Brain Oscillations and Autonomic Synthonization via Comodulation in Collaborative Negotiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Exerimental Procedure
2.2.1. Negotiation Task
2.2.2. Individual Differences Assessment
2.3. EEG and Autonomic Data: Acquisition and Processing
2.4. Statistical Data Analyses
2.4.1. Synth Analysis
2.4.2. Cluster Analysis
3. Results
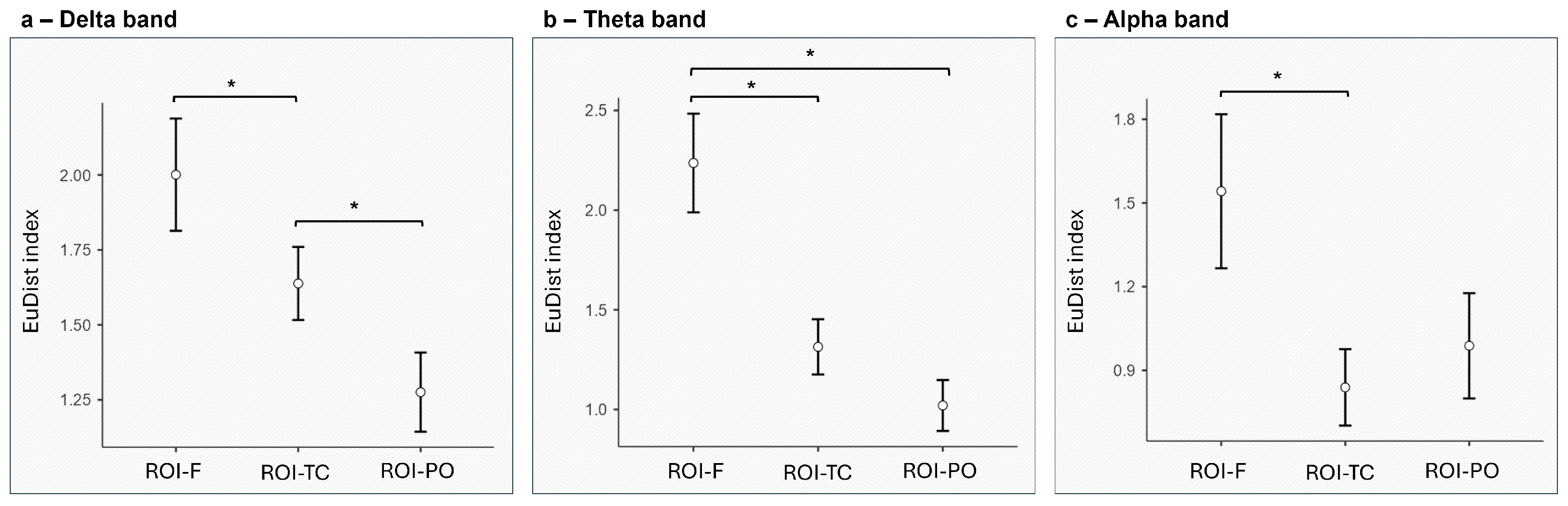
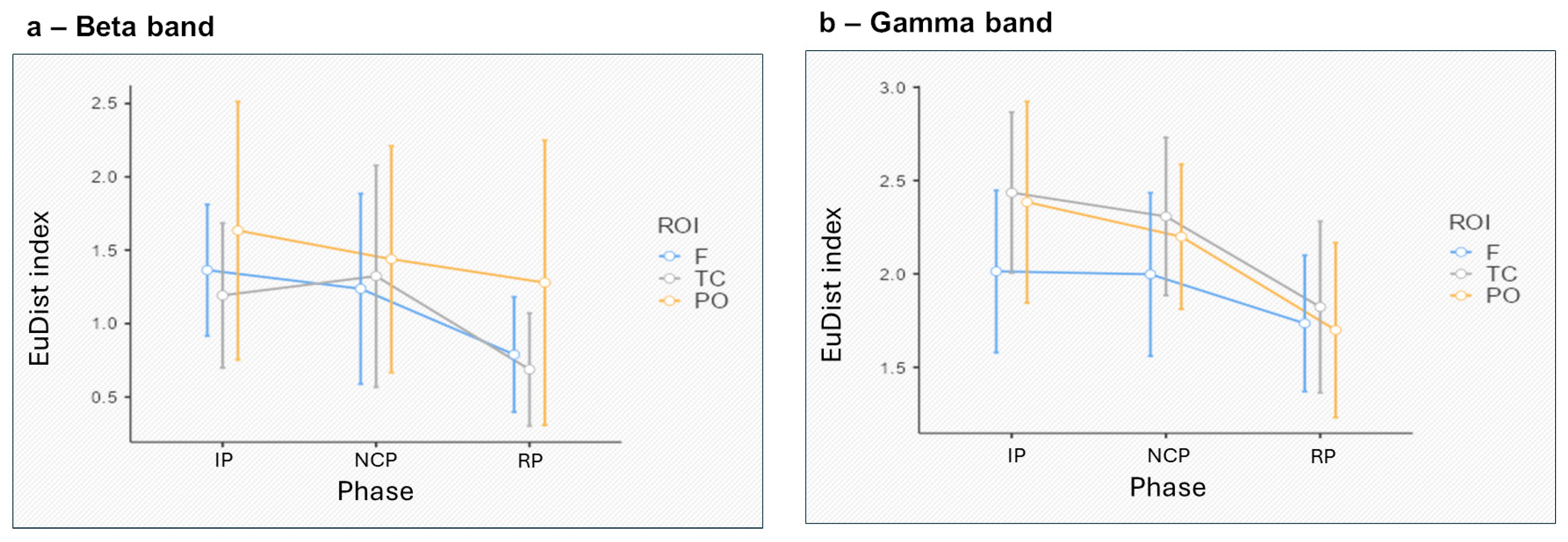
3.1. EEG Synth Results
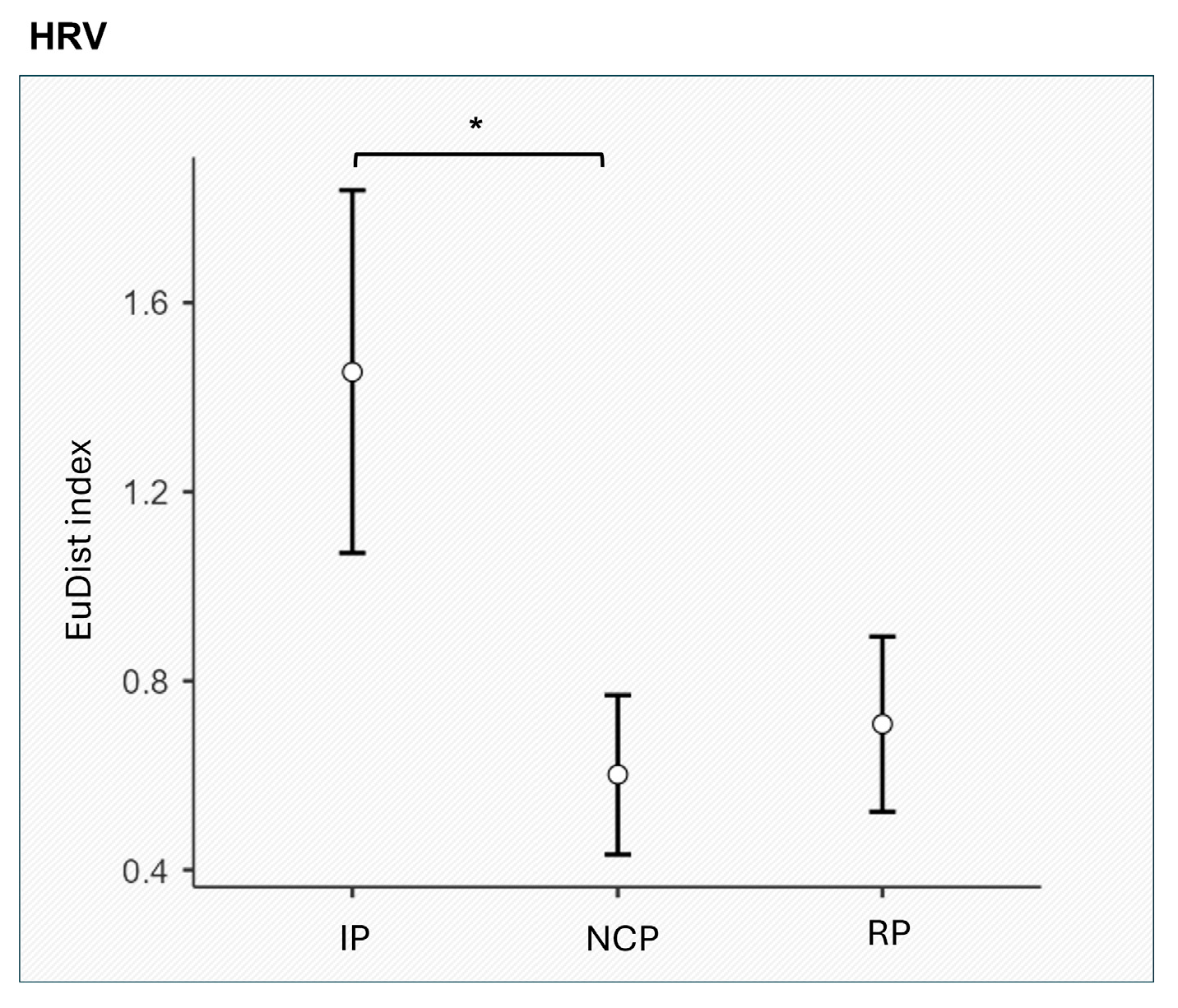
3.2. Autonomic Synth Results
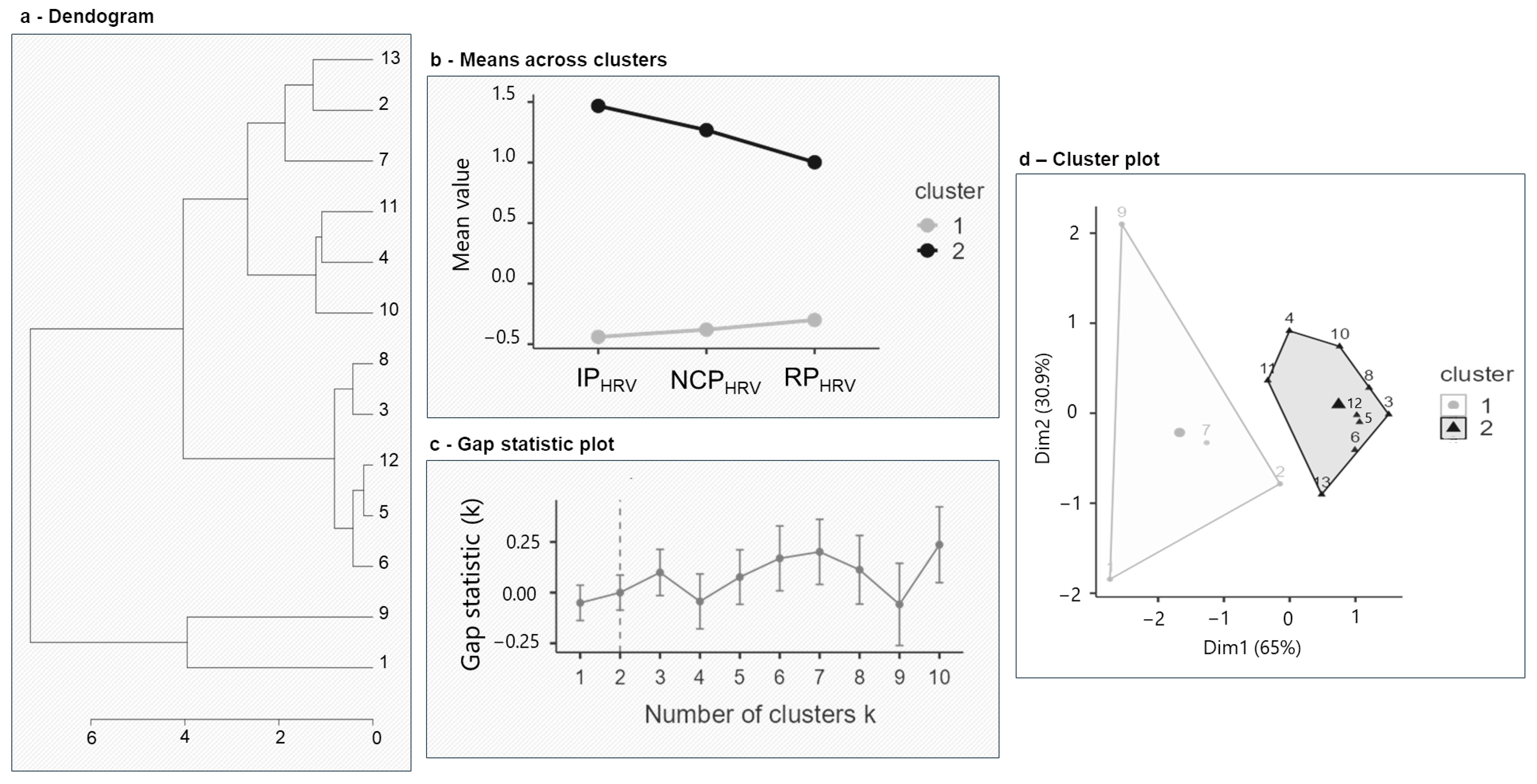
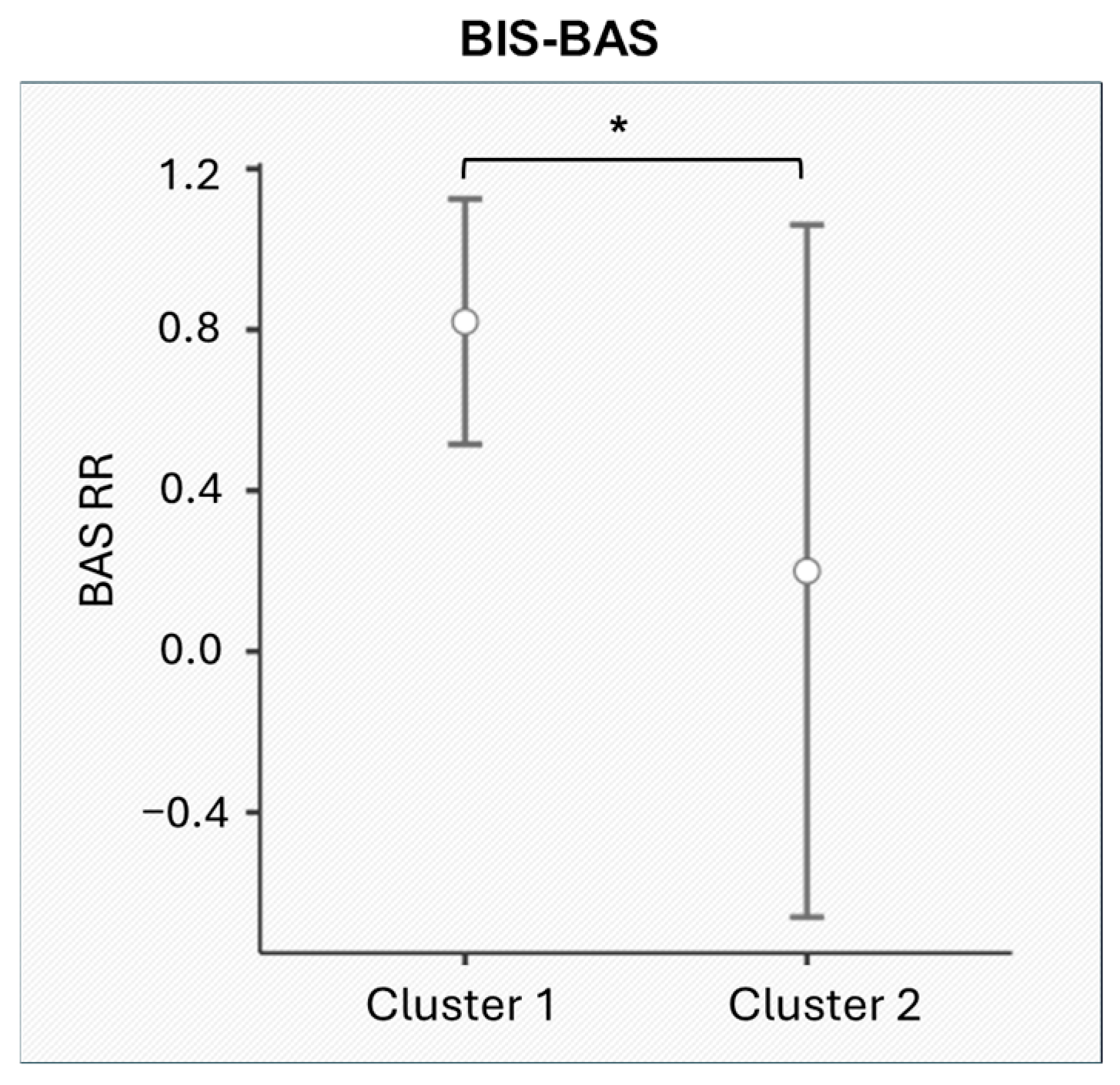
3.3. Cluster Analysis Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chater, N.; Zeitoun, H.; Melkonyan, T. The Paradox of Social Interaction: Shared Intentionality, We-Reasoning, and Virtual Bargaining. Psychol. Rev. 2022, 129, 415. [Google Scholar] [CrossRef] [PubMed]
- Bazerman, M.H.; Curhan, J.R.; Moore, D.A.; Valley, K.L. Negotiation. Annu. Rev. Psychol. 2000, 51, 279–314. [Google Scholar] [CrossRef]
- Cominelli, L.; Folgieri, R.; Lucchiari, C. Empowering Negotiating Skills in Law Professionals: Neuro-Cognitive Applications. In Neuroscience and Law: Complicated Crossings and New Perspectives; Springer: Berlin, Germany, 2020; pp. 139–161. [Google Scholar]
- Varga, S.; Krueger, J. Background Emotions, Proximity and Distributed Emotion Regulation. Rev. Philos. Psychol. 2013, 4, 271–292. [Google Scholar] [CrossRef]
- Barry, B.; Oliver, R.L. Affect in Dyadic Negotiation: A Model and Propositions. Organ. Behav. Hum. Decis. Process. 1996, 67, 127–143. [Google Scholar] [CrossRef]
- Ma, Z. Personality and Negotiation Revisited: Toward a Cognitive Model of Dyadic Negotiation. Manag. Res. News 2008, 31, 774–790. [Google Scholar] [CrossRef]
- Elfenbein, H.A. Individual Differences in Negotiation: A Relational Process Model. Organ. Psychol. Rev. 2021, 11, 73–93. [Google Scholar] [CrossRef]
- Sharma, S.; Bottom, W.P.; Elfenbein, H.A. On the Role of Personality, Cognitive Ability, and Emotional Intelligence in Predicting Negotiation Outcomes: A Meta-Analysis. Organ. Psychol. Rev. 2013, 3, 293–336. [Google Scholar] [CrossRef]
- Evans, J.S.B.T.; Stanovich, K.E. Dual-Process Theories of Higher Cognition: Advancing the Debate. Perspect. Psychol. Sci. 2013, 8, 223–241. [Google Scholar] [CrossRef]
