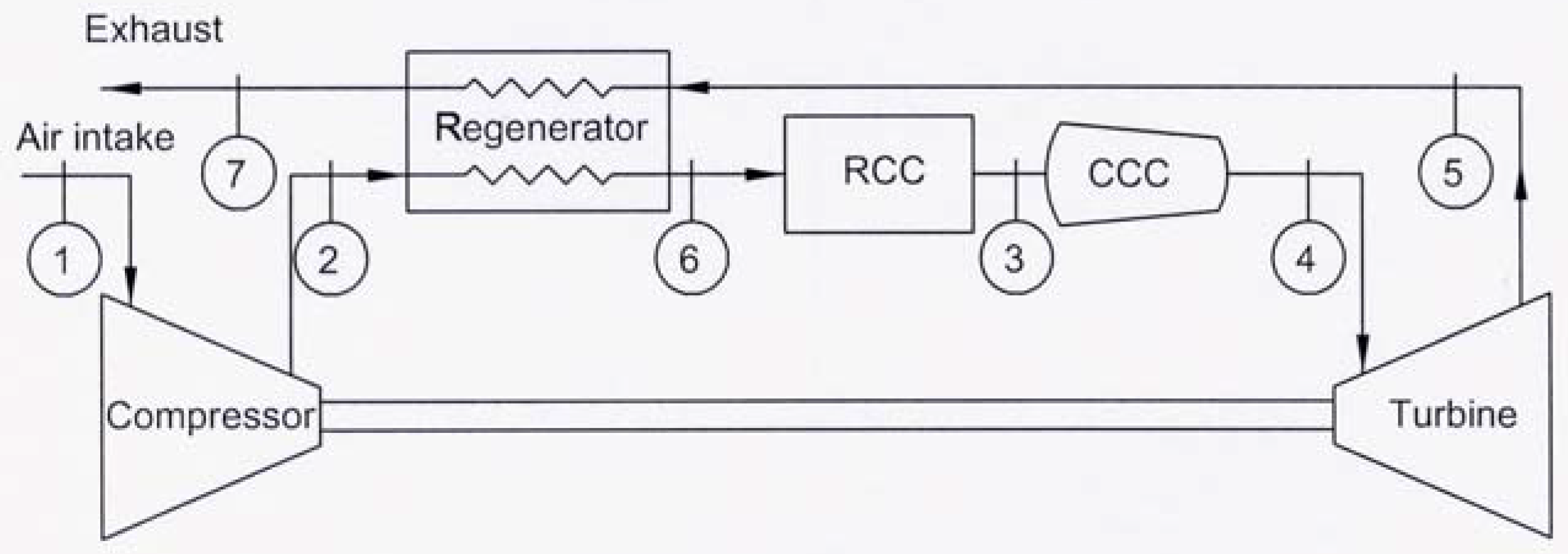
A schematic diagram of a regenerative Brayton cycle with isothermal heat addition process is shown in
Fig. 1, and a
T-
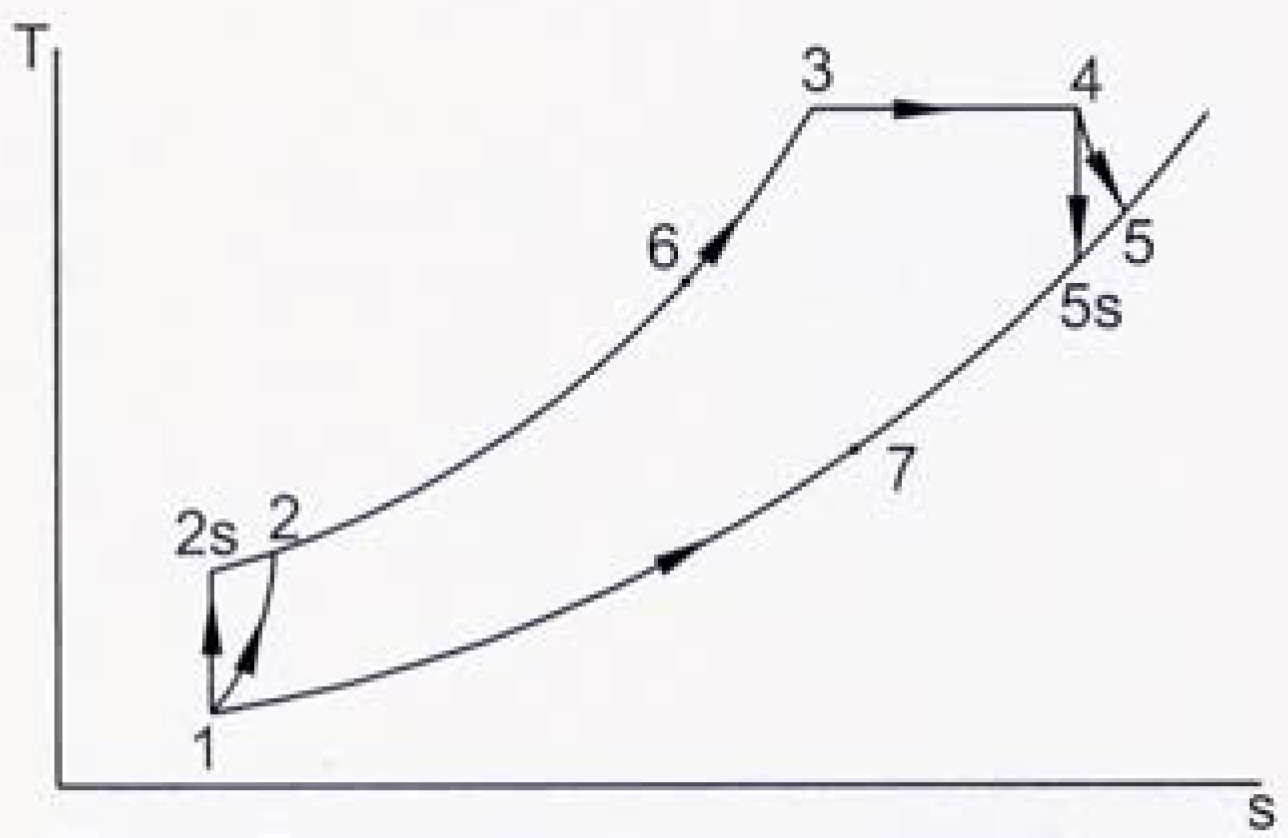
s diagram is shown in
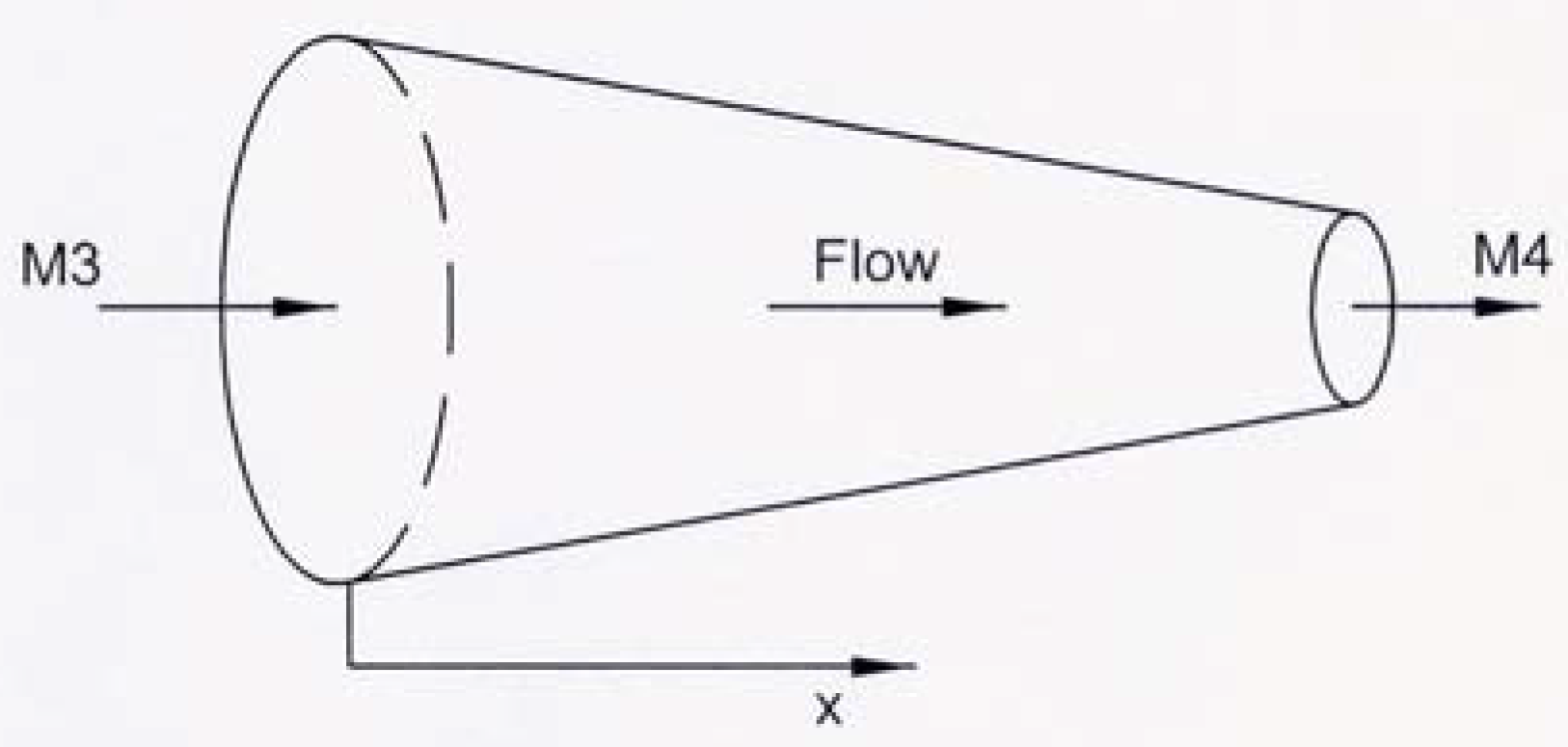
Fig. 2. A converging combustion chamber (CCC) is shown schematically in
Fig. 3. According to
Fig. 2, the gas enters the compressor at state 1 and at state 2 the gas leaving the compressor enters the regenerator, where it is heated to temperature
T6. In an ideal regenerator, the gas will leave the regenerator at the same temperature of the turbine exhaust
T5. The isobaric heat addition process takes place in the regular combustion chamber (RCC) between states 6 and 3. Extra heat addition is accomplished isothermally in the CCC between states 3 and 4. The gas leaving the CCC at state 4 with a lower pressure than that at state 3, but the velocity, and consequently the kinetic energy, of the gas has been increased due to the nature