Epidemic Dynamics and Intervention Measures in Campus Settings Based on Multilayer Temporal Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
- The total student population is approximately 21,800, with a dormitory occupancy rate exceeding 95%, and the dormitories are shared by 2 to 4 students.
- Fixed class hours are scheduled daily (from 7:00 a.m. to 9:15 p.m.), and the course schedule drives the spatiotemporal distribution of the student population.
- Functional areas are concentrated (e.g., classroom buildings, canteens, sports complexes), with frequent interarea movement.
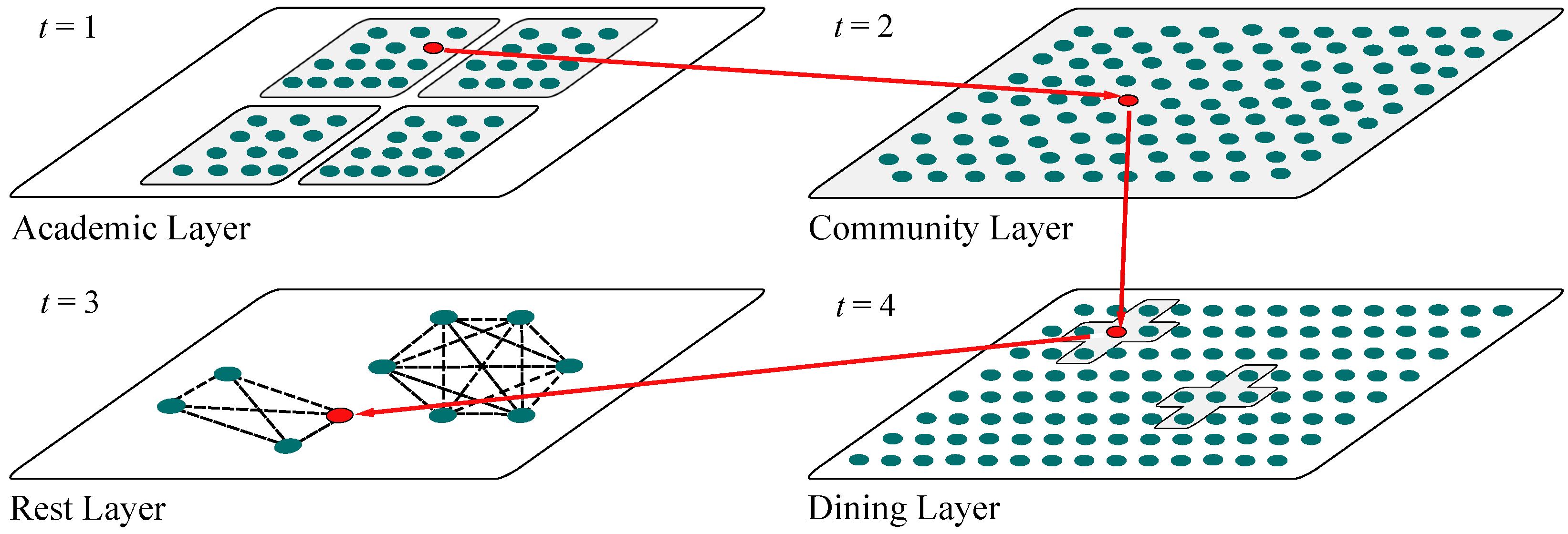
2.2. Multilayer Temporal Campus Network Model
- According to the official timetable, individuals prioritize entering the Academic Layer (A) based on the schedule of the day’s classes;
- When individuals are not in the Academic Layer and the Dining Layer is open, they will enter the Dining Layer (D);
- When individuals are not in the Academic Layer and the Dining Layer is closed, they will enter the Community Layer (C) with probability . This probability is given by , where is an indicator function, which takes the value of 1 when the individual is in the Academic Layer at time i and 0 otherwise. The probability grows non-linearly with time and the accumulation of class duration, making the tendency to enter the Community Layer significantly stronger during the evening hours (when t is larger). To constrain the probability within the [0, 1] range, it is further normalized by the total cumulative amount ;
- Individuals stay in each subnetwork for one time step, completing interactions within the layer before choosing the next active subnetwork.
