Entropy Production in Epithelial Monolayers Due to Collective Cell Migration
Abstract
1. Introduction
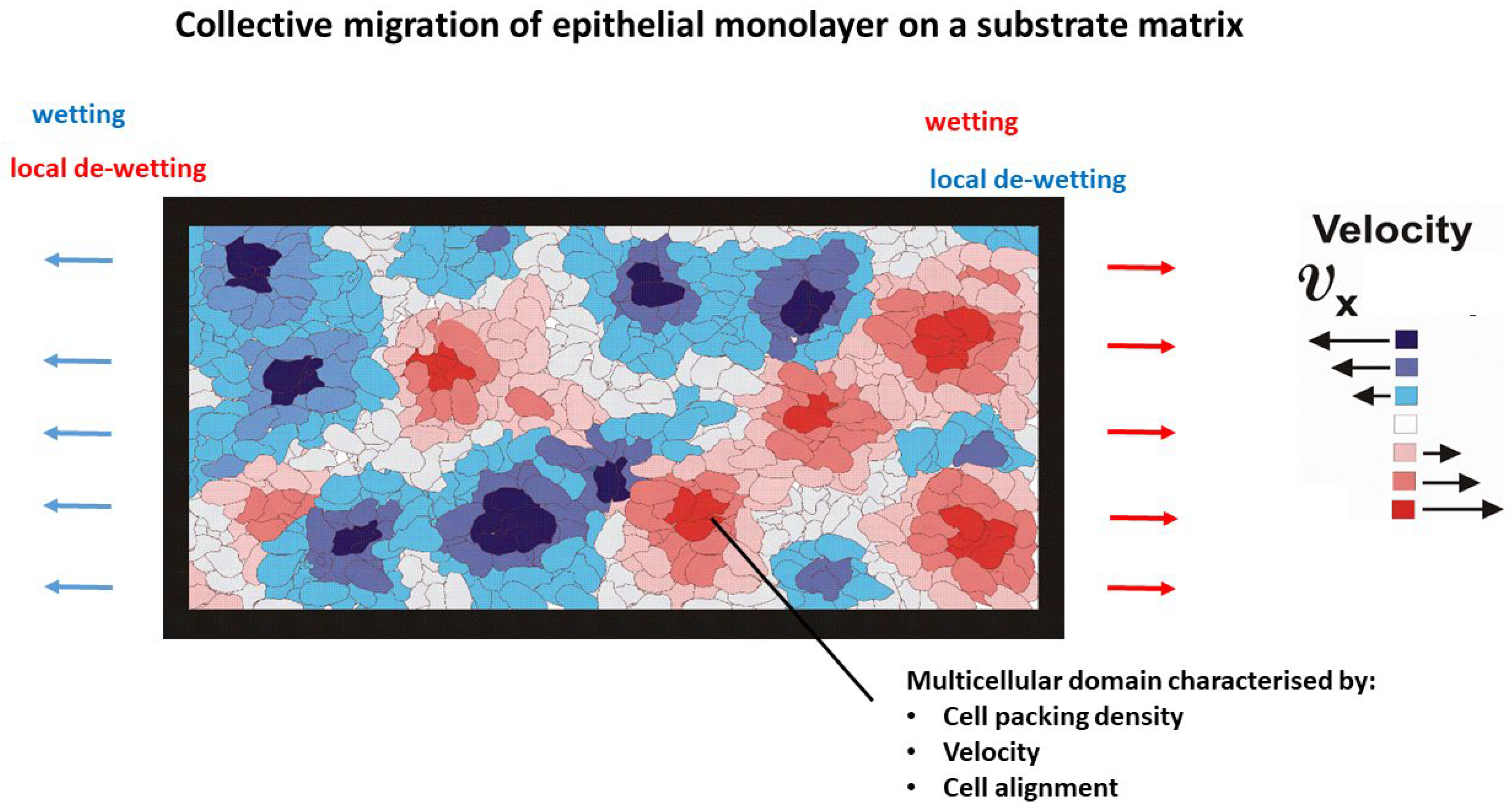
2. Phenomenological Description of Migrating Epithelial Monolayers on Substrate Matrices
- Every domain represents a canonical ensemble of cells and can be described by homogeneous distributions of cell packing density, cell velocity, degree of cell orientation in the direction of movement, and cell mechanical stress.
- The lifetime of domains is on a time scale of hours.
- Vanishing of the domains is caused by (i) domain active and passive wetting/de-wetting and (ii) collisions between neighbouring domains.