- McCrae, R.R.; John, O.P. An Introduction to the Five-factor Model and Its Applications. J. Pers. 1992, 60, 175–215. [Google Scholar] [CrossRef] [PubMed]
- Putnam, L.L. Challenging the Assumptions of Traditional Approaches to Negotiation. Negot. J. 1994, 10, 337. [Google Scholar] [CrossRef]
- Goodpaster, G. Rational Decision-Making in Problem-Solving Negotiation: Compromise, Interest-Valuation, and Cognitive Error. Ohio St. J. Disp. Resol. 1992, 8, 299. [Google Scholar]
- Rhoades, J.A.; Carnevale, P.J. The Behavioral Context of Strategic Choice in Negotiation: A Test of the Dual Concern Model 1. J. Appl. Soc. Psychol. 1999, 29, 1777–1802. [Google Scholar] [CrossRef]
- Tolan, C.; Cai, D.A.; Fink, E.L. Expectations, Conflict Styles, and Anchors in Negotiation. Negot. Confl. Manag. Res. 2023, 16, 247–266. [Google Scholar]
- Tao, X.; Shen, Z.; Miao, C.; Theng, Y.-L.; Miao, Y.; Yu, H. Automated Negotiation through a Cooperative-Competitive Model. In Innovations in Agent-Based Complex Automated Negotiations; Springer: Berlin/Heidelberg, Germany, 2011; pp. 161–178. [Google Scholar]
- Hisaoka, M. Cooperation and Negotiation. In Subjectivity in Asian Children’s Literature and Film: Global Theories and Implications; Routledge: Oxfordshire, UK, 2012; Volume 59. [Google Scholar]
- Micklos, A.; Woensdregt, M. Cognitive and Interactive Mechanisms for Mutual Understanding in Conversation. In Oxford Research Encyclopedia of Communication; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- Roloff, M.E.; Van Swol, L.M. Shared Cognition and Communication within Group Decision Making and Negotiation. In Communication and Social Cognition: Theories and Methods; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2007; pp. 171–195. [Google Scholar]
- Decety, J.; Sommerville, J.A. Shared Representations between Self and Other: A Social Cognitive Neuroscience View. Trends Cogn. Sci. 2003, 7, 527–533. [Google Scholar] [CrossRef]
- Curhan, J.R.; Pentland, A. Thin Slices of Negotiation: Predicting Outcomes from Conversational Dynamics within the First 5 Minutes. J. Appl. Psychol. 2007, 92, 802. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, X.; Li, R.; Huang, H.; Hong, Y.; Li, X.; Li, Z.; Chen, S.; Yang, L.; Ong, S. Exploring the Neural Mechanisms Underlying Cooperation and Competition Behaviour: Insights from SEEG Hyperscanning. iScience 2024, 28, 111506. [Google Scholar]