3. Results
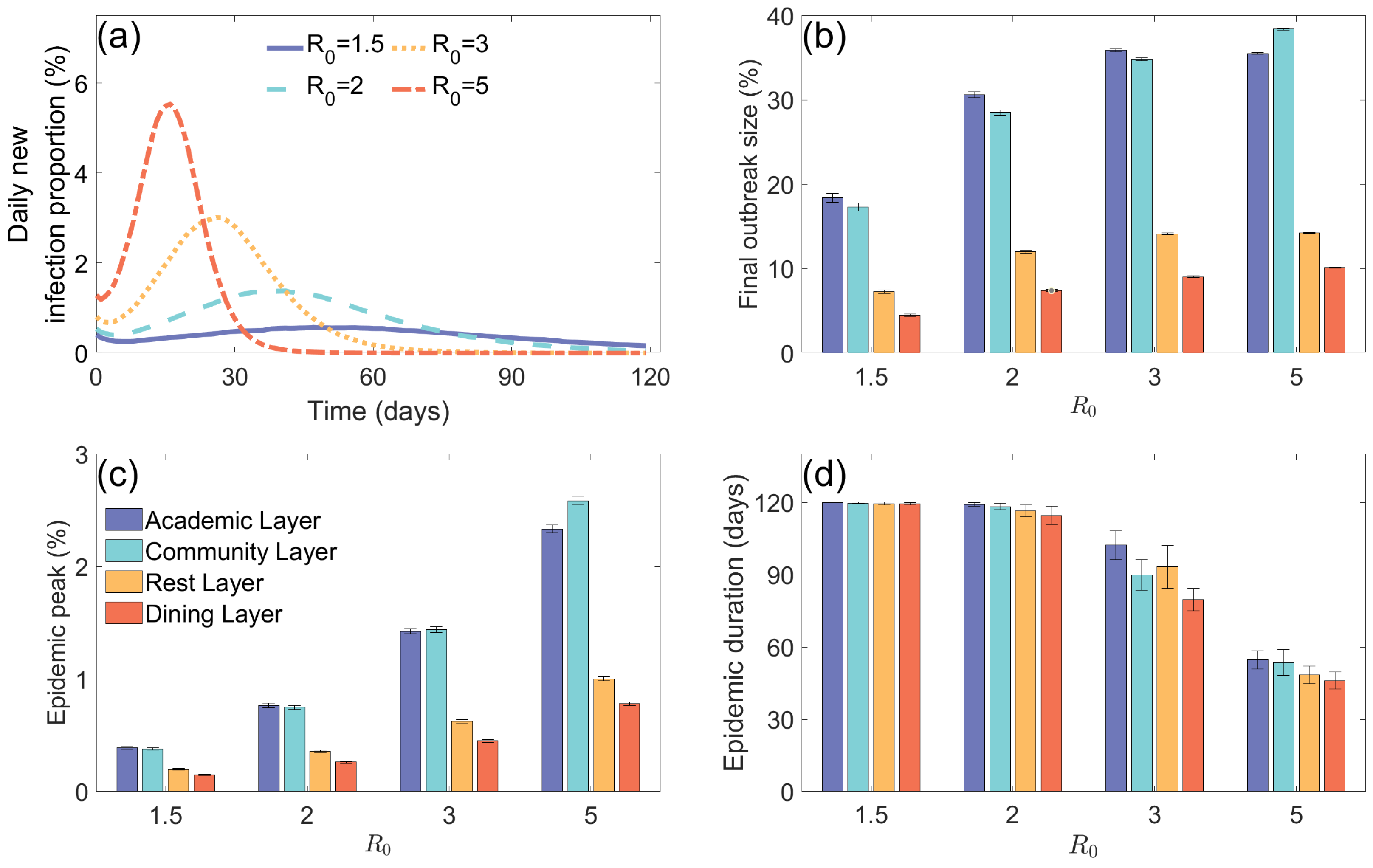
3.1. Epidemic Situation Analysis in Multilayer Coupled Networks and Their Subnetworks
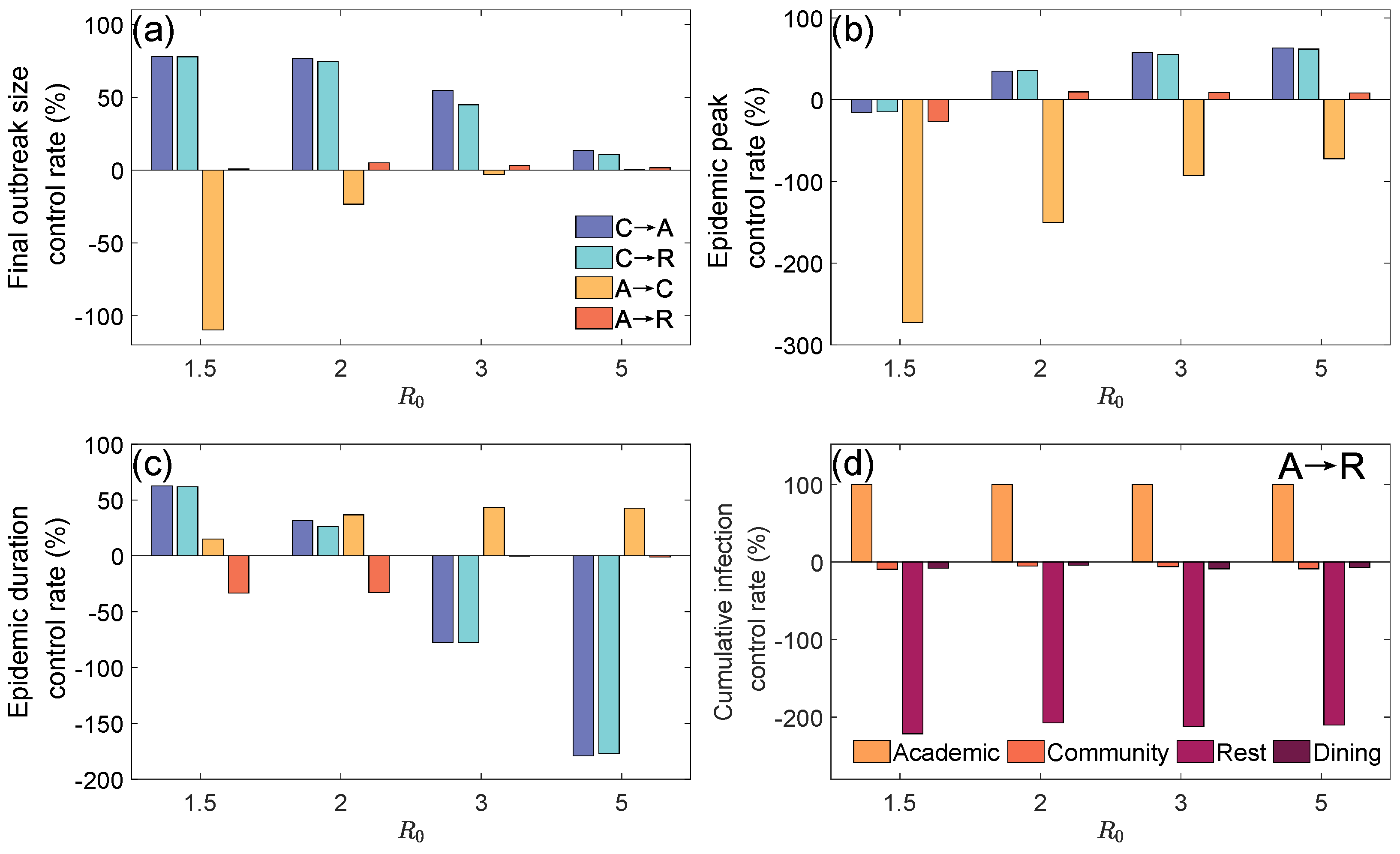
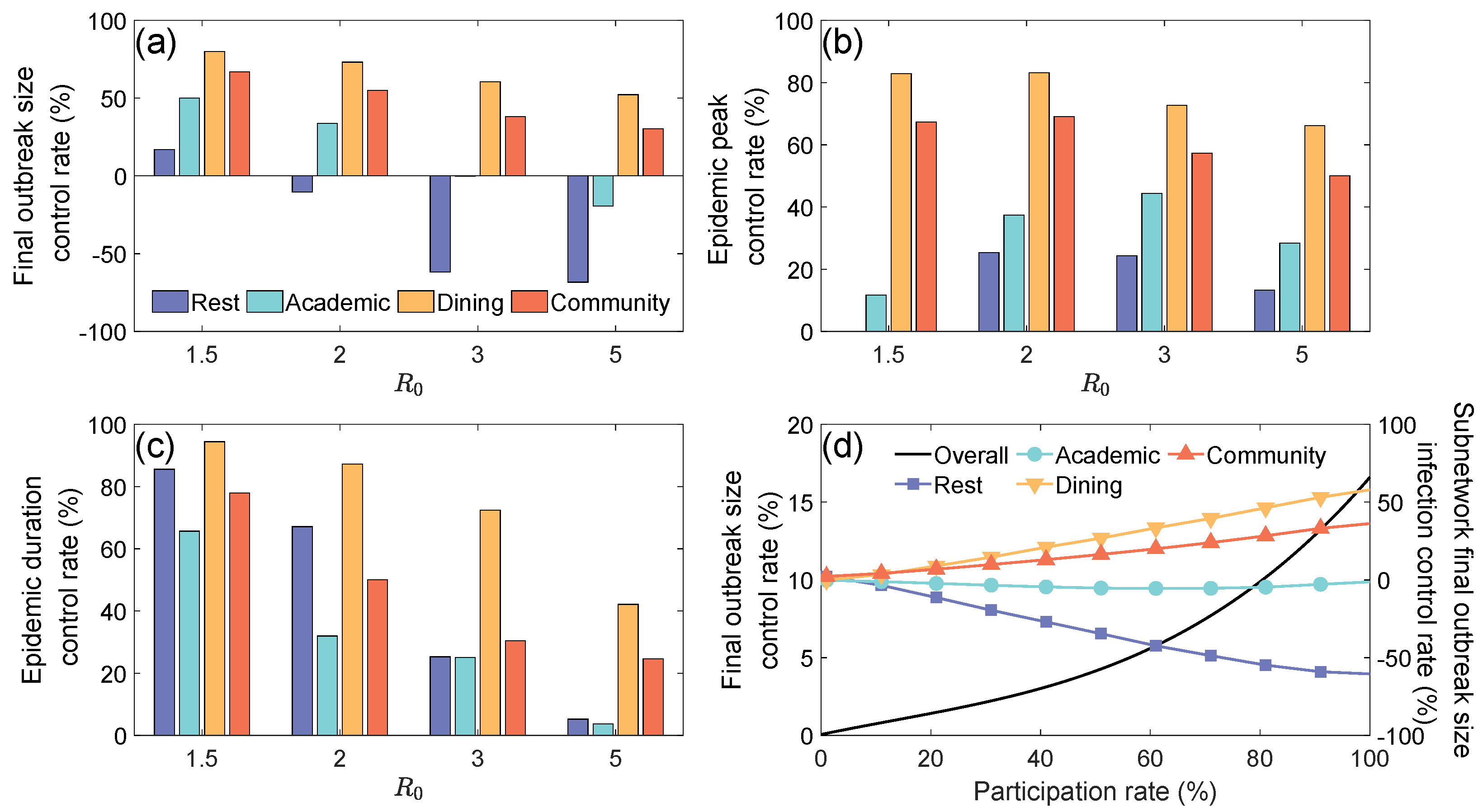
3.2. The Suppressive Effects of Subnetwork (Location) Closure Measures
- Closing the Community Layer: Nodes and edges originally belonging to the Community Layer at time t were entirely redistributed to the Academic Layer () or to the Rest Layer ();
- Closing the Academic Layer: Nodes and edges originally belonging to the Academic Layer at time t were entirely redistributed to the Community Layer () or to the Rest Layer ().