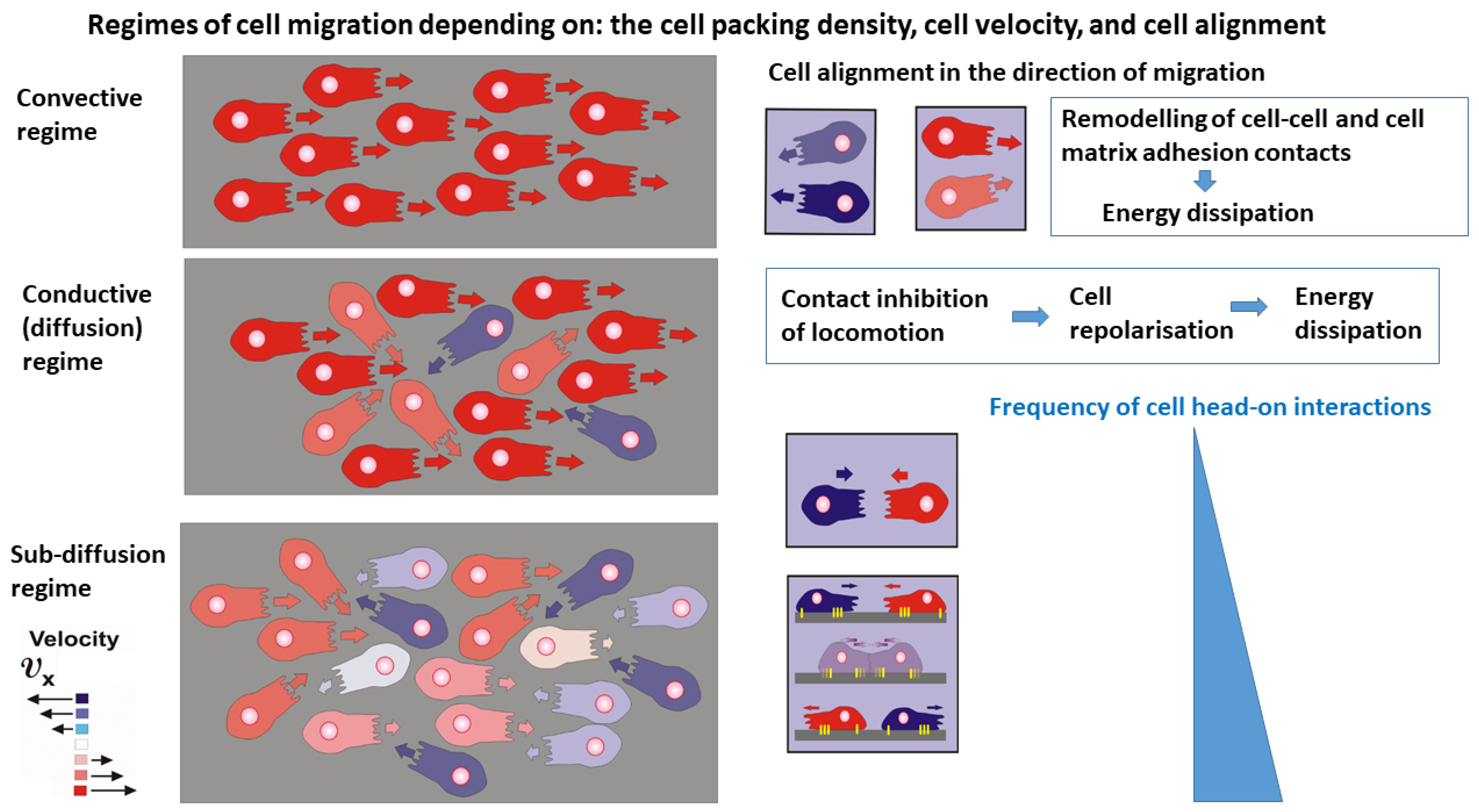
- The convective mechanism of cell migration arises within domains that satisfy the conditions that the cell packing density is and the cell speed is . The velocity correlation length, i.e., the distance over which cells move in a correlated fashion, is about 10 cell lengths [17], indicating an anisotropic behaviour. Contact inhibition of locomotion, caused by cell orientational interactions, is rare. Energy dissipation is caused primarily by the remodelling of cell-cell and cell-matrix adhesion contacts that occurs on a time scale of minutes [19].