of the CCC. The gas then expands in the turbine to state 5. The hot gases enter the regenerator, where it is cooled isobarically to state 7. The cycle is completed by cooling the gas to the initial state. The processes 1-2s and 4-5s are isentropic compression and expansion.
Fig. 1.
Regenerative Brayton cycle with isothermal process
Fig. 1.
Regenerative Brayton cycle with isothermal process
Fig. 2.
T-s diagram of a regenerative Brayton cycle with isothermal heat addition.
Fig. 2.
T-s diagram of a regenerative Brayton cycle with isothermal heat addition.
Fig. 3.
Schematic of a converging combustion chamber.
Fig. 3.
Schematic of a converging combustion chamber.
For one dimensional perfect gas steady flow, application of the steady state second law to each component of the cycle under consideration yields the following:
The rate form of the general exergy balance equation reduces for a steady-flow single-stream systems Cengle and Boles [
12], is:
The first three terms of Eq. (1) represent the exergy transfer by heat, by work, and by mass respectively, while the last term represents the exergy destroyed. The change in the flow exergy by mass is:
The exergy destroyed in the rate form is proportional to the rate of the entropy generated, and can be expressed as:
For a general steady-flow single stream process the rate of the entropy generated is:
The second law efficiency is intended to serve as a measure of approximation to reversible operation, and thus its value should range from zero in the worst case of complete destruction of exergy, to one in the best case of no destruction of exergy Cengle and Boles [
12]. With this in mined, the second law efficiency of a system is defined as:
For a heat engine, such as the Brayton cycle considered, the exergy supplied is the decrease in the exergy of the heat transferred to the engine, which is the difference between the exergy of the heat supplied and the exergy of the heat rejected. The net work output is the recovered exergy.