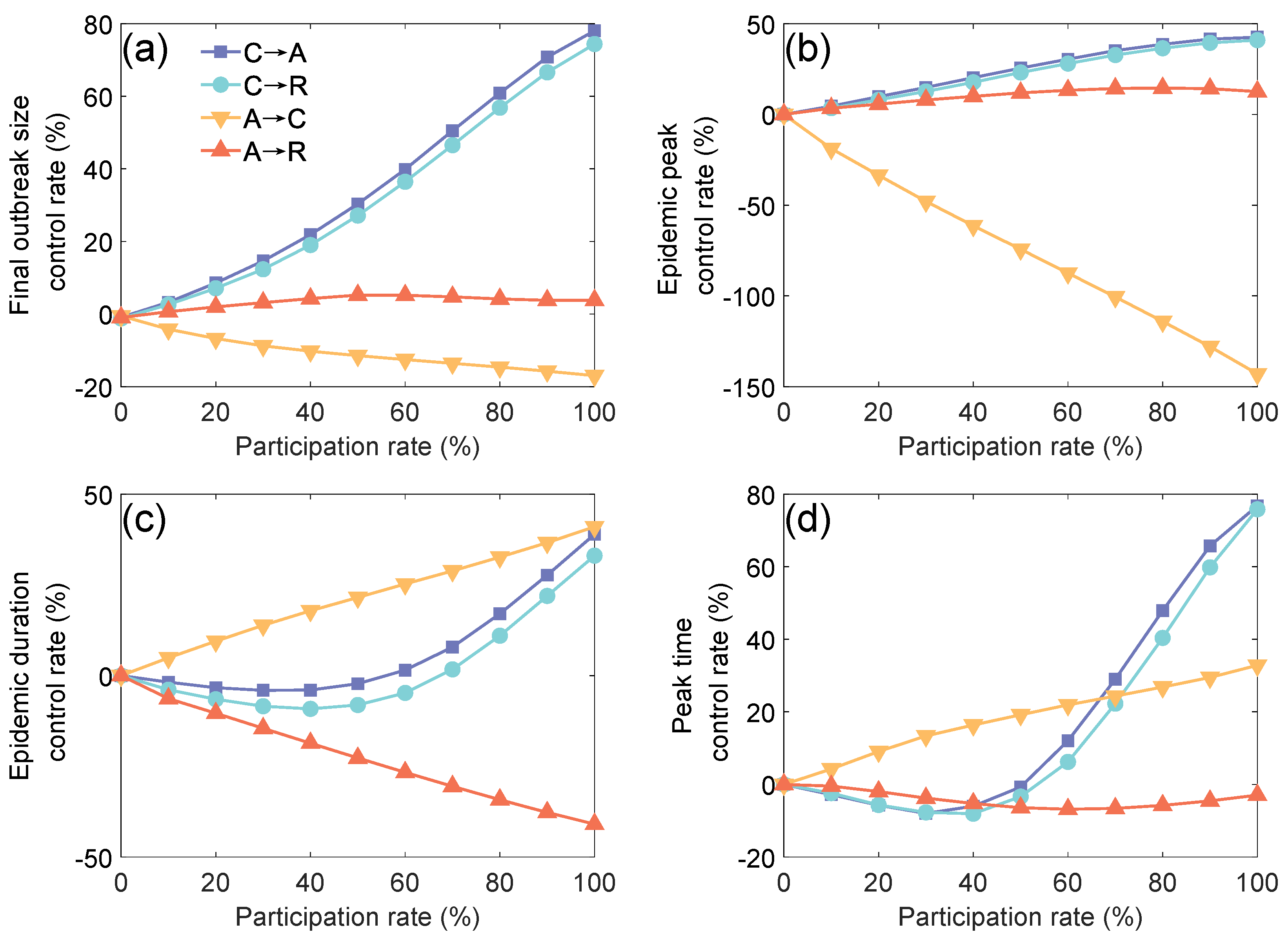
3.3. Impact of Zoned Management on Campus Epidemic Spreading
4. Discussion and Conclusions
- Routine Control Phase (): Focus on controlling the Community Layer, implementing reservation-based flow restrictions (≤200 people per time slot) and staggered time slots (strict control between 18:00 and 21:00);
- Emergency Response Phase (): Initiate partitioned management of the Academic Layer, splitting large classes (>100 people) into smaller sessions and restricting cross-area course selections;
- Emergency State (): Implement grid-based management across the entire campus, creating independent living zones by dormitory buildings, along with online teaching and meal delivery measures.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1991; pp. 1–15. [Google Scholar]
- Zhao, B.; Wang, X.; Zhang, T.; Wang, H. Estimating and modeling spontaneous mobility changes during the COVID-19 pandemic without stay-at-home orders. Humanit. Soc. Sci. Commun. 2024, 11, 1–15. [Google Scholar] [CrossRef]
- Ingelbeen, B.; van Kleef, E.; Mbala, P.; Danis, K.; Macicame, I.; Hens, N.; Cleynen, E.; van der Sande, M.A. Embedding risk monitoring in infectious disease surveillance for timely and effective outbreak prevention and control. BMJ Glob. Health 2025, 10, e016870. [Google Scholar] [CrossRef]
- Calmon, L.; Colosi, E.; Bassignana, G.; Colizza, V. Preserving friendships in school contacts: An algorithm to construct synthetic temporal networks for epidemic modelling. PLoS Comput. Biol. 2024, 20, e1012661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhang, Y. Numerical sensitivity analysis of the susceptible-infected-susceptible model on two-layer interconnected networks. Sci. Rep. 2025, 15, 9723. [Google Scholar] [CrossRef]
- Ali, A.; Sun, M.; Ali, M.R. Data analysis of dynamical system for the optimization of disease dynamics through Neural Networks Paradigm. Chaos Solitons Fractals 2025, 196, 116284. [Google Scholar] [CrossRef]
- Zhu, W.; Meng, X.; Sheng, J.; Zhang, D. Identifying vital spreaders in large-scale networks based on neighbor multilayer contributions. Front. Phys. 2025, 13, 1529904. [Google Scholar] [CrossRef]
- Khomami, M.M.D.; Meybodi, M.R.; Rezvanian, A. Efficient identification of maximum independent sets in stochastic multilayer graphs with learning automata. Results Eng. 2024, 24, 103224. [Google Scholar] [CrossRef]
- Boccaletti, S.; Bianconi, G.; Criado, R.; del Genio, C.I. The structure and dynamics of multilayer networks. Phys. Rep. 2014, 544, 1–122. [Google Scholar] [CrossRef]