- Smith, R.; Badcock, P.; Friston, K.J. Recent Advances in the Application of Predictive Coding and Active Inference Models within Clinical Neuroscience. Psychiatry Clin. Neurosci. 2021, 75, 3–13. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L.; Rovelli, K. Neurophysiological Response to Social Feedback in Stressful Situations. Eur. J. Neurosci. 2024, 60, 6030–6045. [Google Scholar] [CrossRef]
- Rovelli, K.; Balconi, M. Mind in Others’ Shoes: Neuroscientific Protocol for External Referent Decision Awareness (ERDA) in Organizations. Neuroscience 2025, 567, 249–260. [Google Scholar] [CrossRef]
- Dumas, G.; Martinerie, J.; Soussignan, R.; Nadel, J. Does the Brain Know Who Is at the Origin of What in an Imitative Interaction? Front. Hum. Neurosci. 2012, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Nácher, V.; Ledberg, A.; Deco, G.; Romo, R. Coherent Delta-Band Oscillations between Cortical Areas Correlate with Decision Making. Proc. Natl. Acad. Sci. USA 2013, 110, 15085–15090. [Google Scholar] [CrossRef]
- Tan, E.; Troller-Renfree, S.V.; Morales, S.; Buzzell, G.A.; McSweeney, M.; Antúnez, M.; Fox, N.A. Theta Activity and Cognitive Functioning: Integrating Evidence from Resting-State and Task-Related Developmental Electroencephalography (EEG) Research. Dev. Cogn. Neurosci. 2024, 67, 101404. [Google Scholar] [CrossRef]
- Karakaş, S. A Review of Theta Oscillation and Its Functional Correlates. Int. J. Psychophysiol. 2020, 157, 82–99. [Google Scholar] [CrossRef]
- Müller, V. Neural Synchrony and Network Dynamics in Social Interaction: A Hyper-Brain Cell Assembly Hypothesis. Front. Hum. Neurosci. 2022, 16, 848026. [Google Scholar] [CrossRef]
- Friston, K. The Free-Energy Principle: A Unified Brain Theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Basar, E. Memory and Brain Dynamics: Oscillations Integrating Attention, Perception, Learning, and Memory; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0429219075. [Google Scholar]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, R.M.; Foxe, J.J.; Mazaheri, A. The Functional Role of Alpha-Band Activity in Attentional Processing: The Current Zeitgeist and Future Outlook. Curr. Opin. Psychol. 2019, 29, 229–238. [Google Scholar] [CrossRef]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Frith, C.D.; Frith, U. The Neural Basis of Mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef]