- The conductive (diffusion) mechanism of cell migration arises within domains of higher cell packing density, i.e., for cell packing density and (where is the cell packing density under cell jamming). An increase in cell packing density perturbs the cell alignment, quantified by a decrease in the velocity correlation length [17,32]. This regime of cell packing densities could be considered as an isotropic cell rearrangement. The perturbation of cell alignment leads to an increase in contact inhibition of locomotion [25,29,30]. Altered cell re-polarisation accompanied by a weakening of cell-cell and cell-matrix adhesion contacts is the main cause of energy dissipation in this regime [29,30]. Consequently, energy dissipation occurs on a time scale of hours. The main characteristic of this regime is that the re-polarisation time is shorter than the time between collisions, which enables cells to finalise the re-polarisation process and continue migration in the opposite direction by preventing cell jamming.
- The damped conductive (sub-diffusion) mechanism occurs under high cell packing density near cell jamming within domains satisfying the conditions that and . The velocity correlation length corresponds to the length of a single cell [17] indicating isotropic cell rearrangement. This high cell packing density causes intensive cell-cell interactions leading to collective contact inhibition of locomotion (Box 1). However, in contrast to the previous case, the time necessary for cell re-polarisation is longer than the time between two cell collisions and cells are unable to finalise the re-polarisation process. The cells then undergo jamming. Consequently, energy dissipation within cell collectives near jamming occurs on a time scale of hours.
3. Long Term Change of Internal Entropy Caused by Collective Cell Migration
3.1. Surface Characteristics of Epithelial Monolayers
3.2. Viscoelasticity of Epithelial Monolayers: The Energy Dissipation
4. Physical Parameters That Govern Cell Rearrangement and Entropy Production
4.1. The Degree of Cell Orientation in the Direction of Cell Migration
4.2. The Mass Balance
4.3. The Force Balance
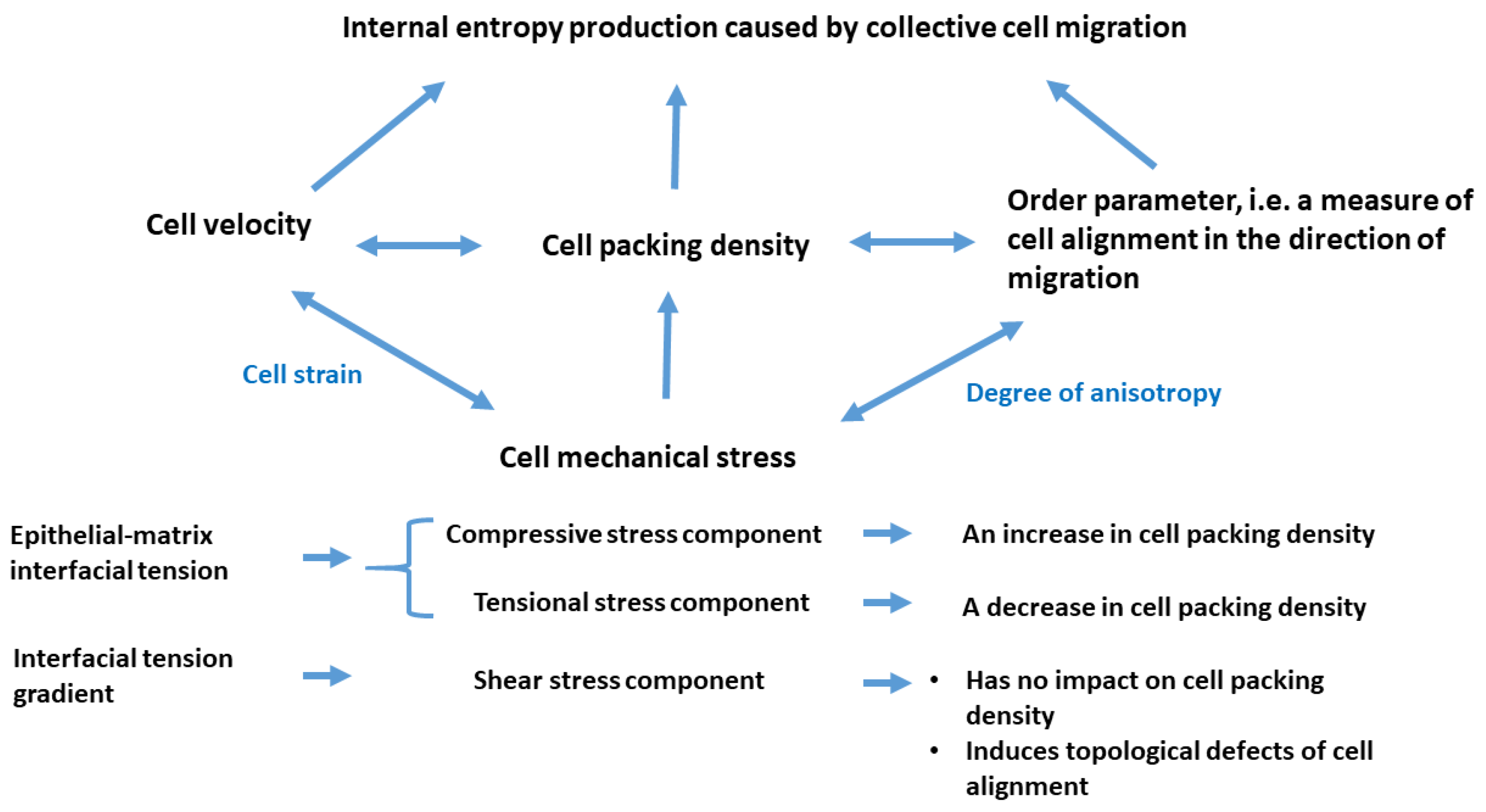
- An inhomogeneous accumulation of cell compressive residual stress within the epithelial monolayer caused by collective cell migration leads to an increase in the viscoelastic force [40]. The viscoelastic force and traction force cause a decrease in the velocity of epithelial cells and an increase in the epithelial packing density. An induced decrease in the velocity causes a decrease in the traction force [48].
- An increase in the epithelial packing density leads to intensive cell-cell interactions, resulting in energy dissipation within the epithelial monolayer and, consequently, decreases in (1) the residual stress accumulation within the epithelium; and (2) the epithelial surface tension.
- A reduction in the residual stress present in the epithelial monolayer leads to a decrease in viscoelastic force. As a result, the suppression of both resistive forces, namely the viscoelastic and traction forces, contributes to an increase once more in the velocity of the epithelial cells.
- Consequently, oscillations of cell velocity are thus a result of variations in energy dissipation alongside the generation of entropy.