For further ascertainment of the results obtained, the exergy destroyed during each process in each model of the cycle is calculated separately, then the sum of these exergy destroyed is calculated and compared with the exergy destroyed calculated for the whole cycle as a closed system. Further more the second law efficiency of each device and for the whole cycle is calculated.
The exergy destroyed during the isentropic process through the compressor and the turbine is zero, so the second law efficiency of the compressor and the turbine in this case is 100 %. The exergy destroyed during each process and the second law efficiency for each device of the cycle is calculated according to the following equations. The kinetic and potential energy change is neglected, and the equations are given for a unit mass flow rate.
- -
- -
- -
Regular combustion chamber:
- -
Converging combustion chamber:
where
- -
- -
- -
The whole cycle:
or simply
Results and discussion
For increasing the performance and decreasing the NOx emission, a hypothetical modification of the Brayton cycle is considered in this study. This modification includes a converging combustion chamber in which an isothermal heat addition process is approximated at a peak temperature ~ 1100 K less than the peak temperature ~ 1300 K in the conventional Brayton cycle. The pressure drop at the exit of the CCC yields a new design parameter for the modified Brayton cycle, named the isothermal pressure drop ratio
rt. Analysis of such modified cycle has shown that the parameter
rt has a strong effect on the validity of the second law of thermodynamics. From Eq. (12), the first term is zero, because
T4 =
T3 , then for positive entropy generation
which means that any greater value of
rt than 0.7308194 will violate the second law of thermodynamics. Taking this into account,
rt is assumed to be o.65 in this study.
For further approximation of the real case, the constant pressure specific heat of air Cp is calculated at the average temperature of each process separately, by iteration.
The Brayton cycle models taking into consideration are:
- A-
Ideal air-standard simple cycle,
- B-
Simple cycle with loses,
- C-
Regenerative air cycle,
- D-
Air cycle with isothermal heat addition,
- E-
Regenerative air cycle with isothermal heat addition,
For these cycles, the compressor and turbine efficiencies are assumed to be 0.85, the generator effectiveness is assumed to be 0.7. Since the maximum and minimum temperature of the cycles are known, then an optimal value of a pressure ratio could be found to be
, where
k is the specific heats ratio
k =
Cp/
Cv, which is calculated accordingly with the appropriate value of
Cp. A reference “design” point of the cycles is assumed to be as follows:
rp = 10, TIT = 1104 K, ambient and dead state conditions are
P = 1.01325 bar,
T0 = 300 K. For Mach numbers, the values assumed in Vecchiarelli et al. [
12] are used, that is
M3 = 0.2 and
M4 = 0.8 .
Table (1) contains the design point and the off-design operating variables for which the off-design calculations and analysis were done.
Table (1).
Operating Variable
Table (1).
Operating Variable
| | D. P. | |
| rp | 1 | 2 | 3 | 4 | 5 | 10 | 15 | 20 | 25 |
| TIT[K] | 850 | 900 | 950 | 1000 | 1050 | 1104 | 1150 | 1200 | 1250 |
| T0[K] | 275 | 280 | 285 | 290 | 295 | 300 | 305 | 310 | 315 |
| P[bar] | 0.9546 | 0.9546 | 0.8456 | 0.795 | 0.7469 | 1.01325 | 0.7012 | 0.6578 | 0.6166 |
| Alt.[m] | 0 | 500 | 1000 | 1500 | 2000 | 2500 | 3000 | 3500 | 4000 |
| P[bar] | 1.01325 | 0.9546 | 0.8988 | 0.8456 | 0.795 | 0.7469 | 0.7012 | 0.6578 | 0.6166 |
| T0[K] | 288.15 | 284.9 | 281.7 | 278.4 | 275.2 | 271.9 | 268.7 | 265.4 | 262.2 |
It can be seen in
Fig. (4), that the exergy destroyed ratio decreases for the models A, B, and D, and increases for the models C and E with increasing the pressure ratio of the cycle.
Fig. (4).
Exergy destroyed ratio vs. pressure ratio
Fig. (4).