- Beers, P.J.; Boshuizen, H.P.A.; Kirschner, P.A.; Gijselaers, W.H. Common Ground, Complex Problems and Decision Making. Group. Decis. Negot. 2006, 15, 529–556. [Google Scholar] [CrossRef]
- Billeke, P.; Zamorano, F.; Cosmelli, D.; Aboitiz, F. Oscillatory Brain Activity Correlates with Risk Perception and Predicts Social Decisions. Cereb. Cortex 2013, 23, 2872–2883. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L. Dyadic Inter-Brain EEG Coherence Induced by Interoceptive Hyperscanning. Sci. Rep. 2023, 13, 4344. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Theory: New Insights into Adaptive Reactions of the Autonomic Nervous System. Cleve Clin. J. Med. 2009, 76, S86. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Fronda, G.; Cassioli, F.; Crivelli, D. Face-to-Face vs. Remote Digital Settings in Job Assessment Interviews: A Multilevel Hyperscanning Protocol for the Investigation of Interpersonal Attunement. PLoS ONE 2022, 17, e0263668. [Google Scholar] [CrossRef]
- Balconi, M.; Lucchiari, C. In the face of emotions: Event-related potentials in supraliminal and subliminal facial expression recognition. Genet Soc Gen Psychol Monogr. 2005, 131, 41–69. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Ostrowski, M.; Hanly, P.; Younes, M. Relationship between Arousal Intensity and Heart Rate Response to Arousal. Sleep 2014, 37, 645–653. [Google Scholar] [CrossRef]
- Balconi, M.; Venturella, I.; Fronda, G.; de Filippis, D.; Salati, E.; Vanutelli, M.E. To Rate or Not to Rate? Autonomic Response and Psychological Well-Being of Employees during Performance Review. Health Care Manag. (Frederick) 2019, 38, 179–186. [Google Scholar] [CrossRef]
- McCraty, R.; Zayas, M.A. Cardiac Coherence, Self-Regulation, Autonomic Stability, and Psychosocial Well-Being. Front. Psychol. 2014, 5, 1090. [Google Scholar] [CrossRef] [PubMed]
- Mauss, I.B.; Zerwas, F.K.; Wilhelm, F.H.; John, O.P. Coherence of Emotional Response Systems: Theory, Measurement, and Benefits. Adv. Exp. Soc. Psychol. 2024, 69, 59–149. [Google Scholar]