5. Short-Term Dynamics of Entropy Generation Associated with the Convective Regime
6. Conclusions
- Our theoretical investigation reveals that cells can effectively control entropy generation linked to the dissipation of mechanical energy during collective migration. This collective movement of cells triggers both active and passive wetting and de-wetting on the substrate matrix, leading to an accumulation of mechanical stress within epithelial monolayers. This mechanical stress exerts a feedback influence on various parameters, including cell packing density, alignment, and velocity. Notably, an increase in compressive stress results in an increase in cell packing density, while shear stress can create topological defects in cell alignment, which in turn amplify cell-cell interactions. The principal outcomes of this study were derived from the synthesis of physical models and experimental data in the domains of mechanobiology and biological physics. We can summarize them as follows:
- Cell-cell interactions cause remodelling of cell-cell and cell-matrix adhesion contacts and can induce the re-polarisation of cells accompanied by a weakening of adhesion contacts. These interactions are pivotal in the dynamic alteration of energy dissipation within cells, which is associated with the viscoelastic properties and surface characteristics of multicellular systems, ultimately contributing to entropy generation. The regulation of energy dissipation serves as a cellular mechanism to decrease mechanical stress in epithelial monolayers, thereby inhibiting cell migration.
- Energy dissipation caused by the remodelling of cell-cell and cell-matrix adhesion contacts during relaxation of migrating epithelial collectives under mechanical stress occurs on a time scale of minutes. This phenomenon of entropy production is indicative of a convective regime. The continuous remodelling of adhesion contacts is a fundamental aspect of cellular adaptation to varying microenvironmental conditions and occurs repeatedly throughout the process of collective cell migration.
- Contact inhibition of locomotion, which is pronounced in an overcrowded environment characterized by an increase in cell packing density in a conductive diffusion regime, leads to energy dissipation during the process of cell re-polarization. This re-polarization, accompanied by a weakening of cell-cell and cell-matrix adhesion contacts, occurs over a time frame of several hours. When cells are afforded sufficient time to complete the re-polarization process and re-establish robust adhesion contacts with both neighbouring cells and the extracellular matrix, they initiate movement in the opposite direction.
- When the interval between two collisions is less than the duration required for the re-polarization process, which is typical of the sub-diffusion regime, cells experience jamming. This phenomenon results in a transition from a contractile to a non-contractile state, leading to prolonged energy dissipation and a reduction in mechanical stress. The latter facilitates the formation of robust adhesion contacts between cells and the extracellular matrix, subsequently initiating collective migration once more.