Exergy destroyed ratio vs. pressure ratio
This is due to the very high drop in the entropy generated by the rejected heat compared with a very low drop in the entropy generated by the added heat in A, B, and D models, and by the same time a high increase in the entropy generated by the rejected heat compared with a very lower increase in the entropy generated by the added heat in C and E models.
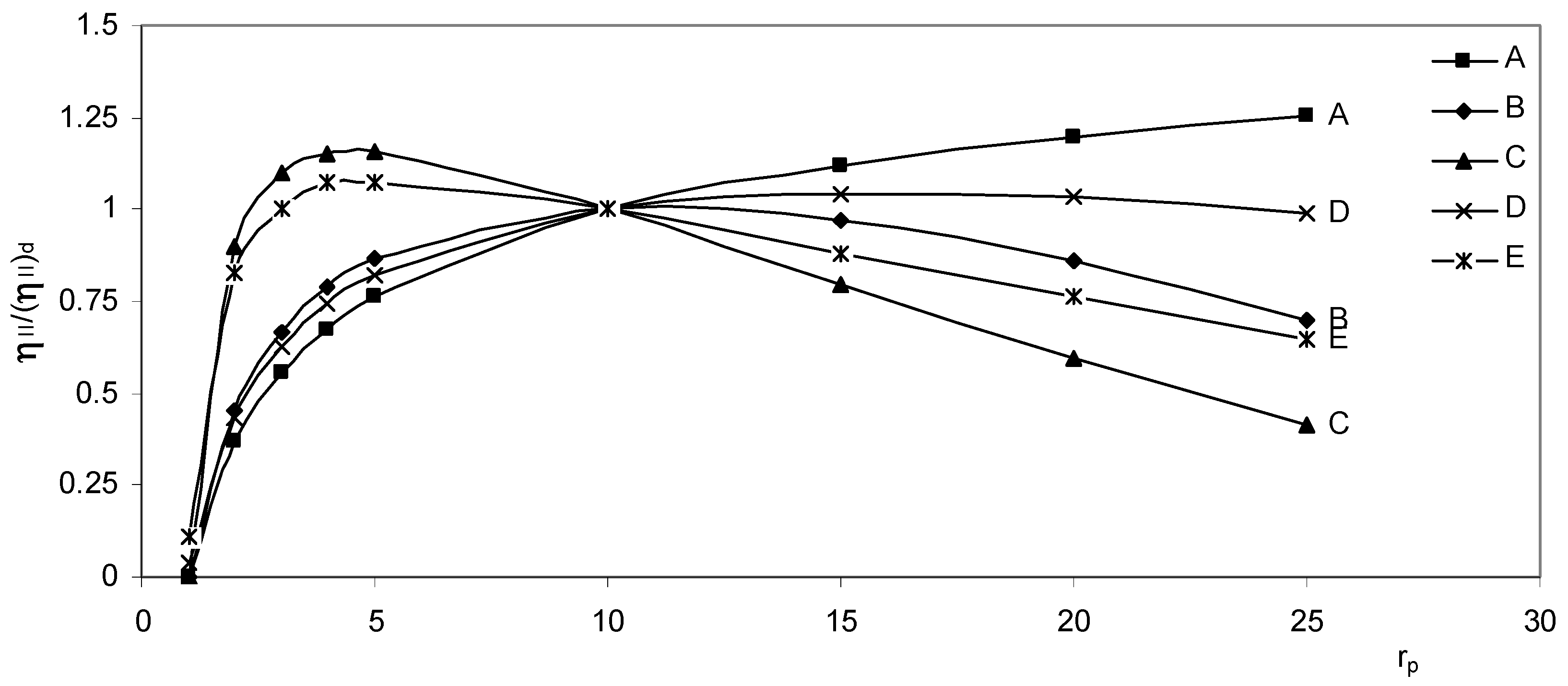
The behavior of the second law efficiency ratio versus pressure ratio is seen in
Fig. (5), it is almost similar to the behavior of the thermal efficiency versus pressure ratio with minor differences due to the variation of constant pressure specific heat with temperature.
Fig. (5).
Second law efficiency ratio vs. pressure ratio
Fig. (5).
