Analytic Solutions and Entropy Production of the Double-Diffusive Equation System
Abstract
1. Introduction
2. Theory and Results
2.1. Double-Diffusion System Without Extra Source Terms
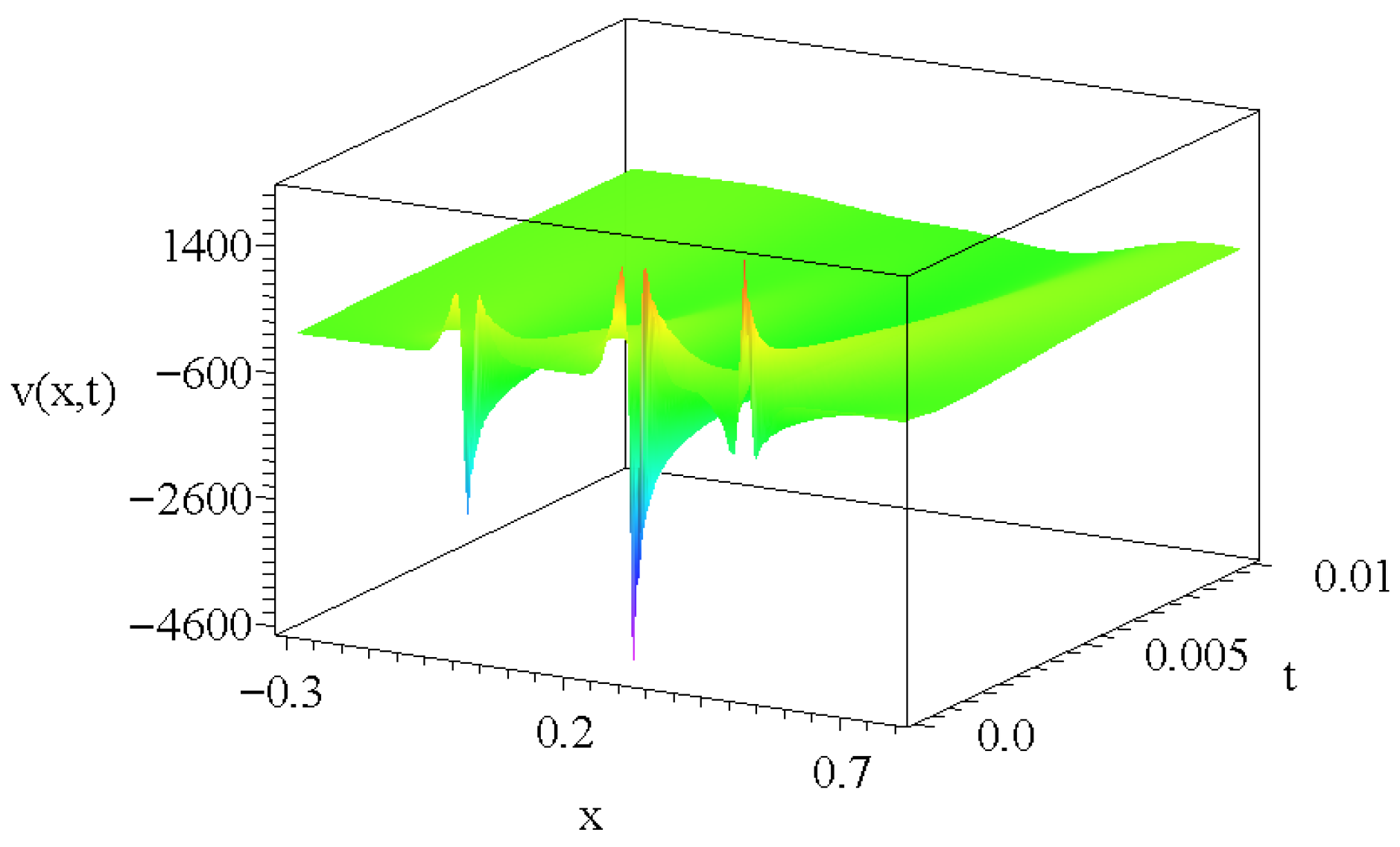
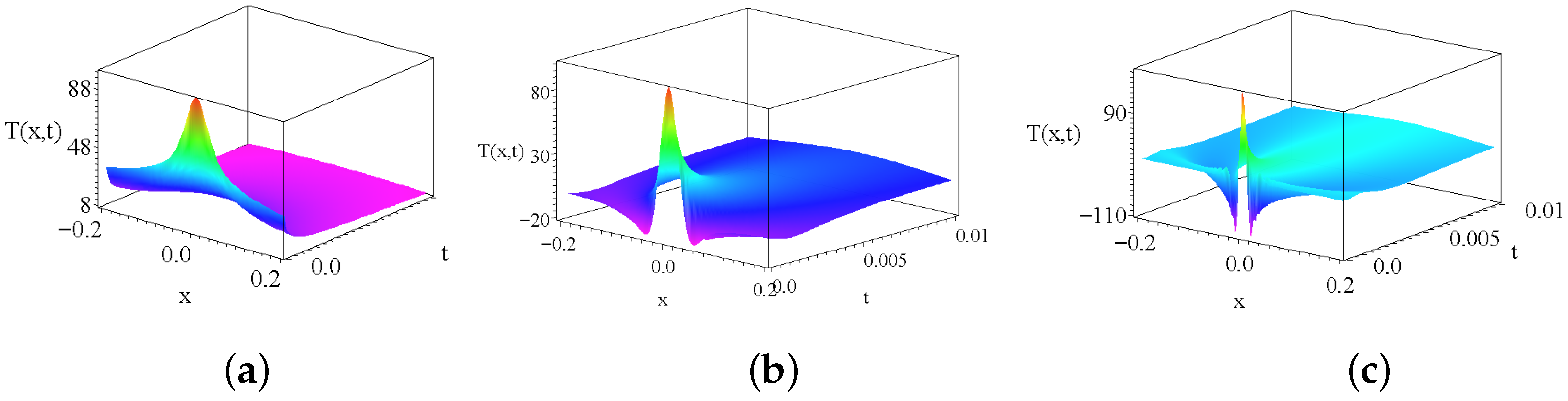
2.2. The Role of Possible Additional Source Terms
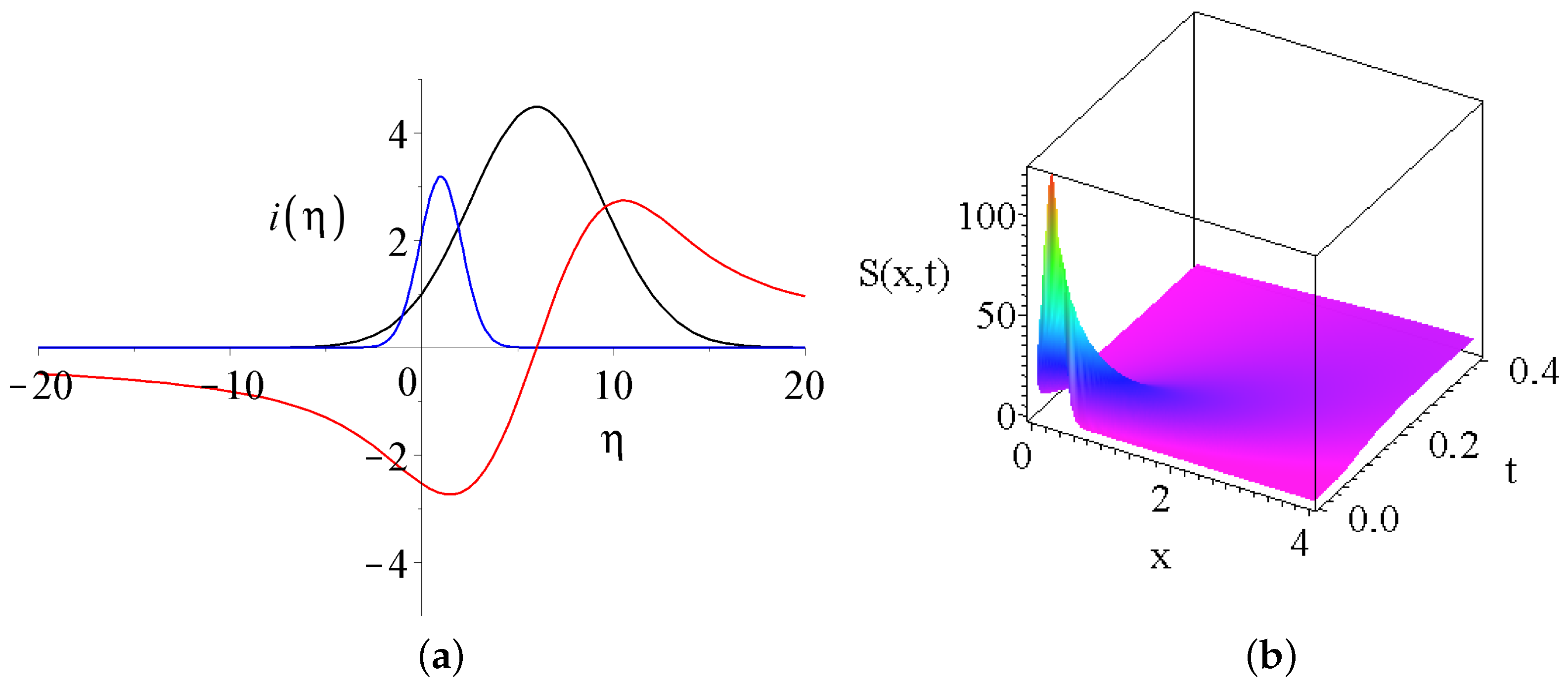
- where the derived solutions are divergent for large s; these are the cyan and the gray lines on Figure 3a. If the first parameter of the Kummer’s M function is a negative integer then the function is a finite order polynomial in . A nice example is whereNote that the first term on the right hand side is a constant (formally Kummer’s function of the first kind is equivalent to the generalized confluent hypergeometric series with the notation of ).The smaller the first negative parameter of the Kummer’s function, the larger the power of the polynomial. Thanks to the exponent, the final temperature distribution will be decaying, but we will see that this parameter regime will not attract the largest interest among the solutions.
- the solution is constant on the whole axis, this is presented by the brown line.
- the solution is positive on the whole axis, and has a decay to zero at large s. Such solutions are plotted with pink and green lines. These are well-behaving solutions with a global maxima in the origin, and in this sense similar to Gaussian solutions.
- the solutions has a maxima in the origin following quick oscillatory decay to zero with growing number of zero transitions as d growing. Black, blue and red curves present such solutions. Unfortunately, the defining series of the Kummer’s M function Equation (26) converges very slowly for highly oscillatory functions.In some sense these are the most interesting solutions.
3. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Ghez, R. Diffusion Phenomena; Dover Publication Inc.: New York, NY, USA, 2001. [Google Scholar]
- Thambynayagam, R. The Diffusion Handbook: Applied Solutions for Engineers; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Latif, M. Heat Convection; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Ashan, A. Convection and Conduction Heat Transfer; Intec: London, UK, 2011. [Google Scholar] [CrossRef]
- Vogel, G. Adventure Diffusion; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Bunde, A.; Caro, J.; Kärger, J.; Vogl, G. Diffusive Spreading in Nature, Technology and Society; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Kovács, E. A class of new stable, explicit methods to solve the non-stationary heat equation. Numer. Methods Partial. Differ. Equ. 2021, 37, 2469–2489. [Google Scholar] [CrossRef]
- Kareem, H.; Kovács, E.; Barna, I.F.; Mátyás, L. Analytical solution and numerical simulation of heat transfer in cylindrical- and spherical-shaped bodies. Computation 2023, 11, 131. [Google Scholar] [CrossRef]
- Bognár, G.; Rozgonyi, E. The local analytic solution to some nonlinear diffusion-reaction problems. WSEAS Trans. Math. 2008, 7, 382–395. [Google Scholar]
- Guedda, M.; Véron, L. Bifurcation phenomena associated to the p-Laplace operator. Trans. Am. Math. Soc. 1988, 310, 419–431. [Google Scholar] [CrossRef]
- Schlichting, H.; Gersten, K. Boundary-Layer Theory; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Goluskin, D. Internally Heated Convection and Rayleigh-Bénard Convection; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Zdunkowski, D.; Bott, A. Dynamics of the Atmosphere: A Course in Theoretical Meteorology; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Tartar, L. An Introduction to Navier Stokes Equation and Oceanography; Springer: New york, NY, USA, 2006. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics, from Air Polution to Climate Change; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- Lorenz, E.N. Deterministic Nonperiodic Flow. J. Atmos. Sci. 1963, 20, 130–141. [Google Scholar] [CrossRef]
- Mátyás, L.; Barna, I.F. General Self-Similar Solutions of Diffusion Equation and Related Constructions. Rom. J. Phys. 2022, 67, 101–117. [Google Scholar]
- Barna, I.F.; Mátyás, L. Analytic self-similar solutions of the Oberbeck–Boussinesq equations. Chaos Solitons Fractals 2015, 78, 249–255. [Google Scholar] [CrossRef]
- Barna, I.F.; Bognár, G.; Mátyás, L.; Hriczó, K. Self-similar analysis of the time-dependent compressible and incompressible boundary layers including heat conduction. J. Therm. Anal. Calorim. 2022, 147, 13625–13632. [Google Scholar] [CrossRef]
- Barna, I.F.; Mátyás, L. Diffusion Cascades and Mutually Coupled Diffusion Processes. Mathematics 2024, 12, 3298. [Google Scholar] [CrossRef]
- Baines, P.G.; Gill, A. On thermohaline convection with linear gradients. J. Fluid Mech. 1969, 37, 289–306. [Google Scholar] [CrossRef]
- Garaud, P. Double-Diffusive Convection at Low Prandtl Number. Annu. Rev. Fluid Mech. 2018, 50, 275–298. [Google Scholar] [CrossRef]
- Radko, T. The sub-microscale dynamics of double-diffusive convection. J. Fluid Mech. 2024, 982, A25. [Google Scholar] [CrossRef]
- Ba, T.L.; Bohus, M.; Lukács, I.E.; Wongwises, S.; Gróf, G.; Hernadi, K.; Szilágyi, I.M. Comparative study of carbon nanosphere and carbon nanopowder on viscosity and thermal conductivity of nanofluids. Nanomaterials 2021, 11, 608. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y. Double-diffusive convection with gravitationally unstable temperature and concentration gradients in homogeneous and heterogeneous porous media. J. Fluid Mech. 2024, 999, A62. [Google Scholar] [CrossRef]