- These oscillations of energy storage and energy dissipation caused by collective cell migration result in oscillations in the production of entropy.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugdale, J.S. Entropy and Low Temperature Physics; Hutchinson: London, UK, 1966. [Google Scholar]
- Prigogine, I. Introduction to Thermodynamics of Irreversible Processes, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1967. [Google Scholar]
- Kleidon, A.; Lorentz, R.D. Non-Equilibrium Thermodynamics and the Production of Entropy, Life, Earth, and Beyond; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Wu, K.; Nan, Q.; Wu, T. Philosophical analysis of the meaning and nature of entropy and negative entropy theories. Complexity 2020, 2020, 8769060. [Google Scholar] [CrossRef]
- Ding, C.; Luo, L. Measurement of entropy production in living cells under an alternating electric field. Cell Biol. Int. 2013, 37, 233–238. [Google Scholar] [CrossRef]
- Himeoka, Y.; Kaneko, K. Entropy production of a steady-growth cell with catalytic reactions. Phys. Rev. E 2014, 90, 042714. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Oloyede, A.; Singh, S.; Gu, Y. Investigation of the effects of extracellular osmotic pressure on morphology and mechanical properties of individual chondrocyte. Cell Biochem. Biophys. 2016, 74, 229–240. [Google Scholar] [CrossRef] [PubMed]
- McClintock, P.V.E. What is life? Contemp. Phys. 2012, 53, 433–435. [Google Scholar] [CrossRef]
- Mayer, C. Life in The Context of Order and Complexity. Life 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Arango-Restrepo, A.; Rubi, J.M. Predicting cancer stages from tissue energy dissipation. Sci. Rep. 2023, 13, 15894. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef]
- Brücher, B.L.D.M.; Jamall, I.S. Cell-Cell Communication in the tumor microenvironment, carcinogenesis, and anticancer treatment. Cell Physiol. Biochem. 2014, 34, 213–243. [Google Scholar] [CrossRef]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The mechanical regulation of integrin–cadherin crosstalk organizes cells, signalling and forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef]
- Barriga, E.H.; Mayor, R. Adjustable viscoelasticity allows for efficient collective cell migration. Sem. Cell Dev. Biol. 2019, 93, 55–68. [Google Scholar]
- Iyer, K.V.; Gomez, R.P.; Paijmans, J.; Julicher, F.; Eaton, S. Epithelial viscoelasticity is regulated by mechanosensitive E-cadherin turnover. Curr. Biol. 2019, 29, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Alert, R.; Trepat, X. Physical models of collective cell migration. Annu. Rev. Condens. Matter Phys. 2020, 11, 77–101. [Google Scholar] [CrossRef]
- Petrolli, V.; Boudou, T.; Balland, M.; Cappello, G. Oscillations in collective cell migration. In Viscoelasticity and Collective Cell Migration: An Interdisciplinary Perspective Across Levels of Organization; Chapter 8; Pajic-Lijakovic, I., Barriga, E., Eds.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Pajic-Lijakovic, I.; Milivojevic, M.; McClintock, P.V.E. Physics of cancer spreading through epithelium. Contemp. Phys. 2024, 64, 296–314. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M.; McClintock, P.V.E. Epithelial cell-cell interactions in an overcrowded environment: Jamming or live cell extrusion. J. Biol. Eng. 2024, 18, 47. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M.; McClintock, P.V.E. Physical aspects of epithelial cell-cell interactions: Hidden system complexities. Eur. Biophys. J. 2024, 53, 355–372. [Google Scholar] [CrossRef]
- Serra-Picamal, X.; Conte, V.; Vincent, R.; Anon, E.; Tambe, D.T.; Bazellieres, E.; Butler, J.P.; Fredberg, J.J.; Trepat, X. Mechanical waves during tissue expansion. Nat. Phys. 2012, 8, 628–634. [Google Scholar] [CrossRef]
- Nnetu, K.D.; Knorr, M.; Pawlizak, S.; Fuhs, T.; Kaes, J. Slow and anomalous dynamics of an MCF-10A epithelial cell monolayer. Soft Matter 2013, 9, 9335–9341. [Google Scholar] [CrossRef]
