Maximum Entropy Production Principle of Thermodynamics for the Birth and Evolution of Life
Abstract
1. Introduction
- (1)
- There has been no thermodynamic research on the birth of life in relation to MEPP, until recently when work by this paper’s authors was published.
- (2)
- A consistent thermodynamic understanding by MEPP of the birth and early period of evolution of life to the latest human evolution is important, even though the number of quantitative thermodynamic research was limited.
2. MEPP Exhibited for the Birth and Evolution of Life
2.1. MEPP
2.2. A Local System Far from Equilibrium Embedded in a Large Reservoir
2.3. History of MEPP Research of the Birth and Evolution of Life
3. Birth of Life from Materials
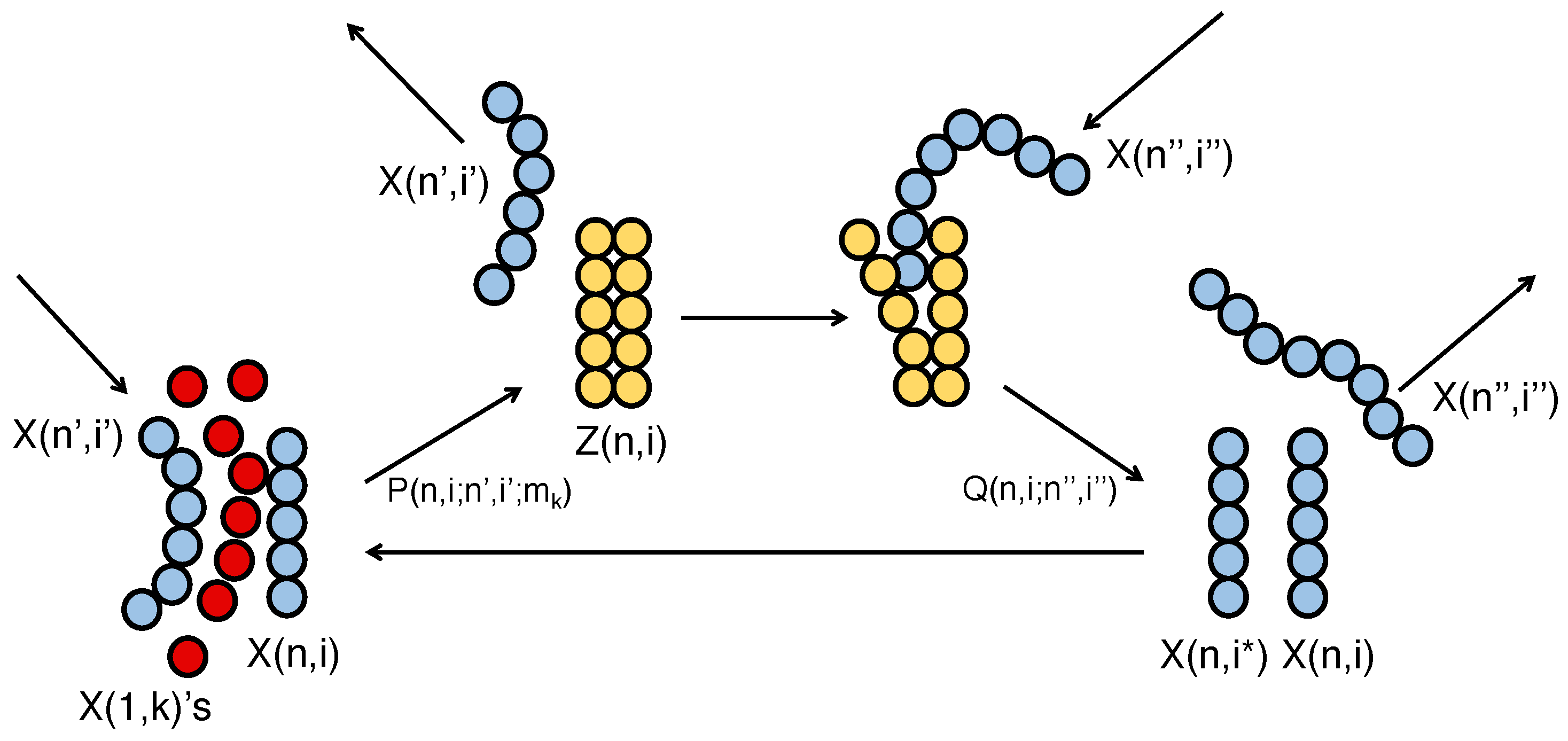
3.1. Birth of Life by Self-Replicative Molecular Reaction with MEPP
3.2. Requirements for the Dynamical Theory on the Birth of Life
- (i)
- To discuss a transition from material world to the pre-RNA world, use molecules of special functions, such as ligase and other ribozymes, should be avoided.