- Hildebrandt, L.K.; McCall, C.; Engen, H.G.; Singer, T. Cognitive Flexibility, Heart Rate Variability, and Resilience Predict Fine-grained Regulation of Arousal during Prolonged Threat. Psychophysiology 2016, 53, 880–890. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Nes, L.S. Heart Rate Variability Reflects Self-Regulatory Strength, Effort, and Fatigue. Psychol. Sci. 2007, 18, 275–281. [Google Scholar] [CrossRef]
- Critchley, H.D. Electrodermal Responses: What Happens in the Brain. Neuroscientist 2002, 8, 132–142. [Google Scholar] [CrossRef]
- McCraty, R.; Shaffer, F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-Regulatory Capacity, and Health Risk. Glob. Adv. Health Med. 2015, 4, 46. [Google Scholar] [CrossRef]
- Scott, S.G.; Bruce, R.A. Decision-Making Style: The Development and Assessment of a New Measure. Educ. Psychol. Meas. 1995, 55, 818–831. [Google Scholar] [CrossRef]
- Gambetti, E.; Fabbri, M.; Bensi, L.; Tonetti, L. A Contribution to the Italian Validation of the General Decision-Making Style Inventory. Pers. Individ. Dif. 2008, 44, 842–852. [Google Scholar] [CrossRef]
- Nenkov, G.Y.; Morrin, M.; Ward, A.; Schwartz, B.; Hulland, J. A Short Form of the Maximization Scale: Factor Structure, Reliability and Validity Studies. Judgm. Decis. Mak. 2008, 3, 371–388. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A Neural Substrate of Prediction and Reward. Science (1979) 1997, 275, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Pierro, A.; Mannetti, L. Validità Della Versione Italiana Delle Scale BIS/BAS Di Carver e White (1994): Generalizzabilità Della Struttura e Relazioni Con Costrutti Affini. G. Ital. Psicol. 2002, 29, 413–436. [Google Scholar]
- Carver, C.S.; White, T.L. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319. [Google Scholar] [CrossRef]
- Guido, G.; Peluso, A.M.; Capestro, M.; Miglietta, M. An Italian Version of the 10-Item Big Five Inventory: An Application to Hedonic and Utilitarian Shopping Values. Pers. Individ. Dif. 2015, 76, 135–140. [Google Scholar] [CrossRef]
- Balconi, M.; Vandelli, G.V.; Angioletti, L. Be Creative to Innovate! EEG Correlates of Group Decision-Making in Managers. Sustainability 2024, 16, 2175. [Google Scholar] [CrossRef]
- Rovelli, K.; Angioletti, L.; Acconito, C.; Balconi, M. Neurosciences Tell Us How to Be Adaptable, Creative, and Proactive Agents in Decision Making: A Pilot Study. J. Neurosci. Psychol. Econ. 2023, 17, 30–45. [Google Scholar] [CrossRef]