- Tlili, S.; Gauquelin, E.; Li, B.; Cardoso, O.; Ladoux, B.; Delanoë-Ayari, H.; Graner, F. Collective cell migration without proliferation: Density determines cell velocity and wave velocity. R. Soc. Open Sci. 2018, 5, 172421. [Google Scholar] [CrossRef]
- Kang, W.; Ferruzzi, J.; Spatarelu, C.-P.; Han, Y.L.; Sharma, Y.; Koehler, S.A.; Mitchel, J.A.; Khan, A.; Butler, J.P.; Roblyer, D.; et al. A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation. iScience 2021, 24, 103252. [Google Scholar] [CrossRef]
- Lin, S.Z.; Ye, S.; Xu, G.K.; Li, B.; Feng, X.Q. Dynamic migration modes of collective cells. Biophys. J. 2018, 115, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Deforet, M.; Hakim, V.; Yevick, H.; Duclos, G.; Silberzan, P. Emergence of collective modes and tri-dimensional structures from epithelial confinement. Nat. Commun. 2014, 5, 3747. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Wolgemuth, C.W. Crawling cells can close wounds without purse strings or signalling. PLoS Comp. Biol. 2011, 7, e1002007. [Google Scholar] [CrossRef]
- Saw, T.B.; Doostmohammadi, A.; Nier, V.; Kocgozlu, L.; Thampi, S.; Toyama, Y.; Marcq, P.; Lim, C.T.; Yeomans, J.M.; Ladoux, B. Topological defects in epithelia govern cell death and extrusion. Nature 2017, 544, 212–216. [Google Scholar] [CrossRef]
- Roycroft, A.; Mayor, R. Molecular basis of contact inhibition of locomotion. Cell. Mol. Life Sci. 2016, 73, 1119–1130. [Google Scholar] [CrossRef]
- Zimmermann, J.; Camley, B.A.; Rappel, W.J.; Herbert, L.H. Contact inhibition of locomotion determines cell–cell and cell–substrate forces in tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Notbohm, J.; Banerjee, S.; Utuje, K.J.C.; Gweon, B.; Jang, H.; Park, Y.; Shin, J.; Butler, J.P.; Fredberg, J.J.; Marchetti, M.C. Cellular contraction and polarization drive collective cellular motion. Biophys. J. 2016, 110, 2729–2738. [Google Scholar] [CrossRef]
- Petitjean, L.; Reffay, M.; Grasland-Mongrain, E.; Poujade, M.; Ladoux, B.; Buguin, A.; Silberzan, P. Velocity fields in a collectively migrating epithelium. Biophys. J. 2010, 98, 1790–1800. [Google Scholar] [CrossRef]
- Garcia, S.; Hannezo, E.; Elgeti, J.; Joanny, J.-F.; Silberzan, P.; Gov, N.S. Physics of active jamming during collective cellular motion in a monolayer. Proc. Natl. Acad. Sci. USA 2015, 112, 15314–15319. [Google Scholar]
- Pajic-Lijakovic, I.; Milivojevic, M. The role of viscoelasticity in long time cell rearrangement. Progr. Biophys. Mol. Biol. 2022, 173, 60–71. [Google Scholar] [CrossRef]
- Schulze, K.D.; Zehnder, S.M.; Urueña, J.M.; Bhattacharjee, T.; Sawyer, W.G.; Angelini, T.E. Elastic modulus and hydraulic permeability of MDCK monolayers. J. Biomech. 2017, 53, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.C.; Eaton, M.J.; Karasek, M.A. Growth characteristics of human epidermal kerationcytes from newborn foreskin in primary and serial cultures. In Vitro 1979, 15, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Thompson, D.P.; Cramer, C.T.; Vidmar, T.J.; Scieszka, J.F. The Madin Darby canine kidney (MDCK) epithelial cell monolayer as a model cellular transport barrier. Pharm. Res. 1989, 6, 71–77. [Google Scholar] [CrossRef]
- Adak, A.; Unal, Y.C.; Yucel, S.; Vural, Z.; Turan, F.B.; Yalcin-Ozuysal, O.; Ozcivici, E.; Mese, G. Connexin 32 induces pro-tumorigenic features in MCF10A normal breast cells and MDA-MB-231 metastatic breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118851. [Google Scholar] [CrossRef]
- Volfson, D.; Cookson, S.; Hasty, J.; Tsimring, L.S. Biomechanical ordering of dense cell populations. Proc. Natl. Acad. Sci. USA 2008, 105, 15346–15351. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M. Multiscale nature of cell rearrangement caused by collective cell migration. Eur. Biophys. J. 2021, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, S.; Vaughen, J.; Igaki, T. Cell Extrusion: A Stress-Responsive Force for Good or Evil in Epithelial Homeostasis. Develop. Cell 2018, 44, 284–296. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M.; McClintock, P.V.E. Role of viscoelasticity in the appearance of low-Reynolds turbulence: Considerations for modelling. J. Biol. Eng. 2024, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, C.; Alert, R.; Blanch-Mercader, C.; Gómez-González, M.; Kolodziej, T.; Bazellieres, E.; Casademunt, J.; Trepat, X. Active wetting of epithelial tissues. Nat. Phys. 2019, 15, 79–88. [Google Scholar] [CrossRef]