- Salari, L.; Rondoni, L.; Giberti, C.; Klages, R. A simple non-chaotic map generating subdiffusive, diffusive, and superdiffusive dynamics. Chaos 2015, 25, 073113. [Google Scholar] [CrossRef]
- de Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; Dover Publication Inc.: New York, NY, USA, 2013. [Google Scholar]
- Siegmann, W.L.; Rubenfeld, L.A. A Nonlinear Model for Double-Diffusive Convection. SIAM J. Appl. Math. 1975, 29, 540–557. [Google Scholar] [CrossRef]
- Vafai, K. Double-Diffusive Convection in Porous Media. In Handbook of Porous Media; Mojtabi, A., Charrier-Mojtabi, M.-C., Eds.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Radko, T. Double-Diffusive Convection; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Brandt, A. and Fernando, H.J.S. Double-Diffusive Convection; American Geophysical Union: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Schmitt, R.W. Double Diffusion in Oceanography. Annu. Rev. Fluid Mech. 2003, 26, 255–285. [Google Scholar] [CrossRef]
- Huppert, H.E.; Sparks, R.J. Double-Diffusive Convection Due to Crystallization in Magmas. Annu. Rev. Earth Planet. Sci. 1984, 12, 11–37. [Google Scholar] [CrossRef]
- Leconte, J.; Chabrier, G. A new vision of giant planet interiors: Impact of double diffusive convection. Astron. Astrophys. 2012, 540, A20. [Google Scholar] [CrossRef]
- Mirouh, G.M.; Garaud, P.; Stellmach, S.; Traxler, A.L.; Wood, T.S. A New Model for Mixing by Double-Diffusive Cconvection (Semi-Convection). I. The Conditions for Layer Formation. Astrophys. J. 2012, 750, 61. [Google Scholar] [CrossRef]
- Hughes, D.W.; Brummell, N.H. Double-diffusive Magnetic Layering. Astrophys. J. 2021, 922, 195. [Google Scholar] [CrossRef]
- Parsons, J.D.; Bush, J.W.M.; Syvitski, J.P.M. Hyperpycnal plume formation from riverine outflows with small sediment concentrations. Sedimentology 2001, 48, 465–478. [Google Scholar] [CrossRef]
- Davarpanah Jazi, S.; Wells, M.G. Enhanced sedimentation beneath particle-laden flows in lakes and the ocean due to double-diffusive convection. Geophys. Res. Lett. 2016, 43, 10883–10890. [Google Scholar] [CrossRef]
- Schmitt, R.W. The characteristics of salt fingers in a variety of fluid systems, including stellar interiors, liquid metals, oceans, and magmas. Phys. Fluids 1983, 26, 2373–2377. [Google Scholar] [CrossRef]
- Turner, J.S. Double-Diffusive Phenomena. Annu. Rev. Fluid Mech. 1974, 6, 37–54. [Google Scholar] [CrossRef]
- Turner, J.S. Multicomponent Convection. Annu. Rev. Fluid Mech. 1985, 17, 11–44. [Google Scholar] [CrossRef]
- Debure, M.; Lassin, A.; Marty, N.; Claret, F.; Virgone, A.; Calassou, S.; Gaucher, E. Thermodynamic evidence of giant salt deposit formation by serpentinization: An alternative mechanism to solar evaporation. Sci. Rep. 2019, 9, 11720. [Google Scholar] [CrossRef]
- Farkas, C.; Winkert, P. An existence result for singular Finsler double phase problem. J. Differ. Equ. 2021, 286, 455–473. [Google Scholar] [CrossRef]
- Máthé, I.; Borsodi, A.K.; Tóth, E.M.; Felföldi, T.; Jurecska, L.; Krett, G.; Kelemen, Z.; Elekes, E.; Barkács, K.; Márialigeti, K. Vertical physico-chemical gradients with distinct microbial communities in the hypersaline and heliothermal Lake Ursu (Sovata, Romania). Extremophiles 2014, 18, 501–514. [Google Scholar] [CrossRef]