Second law efficiency ratio vs. pressure ratio
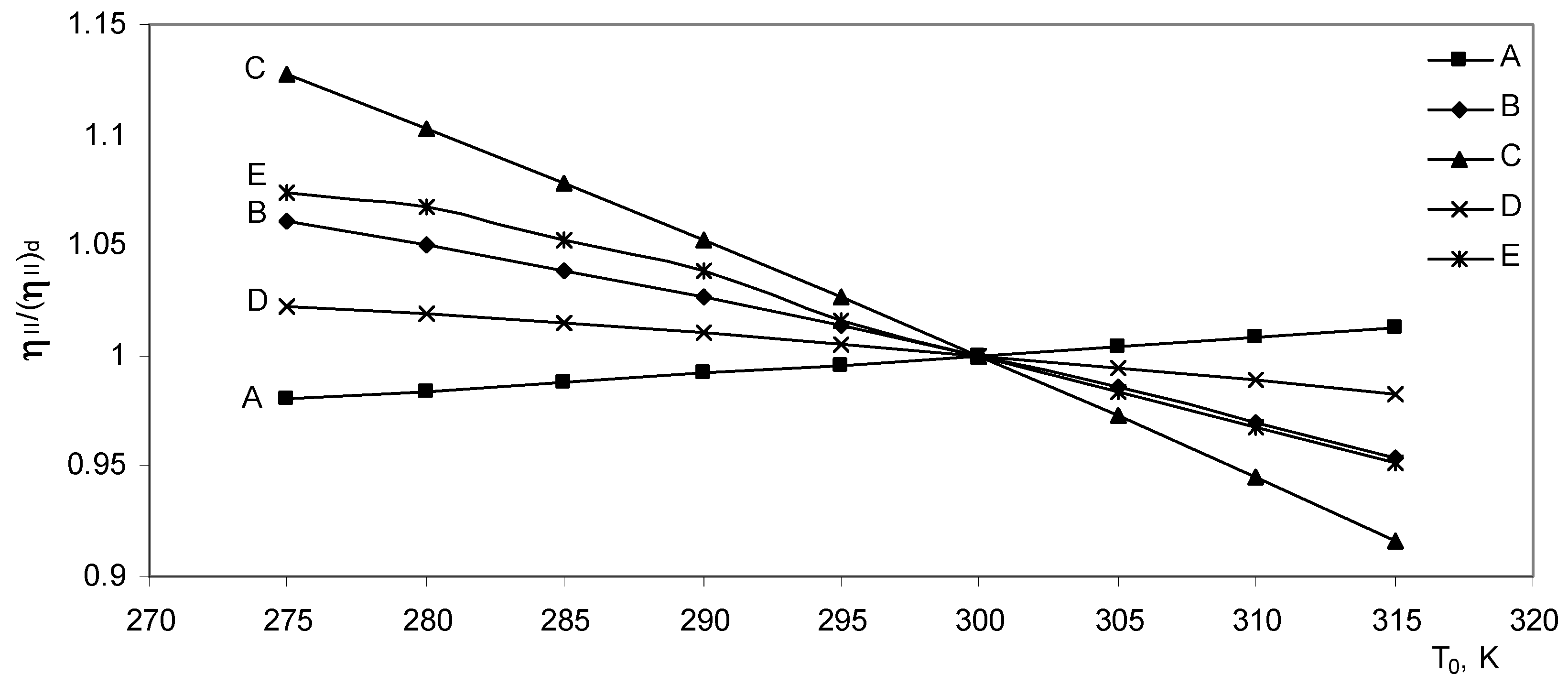
The effect of ambient temperature on the exergy destroyed ratio is shown in
Fig (6). The linear character of the curves is due to the linear relationship between the exergy destroyed and the ambient “dead state” temperature.
Fig. (6).
Exergy destroyed ratio vs. ambient temperature
Fig. (6).
Exergy destroyed ratio vs. ambient temperature
The second law efficiency ratio dependence on the ambient temperature is presented in
Fig. (7). It is known that if the thermal and reversible efficiency decreases or increases almost by the same amount, then the second law efficiency decreases or increases respectively.
The gradient of the decreasing or increasing process of the second law efficiency depends on the speed of decreasing or increasing the thermal and reversible efficiency of the system.
Fig. (7).