- Karbalaei, A.; Kumar, R.; Cho, H.J. Thermocapillarity in microfluidics—A review. Micromachines 2016, 7, 13. [Google Scholar] [CrossRef]
- Murray, J.D.; Maini, P.K.; Tranquillo, R.T. Mechanochemical models for generating biological pattern and form in development. Phys. Rep. 1988, 171, 59–84. [Google Scholar] [CrossRef]
- Stirbat, T.V.; Mgharbel, A.; Bodennec, S.; Ferri, K.; Mertani, H.C.; Rieu, J.P.; Delanoë-Ayari, H. Fine tuning of tissues’ viscosity and surface tension through contractility suggests a new role for a-Catenin. PLoS ONE 2013, 8, e52554. [Google Scholar] [CrossRef] [PubMed]
- Koride, S.; Loza, A.J.; Sun, S.X. Epithelial vertex models with active biochemical regulation of contractility can explain organized collective cell motility. APL Bioeng. 2018, 2, 031906. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M. Physics of collective cell migration. Eur. Biophys. J. 2023, 52, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Gsell, S.; Tlili, S.; Merkel, M.; Lenne, P.-F. Marangoni-like tissue flows enhance symmetry breaking of embryonic organoids. bioRxiv 2023. [Google Scholar] [CrossRef]
- Devanny, A.J.; Vancura, M.B.; Kaufman, L.J. Exploiting differential effects of actomyosin contractility to control cell sorting among breast cancer cells. Mol. Biol. Cell 2021, 32, ar24. [Google Scholar] [CrossRef]
- Guevorkian, K.; Brochard-Wyart, F.; Gonzalez-Rodriguez, D. Flow dynamics of 3D multicellular systems into capillaries. In Viscoelasticity and Collective Cell Migration: An Interdisciplinary Perspective Across Levels of Organization; Pajic-Lijakovic, I., Barriga, E., Eds.; Academic Press: Cambridge, MA, USA, 2021; p. 193. [Google Scholar]
- Clark, A.G.; Maitra, A.; Jacques, C.; Bergert, M.; Pérez-González, C.; Simon, A.; Lederer, L.; Diz-Muñoz, A.; Trepat, X.; Voituriez, R.; et al. Self-generated gradients steer collective migration on viscoelastic collagen networks. Nat. Mat. 2022, 21, 1200–1210. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M. Viscoelasticity and cell jamming state transition. Eur. Phys. J. Plus 2021, 136, 750. [Google Scholar] [CrossRef]
- Colinas-Armijo, N.; DiPaola, M.; Pinnola, F.P. Fractional characteristic times and dissipated energy in fractional linear viscoelasticity. Commun. Nonlinear Sci. Numer. Simul. 2016, 37, 14–30. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations. In Mathematics in Science and Engineering; London Academic Press: London, UK, 1999; Volume 198, p. 78. [Google Scholar]
- Khalilgharibi, N.; Fouchard, J.; Asadipour, N.; Yonis, A.; Harris, A.; Mosaff, P.; Fujita, Y.; Kabla, A.; Baum, B.; Muñoz, J.J.; et al. Stress relaxation in epithelial monolayers is controlled by actomyosin. Nat. Phys. 2019, 15, 839–847. [Google Scholar] [CrossRef]
- Tambe, D.T.; Croutelle, U.; Trepat, X.; Park, C.Y.; Kim, J.H.; Millet, E.; Butler, J.P.; Fredberg, J.J. monolayer stress microscopy: Limitations, artifacts, and accuracy of recovered intercellular stresses. PLoS ONE 2013, 8, e55172. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Murtra, B.; Petridou, N.I. Viscoelastic networks: Forming cells and tissues. Front. Phys. 2021, 9, 666916. [Google Scholar]
- Juettner, V.V.; Dan, A.; Deborah Leckband, D.; Komarova, Y.; Malik, A. The Role of VE-PTP in Stabilizing VE-cadherin Adhesion. FASEB J. 2016, 30, lb520. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef]
- Campi, G.; Cristofaro, F.; Pani, G.; Fratini, M.; Pascucci, B.; Corsetto, P.A.; Weinhausen, B.; Cedola, A.; Rizzo, A.M.; Visai, L.; et al. Heterogeneous and self-organizing mineralization of bone matrix promoted by hydroxyapatite nanoparticles. Nanoscale 2017, 9, 17274. [Google Scholar] [CrossRef]