- Wang, J.; He, H.; Li, P.; Du, Z.; Xu, X. Epidemic spread dynamics in multilayer networks: Probing the impact of information outbreaks and reception games. Chaos 2025, 35, 033118. [Google Scholar] [CrossRef]
- Liu, Q.-H.; Zhang, J.; Peng, C.; Litvinova, M.; Huang, S.; Poletti, P.; Trentini, F.; Guzzetta, G.; Marziano, V.; Zhou, T.; et al. Model-based evaluation of alternative reactive class closure strategies against COVID-19. Nat. Commun. 2022, 13, 322. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Y.; Liu, Y.; Tang, M.; Liu, Y.; Jin, Z.; Do, Y.; Pelinovsky, E.; Kirillin, M.; Macau, E. The impact of social interventions on COVID-19 spreading based on multilayer commuter networks. Chaos Solitons Fractals 2024, 185, 115160. [Google Scholar] [CrossRef]
- Chen, P.; Guo, X.; Jiao, Z.; Liang, S.; Li, L.; Yan, J.; Huang, Y.; Liu, Y.; Fan, W. A multilayer network model for studying the impact of non-pharmaceutical interventions implemented in response to COVID-19. Front. Phys. 2022, 10, 915441. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Tang, M.; Lai, Y.C. Epidemic dynamics with non-Markovian travel in multilayer networks. Commun. Phys. 2023, 6, 263. [Google Scholar] [CrossRef]
- Ma, J.; Wang, P. Epidemic spreading on multilayer community networks. Phys. Lett. A 2025, 532, 130199. [Google Scholar] [CrossRef]
- Nordholm, A.C.; Søborg, B.; Jokelainen, P.; Møller, K.L.; Sørensen, L.F.; Krause, T.G.; Uldum, S.A.; Emborg, H.-D. Mycoplasma pneumoniae epidemic in Denmark, October to December, 2023. Eurosurveillance 2024, 29, 2300707. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Yang, W.; Wang, C.; Bärnighausen, T. COVID-19 control in China during mass population movements at New Year. Lancet 2020, 395, 764–775. [Google Scholar] [CrossRef]
- Hekmati, A.; Luhar, M.; Krishnamachari, B.; Matarić, M. Simulation-based analysis of COVID-19 spread through classroom transmission on a university campus. In Proceedings of the IEEE ICC Workshops, Montreal, QC, Canada, 14–18 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Tatapudi, H.; Das, T.K. Impact of school reopening on pandemic spread: A case study using an agent-based model for COVID-19. Infect. Dis. Model. 2021, 6, 839–851. [Google Scholar] [CrossRef]
- Aleta, A.; Martin-Corral, D.; Piontti, A.P.Y.; Ajelli, M.; Litvinova, M.; Chinazzi, M.; Dean, N.E.; Halloran, M.E.; Longini, I.M.; Merler, S.; et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 2020, 4, 964–971. [Google Scholar] [CrossRef]