Second law efficiency ratio vs. ambient temperature
Fig. (7).
Second law efficiency ratio vs. ambient temperature
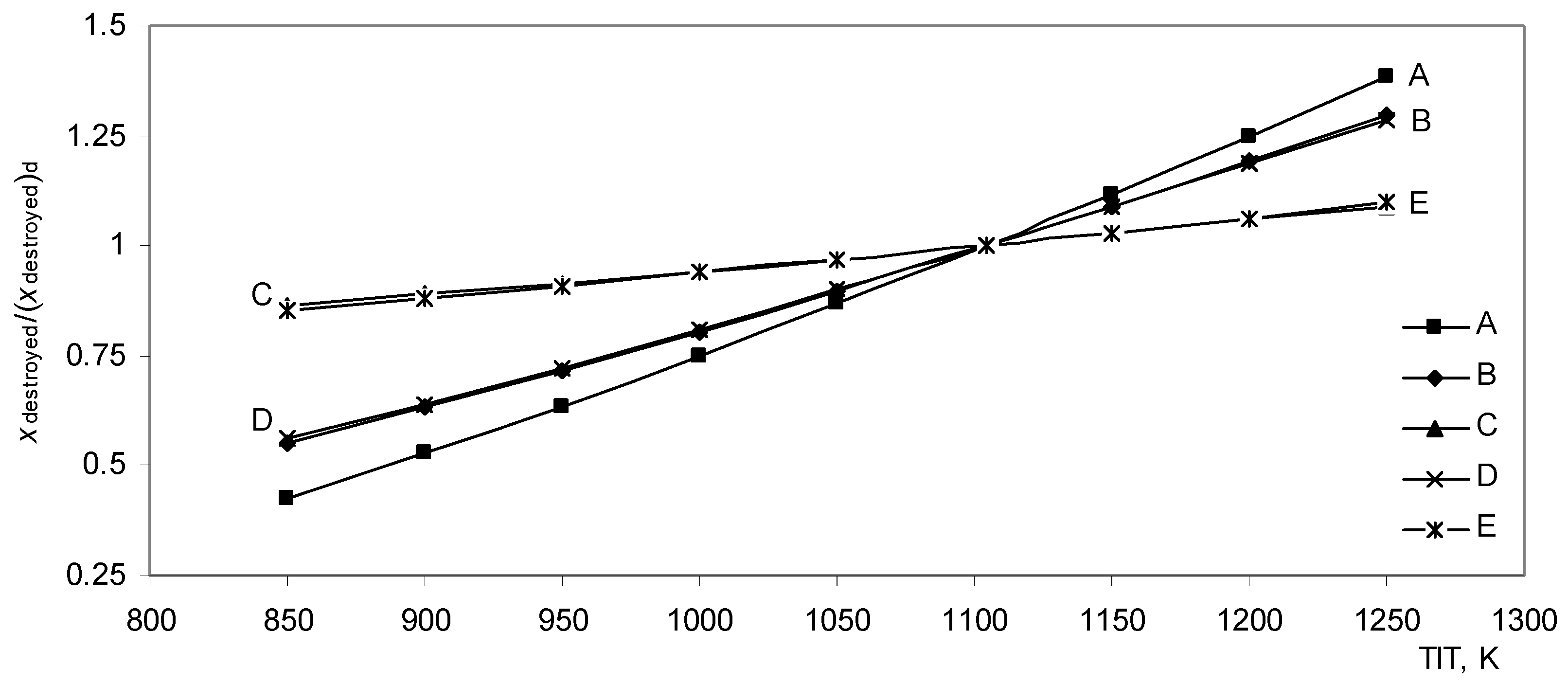
Turbine inlet temperature has the highest effect on the exergy destroyed ratio and the second law efficiency ratio.
As shown in
Fig. (8), the exergy destroyed ratio increases intensively
In A, B, and D models, and moderately in C and E models. The exergy destroyed ratio in model A at 1250 K is more than three times as high as the exergy destroyed ratio at 850 K.
Fig. (8).
Exergy destroyed ratio vs. turbine inlet temperature
Fig. (8).