- (ii)
- The activation of a self-replicator is indispensable. Sharp growth of high-fidelity, informative pn-molecules is achieved only by self-replicators.
- (iii)
- The transition must occur at a specific point on the time axis of the material world. Also, nonlinear dynamics is essential to avoid the poor information quality of linear dynamics at the separation of double strands [67].
- (iv)
- A second-order differential equation, which corresponds to a dynamical system of interacting two molecules, is suited for representing the first transition from the material world.
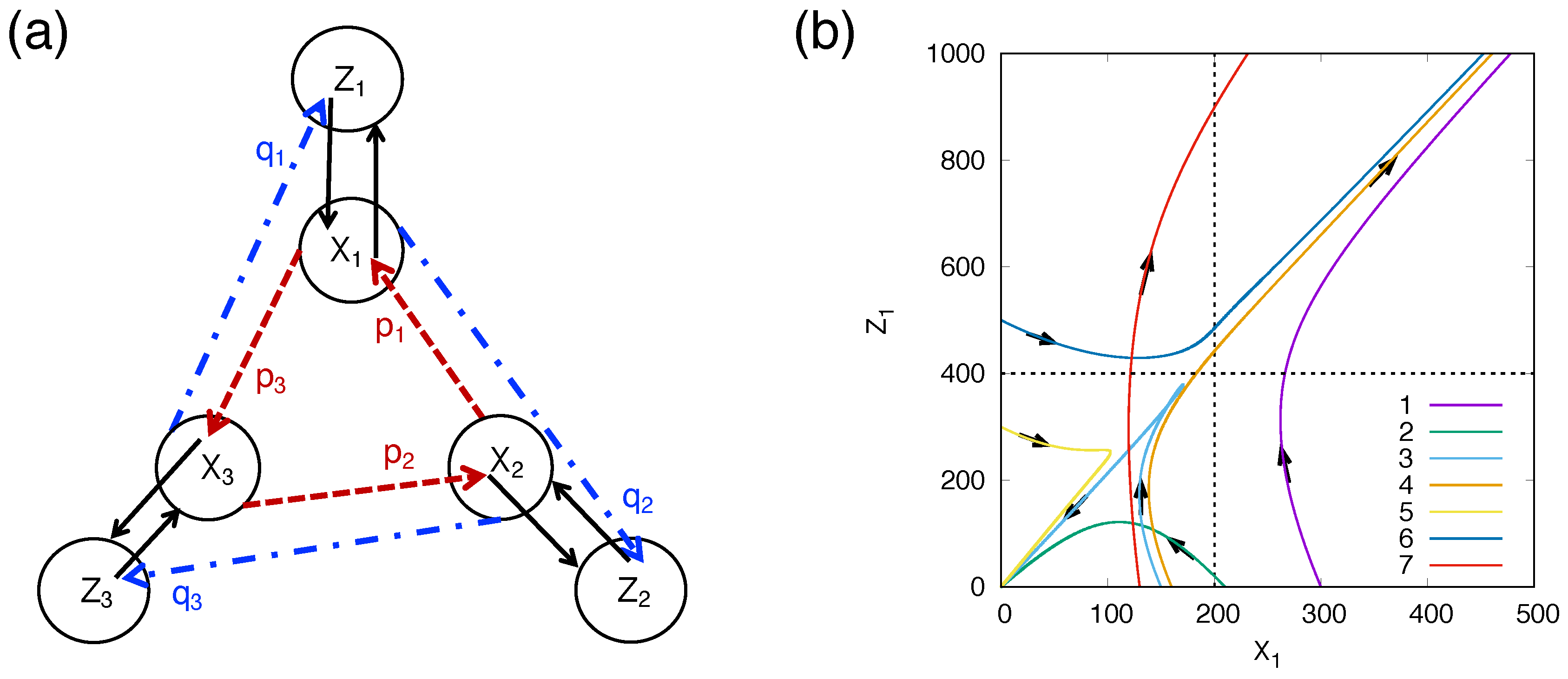
3.3. Dynamical Onset Model of Mutually Catalytic Self-Replication and Transition from Material to Pre-Biotic RNA