- Xu, C.; Kumar, S.; Yang, M.; Wang, H. From node to network: Weaving a global perspective on efficacy and costs of non-pharmaceutical interventions. Sci. Rep. 2025, 15, 2844. [Google Scholar] [CrossRef]
- Gu, W.; Qiu, Y.; Li, W.; Zhang, Z.; Liu, X.; Song, Y.; Wang, W. Epidemic spreading on spatial higher-order network. Chaos 2024, 34, 073105. [Google Scholar] [CrossRef]
- Wang, W.; Nie, Y.; Li, W.; Lin, T.; Shang, M.-S.; Su, S.; Tang, Y.; Zhang, Y.-C.; Sun, G.-Q. Epidemic spreading on higher-order networks. Phys. Rep. 2024, 1056, 1–137. [Google Scholar] [CrossRef]
- Da Silva, P.C.V.; Velásquez-Rojas, F.; Connaughton, C.; Vazquez, F.; Moreno, Y.; Rodrigues, F.A. Epidemic spreading with awareness and different timescales in multiplex networks. Phys. Rev. E 2019, 100, 032313. [Google Scholar] [CrossRef]
- Velásquez-Rojas, F.; Ventura, P.C.; Connaughton, C.; Moreno, Y.; Rodrigues, F.A.; Vazquez, F. Disease and information spreading at different speeds in multiplex networks. Phys. Rev. E 2020, 102, 022312. [Google Scholar] [CrossRef]
- Albi, G.; Bertaglia, G.; Boscheri, W.; Dimarco, G.; Pareschi, L.; Toscani, G.; Zanella, M. Kinetic Modelling of Epidemic Dynamics: Social Contacts, Control with Uncertain Data, and Multiscale Spatial Dynamics. In Predicting Pandemics in a Globally Connected World, Volume 1: Toward a Multiscale, Multidisciplinary Framework through Modeling and Simulation; Bellomo, N., Chaplain, M.A.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 43–108. [Google Scholar]
- Jang, Y.; Lee, H.; Park, H. Surveillance system for infectious disease prevention and management: Direction of Korea’s infectious disease surveillance system. J. Korean Med. Sci. 2025, 40, e108. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Nian, F.; Li, F.; Cui, Y. Information dissemination and phase transition in fractal social networks. Commun. Nonlinear Sci. Numer. Simul. 2025, 146, 108812. [Google Scholar] [CrossRef]
- Indravudh, P.P.; McGee, K.; Sibanda, E.L.; Corbett, E.L.; Fielding, K.; Terris-Prestholt, F. Community-led strategies for communicable disease prevention and management in low-and middle-income countries: A mixed-methods systematic review of health, social, and economic impact. PLoS Glob. Public Health 2025, 5, e0004304. [Google Scholar] [CrossRef]
- Zaidh, S.M.; Vengateswaran, H.T.; Habeeb, M.; Aher, K.B.; Bhavar, G.B.; Irfan, N.; Lakshmi, K.N.V. Network pharmacology and AI in cancer research uncovering biomarkers and therapeutic targets for RALGDS mutations. Sci. Rep. 2025, 15, 1–13. [Google Scholar] [CrossRef]