Exergy destroyed ratio vs. turbine inlet temperature
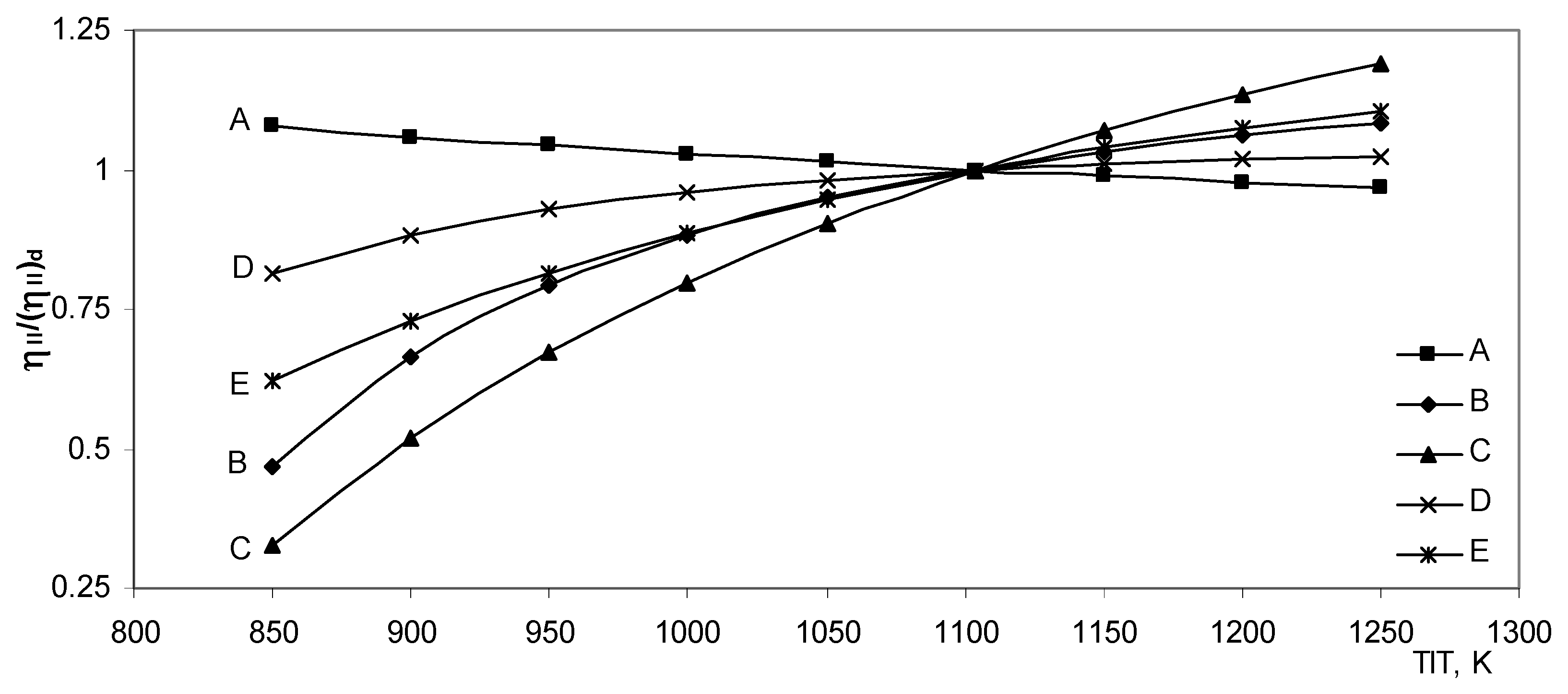
The second law efficiency ratio dependent on the turbine inlet temperature is shown in
Fig. (9). Only for model A the second law efficiency ratio decreases, while for the remaining
Fig. (9).
Second law efficiency ratio vs. turbine inlet temperature
Fig. (9).
Second law efficiency ratio vs. turbine inlet temperature
models it increases with different gradients. The model C has the highest gradient; the second law efficiency for this model at 1250 K is more than 3.6 times that at 850 K.
The remaining parameter in this study, altitude, has no effect, neither on the exergy destroyed nor on the second law efficiency. When the altitude was taken with different ambient temperatures, the effect on the exergy destroyed and second law efficiency was identical with the results obtained from changing only the ambient temperature.