- Ala-Nissila, T.; Majaniemi, K.; Elder, K. Phase-field modeling of dynamical interface phenomena in fluids. In Lecture Notes in Physics 640; Springer: New York, NY, USA, 2004; pp. 357–388. [Google Scholar]
- Tanaka, H. A viscoelastic model of phase separation. Phys. Rev. 1997, 56, 4451–4462. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons Inc.: New York, NY, USA, 1960. [Google Scholar]
- Bent, H.A. The Second Law: An Introduction to Classical and Statistical Thermodynamics; Oxford University Press: New York, NY, USA, 1965. [Google Scholar]
- Blanchard, G.B.; Fletcher, A.G.; Schumacher, L.J. The devil is in the mesoscale: Mechanical and behavioural heterogeneity in collective cell movement. Sem. Cell Dev. Biol. 2019, 93, 46–54. [Google Scholar] [CrossRef]
- Petrungaro, G.; Morelli, L.; Uriu, K. Information flow in the presence of cell mixing and signaling delays during embryonic development. Sem. Cell Dev. Biol. 2019, 93, 26–35. [Google Scholar] [CrossRef] [PubMed]
| Physical Parameter | Short Description of the Physical Parameters |
|---|---|
| Spreading factor [43] | , epithelial cells undergo de-wetting [19,43]. |
| Epithelial-matrix adhesion energy [45] | . |
| Epithelial cohesion energy is the energy necessary for the separation of two previously connected epithelial surfaces and can be expressed as is the epithelial surface tension | serves as an indicator of the cohesiveness of epithelial tissue when in contact with a liquid medium. is the surface area). The surface energy accounts for three contributions: (i) the elastic contribution caused by a change in the surface area of cells, (ii) the contribution of cell-cell adhesion contacts, and (iii) the contractile energy of cells [46,47]. |
| Matrix surface tension | Matrix surface tension depends on inter- and intra-chain interactions and is influenced by cell tractions [48]. |
| Epithelial-matrix interfacial tension [40] | Epithelial-matrix interfacial tension does work in reducing the biointerface area, thus generating the isotropic part of the cell compressive stress [19]. |
| Gradient of epithelial-matrix interfacial tension [40] | The gradient of the epithelial-matrix interfacial tension directs cell movement from regions of lower interfacial tension to regions of higher interfacial tension by leading to passive wetting/de-wetting. The phenomenon has been known as the Marangoni effect [44]. The experimental validation of cell migration across multicellular surfaces, driven by gradients in surface tension, was established by Gsell et al. [49]. |
| Mechanism of Epithelial Cell Migration | Constitutive Models for the Viscoelasticity of Epithelial Monolayers | Energy Dissipation |
|---|---|---|
| The Zener model for viscoelastic solids: Stress relaxation under constant strain conditions per single short-time relaxation cycle: Cell residual stress is elastic. | where the stress difference is Average energy dissipation is is the time necessary for stress relaxation. |
| The Kelvin-Voigt model for viscoelastic solids: , while the viscous part of the stress is and its elastic part The stress cannot relax. | |
| The fraction model for viscoelastic solids: Elastic and viscous parts of stress cannot be dissociated [54]. The stress cannot relax. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajic-Lijakovic, I.; Milivojevic, M. Entropy Production in Epithelial Monolayers Due to Collective Cell Migration. Entropy 2025, 27, 483. https://doi.org/10.3390/e27050483
Pajic-Lijakovic I, Milivojevic M. Entropy Production in Epithelial Monolayers Due to Collective Cell Migration. Entropy. 2025; 27(5):483. https://doi.org/10.3390/e27050483
Chicago/Turabian StylePajic-Lijakovic, Ivana, and Milan Milivojevic. 2025. "Entropy Production in Epithelial Monolayers Due to Collective Cell Migration" Entropy 27, no. 5: 483. https://doi.org/10.3390/e27050483
APA StylePajic-Lijakovic, I., & Milivojevic, M. (2025). Entropy Production in Epithelial Monolayers Due to Collective Cell Migration. Entropy, 27(5), 483. https://doi.org/10.3390/e27050483