- Balconi, M.; Rovelli, K. Does Emotional Valence Affect Cognitive Performance and Neurophysiological Response during Decision Making? A Preliminary Study. Front. Neurosci. 2024, 18, 1408526. [Google Scholar] [CrossRef]
- Teixeira, S.; Machado, S.; Velasques, B.; Sanfim, A.; Minc, D.; Peressutti, C.; Bittencourt, J.; Budde, H.; Cagy, M.; Anghinah, R. Integrative Parietal Cortex Processes: Neurological and Psychiatric Aspects. J. Neurol. Sci. 2014, 338, 12–22. [Google Scholar] [CrossRef]
- Schulte-Rüther, M.; Markowitsch, H.J.; Fink, G.R.; Piefke, M. Mirror Neuron and Theory of Mind Mechanisms Involved in Face-to-Face Interactions: A Functional Magnetic Resonance Imaging Approach to Empathy. J. Cogn. Neurosci. 2007, 19, 1354–1372. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V.; Goldman, A. Mirror Neurons and the Simulation Theory of Mind-Reading. Trends Cogn. Sci. 1998, 2, 493–501. [Google Scholar] [CrossRef]
- Jochemczyk, Ł.; Pietrzak, J.; Zawadzka, A. The Construction of Dynamical Negotiation Networks Depending on Need for Cognitive Closure. Lang. Sci. 2016, 53, 44–57. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The Role of Prefrontal Cortex in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Toba, M.N.; Malkinson, T.S.; Howells, H.; Mackie, M.A.; Spagna, A. Same, Same but Different? A Multi-Method Review of the Processes Underlying Executive Control. Neuropsychol. Rev. 2023, 34, 418–454. [Google Scholar] [CrossRef]
- Yeung, N. Conflict Monitoring and Cognitive Control. In The Oxford Handbook of Cognitive Neuroscience: The Cutting Edges; Oxford University Press: Oxford, UK, 2014; Volume 2, pp. 275–299. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Carter, C.S.; Braver, T.S.; Barch, D.M.; Cohen, J.D. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef]
- Connolly, J.; McNulty, J.P.; Boran, L.; Roche, R.A.P.; Delany, D.; Bokde, A.L.W. Identification of Resting State Networks Involved in Executive Function. Brain Connect. 2016, 6, 365–374. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J. Learning from Errors: Distinct Neural Networks for Monitoring Errors and Maintaining Corrects through Repeated Practice and Feedback. Neuroimage 2023, 271, 120001. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. EEG Brain Oscillations Are Modulated by Interoception in Response to a Synchronized Motor vs. Cognitive Task. Front. Neuroanat. 2022, 16, 991522. [Google Scholar] [CrossRef] [PubMed]