- Gyenge, L.; Erdo, K.; Albert, C.; Laslo, E.; Salamon, R.V. The effects of soaking in salted blackurrant wine on the properties of cheese. Heliyon 2024, 10, e34060. [Google Scholar] [CrossRef] [PubMed]
- Stommel, H.; Arons, A.B.; Blanchard, D. An oceanographical curiosity: The perpetual salt fountain. Deep Sea Res. 1956, 3, 152–153. [Google Scholar] [CrossRef]
- Stern, M.E. The “Salt-Fountain” and Thermohaline Convection. Tellus 1960, 12, 172–175. [Google Scholar] [CrossRef]
- Kunze, E. Limits on growing, finite-length salt fingers: A Richardson number constraint. J. Mar. Res. 1987, 45, 533–556. [Google Scholar] [CrossRef]
- Proctor, M.R.E.; Holyer, J.Y. Planform selection in salt fingers. J. Fluid Mech. 1986, 168, 241–253. [Google Scholar] [CrossRef]
- Schmitt, R.W. The growth rate of super-critical salt fingers. Deep Sea Res. Part A Oceanogr. Res. Pap. 1979, 26, 23–40. [Google Scholar] [CrossRef]
- Schmitt, R.W. The Ocean’s Salt Fingers. Sci. Am. 1995, 5, 70–75. [Google Scholar] [CrossRef]
- Gregg, M. Mixing in the Thermohaline Staircase East of Barbados. In Small-Scale Turbulence and Mixing in the Ocean; Elsevier Oceanography Series; Nihoul, J., Jamart, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 46, pp. 453–470. [Google Scholar] [CrossRef]
- Roquet, F.; Madec, G.; Brodeau, L.; Nycander, J. Defining a Simplified Yet “Realistic” Equation of State for Seawater. J. Phys. Oceanogr. 2015, 45, 2564–2579. [Google Scholar] [CrossRef]
- Mátyás, L.; Barna, I.F. Self-similar and traveling wave solutions of diffusion equations with concentration dependent diffusion coefficients. Rom. J. Phys. 2024, 69, 106. [Google Scholar] [CrossRef]
- Knight, G.; Georgiou, O.; Dettmann, C.P.; Klages, R. Dependence of chaotic diffusion on the size and position of holes. Chaos 2012, 22, 023132. [Google Scholar] [CrossRef]
- Sedov, L.I. Similarity and Dimensional Methods in Mechanics; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar] [CrossRef]
- Boltzmann, L. Zur Integration der Diffusionsgleichung bei variabeln Diffusions-coefficienten. Ann. Der Phys. 1894, 289, 959–964. [Google Scholar] [CrossRef]
- Szigeti, B.E.; Szapudi, I.; Barna, I.F.; Barnaföldi, G.G. Can rotation solve the Hubble Puzzle? Mon. Not. R. Astron. Soc. 2025, 538, 3038–3041. [Google Scholar] [CrossRef]
- Olver, F.W.J.; Lozier, D.W.; Boisvert, R.F.; Clark, C.W. The NIST Handbook of Mathematical Functions; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Márkus, F.; Gambár, K. Minimum Entropy Production Effect on a Quantum Scale. Entropy 2021, 23, 1350. [Google Scholar] [CrossRef] [PubMed]
- Ould Sidi, H.; Babatin, M.; Alosaimi, M.; Hendy, A.S.; Zaky, M.A. Simultaneous numerical inversion of space-dependent initial condition and source term in multi-order time-fractional diffusion models. Rom. Rep. Phys. 2024, 76, 104. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barna, I.F.; Mátyás, L. Analytic Solutions and Entropy Production of the Double-Diffusive Equation System. Entropy 2025, 27, 946. https://doi.org/10.3390/e27090946
Barna IF, Mátyás L. Analytic Solutions and Entropy Production of the Double-Diffusive Equation System. Entropy. 2025; 27(9):946. https://doi.org/10.3390/e27090946
Chicago/Turabian StyleBarna, Imre Ferenc, and László Mátyás. 2025. "Analytic Solutions and Entropy Production of the Double-Diffusive Equation System" Entropy 27, no. 9: 946. https://doi.org/10.3390/e27090946
APA StyleBarna, I. F., & Mátyás, L. (2025). Analytic Solutions and Entropy Production of the Double-Diffusive Equation System. Entropy, 27(9), 946. https://doi.org/10.3390/e27090946