- Eom, J.K.; Stone, J.R.; Ghosh, S.K. Daily activity patterns of university students. J. Urban Plan. Dev. 2009, 135, 141–149. [Google Scholar] [CrossRef]
- Zhang, J.; Litvinova, M.; Liang, Y.; Wang, Y.; Wang, W.; Zhao, S.; Wu, Q.; Merler, S.; Viboud, C.; Vespignani, A.; et al. Changes in contact patterns shape the dynamics of the covid-19 outbreak in China. Science 2020, 368, 1481–1486. [Google Scholar] [CrossRef]
- Sanchez, S.; Lin, Y.T.; Xu, C. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1470–1477. [Google Scholar] [CrossRef]
- Bondesan, A.; Piralla, A.; Ballante, E.; Pitrolo, A.M.G.; Figini, S.; Baldanti, F.; Zanella, M. Predictability of viral load dynamics in the early phases of SARS-CoV-2 through a model-based approach. Math. Biosci. Eng. 2025, 22, 725–743. [Google Scholar] [CrossRef] [PubMed]
| Time | Schedule |
|---|---|
| 7:00–9:00 | Breakfast |
| 8:00–12:15 | Class |
| 11:00–13:00 | Lunch |
| 13:00–17:15 | Class |
| 17:00–19:00 | Dinner |
| 18:00–21:15 | Class |
| 22:30–6:30 | Sleep |
| Parameter | Description | Value |
|---|---|---|
| N | Total population size | 21,800 |
| Number of colleges | 29 | |
| Number of majors | 58 | |
| Number of grades | 4 | |
| Total courses offered | 2160 | |
| Class capacity | 24–278 | |
| Dormitory capacity | 2–4 | |
| Number of dining halls | 10 | |
| Dining hall capacity | 24–278 | |
| Basic reproduction number | 1.5, 2, 3, 5 | |
| Mean incubation period (days) | 5.1 [31] | |
| Latent-to-infectious transition rate | ||
| Mean infectious period (days) | 5 [32] | |
| Recovery rate | ||
| Disease transmission rate | [1] | |
| Participation rate | 0–100% | |
| Time delay (days) | 1, 2, 5, 7 | |
| Average degree of subworks | , , | |
| , [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tang, M. Epidemic Dynamics and Intervention Measures in Campus Settings Based on Multilayer Temporal Networks. Entropy 2025, 27, 543. https://doi.org/10.3390/e27050543
Zhang X, Tang M. Epidemic Dynamics and Intervention Measures in Campus Settings Based on Multilayer Temporal Networks. Entropy. 2025; 27(5):543. https://doi.org/10.3390/e27050543
Chicago/Turabian StyleZhang, Xianyang, and Ming Tang. 2025. "Epidemic Dynamics and Intervention Measures in Campus Settings Based on Multilayer Temporal Networks" Entropy 27, no. 5: 543. https://doi.org/10.3390/e27050543
APA StyleZhang, X., & Tang, M. (2025). Epidemic Dynamics and Intervention Measures in Campus Settings Based on Multilayer Temporal Networks. Entropy, 27(5), 543. https://doi.org/10.3390/e27050543