- Angioletti, L.; Acconito, C.; Balconi, M. An EEG Hyperscanning Study during Persuasion toward Groupness. The Frontal Brain Area Activation as a Function of Role. Soc. Neurosci. 2025, 19, 340–353. [Google Scholar] [CrossRef]
- Dragomir, A.; Omurtag, A. Brain’s Networks and Their Functional Significance in Cognition. In Handbook of Neuroengineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–30. [Google Scholar]
- Parks, E.L.; Madden, D.J. Brain Connectivity and Visual Attention. Brain Connect. 2013, 3, 317–338. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z. A Brief Review of Chimera State in Empirical Brain Networks. Front. Physiol. 2020, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Bera, B.K.; Ghosh, D.; Perc, M. Chimera States in Neuronal Networks: A Review. Phys. Life Rev. 2019, 28, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Alpha-Band Oscillations, Attention, and Controlled Access to Stored Information. Trends Cogn. Sci. 2012, 16, 606. [Google Scholar] [CrossRef]
- Clark, A. Whatever next? Predictive Brains, Situated Agents, and the Future of Cognitive Science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Kok, P.; de Lange, F.P. Predictive Coding in Sensory Cortex. In An Introduction to Model-Based Cognitive Neuroscience; Springer: Berlin/Heidelberg, Germany, 2015; pp. 221–224. [Google Scholar]
- Samuel, I.B.H.; Wang, C.; Hu, Z.; Ding, M. The Frequency of Alpha Oscillations: Task-Dependent Modulation and Its Functional Significance. Neuroimage 2018, 183, 897–906. [Google Scholar] [CrossRef]
- Atzil, S.; Hendler, T.; Feldman, R. The Brain Basis of Social Synchrony. Soc. Cogn. Affect. Neurosci. 2013, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Allegretta, R.A.; Rovelli, K.; Balconi, M. The Role of Emotion Regulation and Awareness in Psychosocial Stress: An EEG-Psychometric Correlational Study. Healthcare 2024, 12, 1491. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. Social Interoception and Autonomic System Reactivity during Synchronization Behavior. Behav. Sci. 2024, 14, 149. [Google Scholar] [CrossRef]
- Banu, A.R.S.; Nagaveni, V. Assessment of Sympathetic and Parasympathetic Activities of Nervous System from Heart Rate Variability Using Machine Learning Techniques. SN Comput. Sci. 2023, 4, 646. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Goldberger, J.J. Sympathovagal Balance: How Should We Measure It? Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H1273–H1280. [Google Scholar] [CrossRef]
- Galván, A. Neural Systems Underlying Reward and Approach Behaviors in Childhood and Adolescence. In The Neurobiology of Childhood; Springer: Berlin/Heidelberg, Germany, 2013; pp. 167–188. ISSN 1866-3389. ISBN 978-3-642-54913-7. [Google Scholar]
- Slavin, R.E. When and Why Does Cooperative Learning Increase Achievement? Theoretical and Empirical Perspectives. In The Routledge Falmer Reader in Psychology of Education; Routledge: London, UK, 2004; pp. 271–293. [Google Scholar]
- Kalyuga, S. Cognitive Load Theory. Managing Cognitive Load in Adaptive Multimedia Learning; IGI Global: Hershey, PA, USA, 2009; pp. 34–57. [Google Scholar]
- Paas, F.; Van Gog, T.; Sweller, J. Cognitive Load Theory: New Conceptualizations, Specifications, and Integrated Research Perspectives. Educ. Psychol. Rev. 2010, 22, 115–121. [Google Scholar] [CrossRef]
- Bandhu, D.; Mohan, M.M.; Nittala, N.A.P.; Jadhav, P.; Bhadauria, A.; Saxena, K.K. Theories of Motivation: A Comprehensive Analysis of Human Behavior Drivers. Acta Psychol. 2024, 244, 104177. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovelli, K.; Acconito, C.; Angioletti, L.; Balconi, M. Brain Oscillations and Autonomic Synthonization via Comodulation in Collaborative Negotiation. Entropy 2025, 27, 873. https://doi.org/10.3390/e27080873
Rovelli K, Acconito C, Angioletti L, Balconi M. Brain Oscillations and Autonomic Synthonization via Comodulation in Collaborative Negotiation. Entropy. 2025; 27(8):873. https://doi.org/10.3390/e27080873
Chicago/Turabian StyleRovelli, Katia, Carlotta Acconito, Laura Angioletti, and Michela Balconi. 2025. "Brain Oscillations and Autonomic Synthonization via Comodulation in Collaborative Negotiation" Entropy 27, no. 8: 873. https://doi.org/10.3390/e27080873
APA StyleRovelli, K., Acconito, C., Angioletti, L., & Balconi, M. (2025). Brain Oscillations and Autonomic Synthonization via Comodulation in Collaborative Negotiation. Entropy, 27(8), 873. https://doi.org/10.3390/e27080873