3.4. Quantitative Condition of the Molecular Systems Necessary for Functioning as a Self-Replicating Dissipative System with MEPP
4. Evolution of Life from Early State to Later Stage
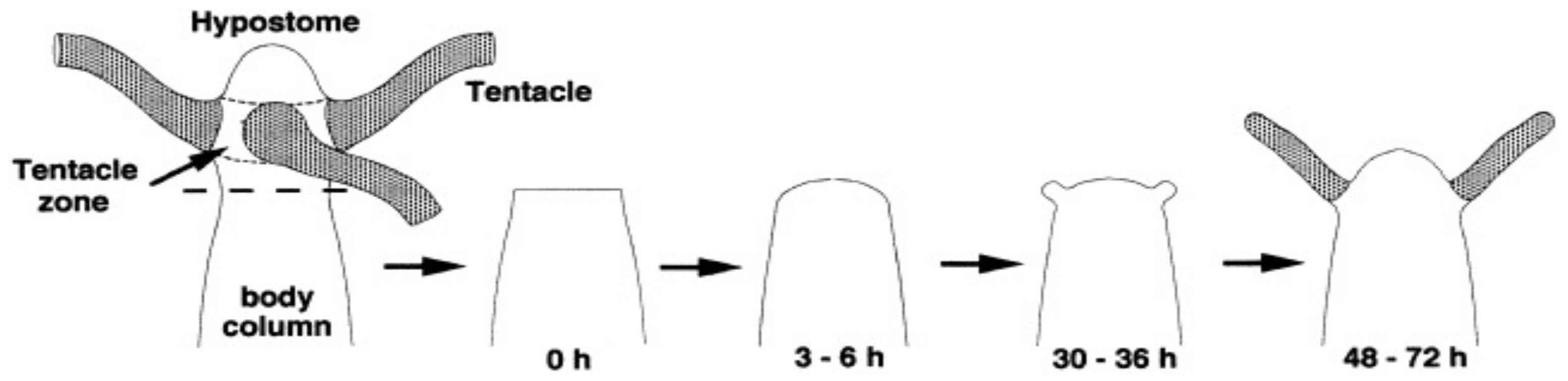
4.1. Single Cell Organization and Geometry of Multicellular Organization
4.2. Multi-Cellular Structures as Examples of Evolution at Early Stage with Limited Number of Cells
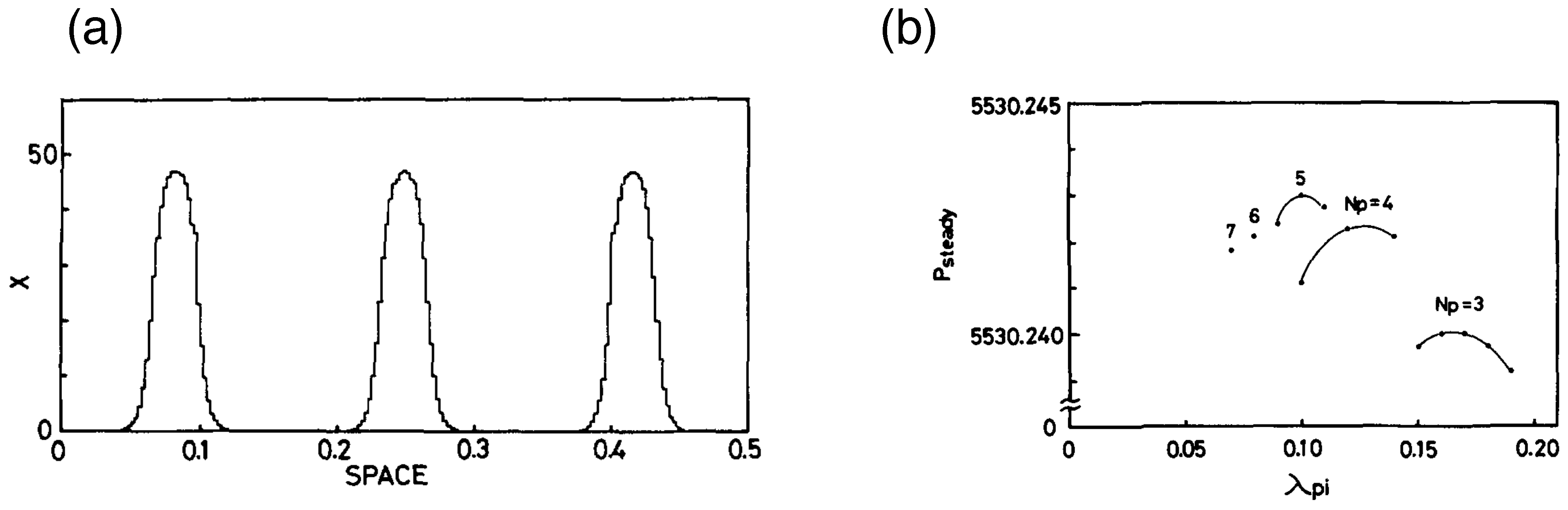
4.3. Numerical Simulation of Differentiation of Multi-Cells Assembly and MEPP
4.4. Formation of Society and External Entropy Production in Later Stage of Evolution
5. Non-MEP State of Multi-Cellular Biology in Severe Circumstances
5.1. Examples of Switching of the Organism from a Normal Metabolic State to a Weak-Metabolic State in Severe Conditions
5.2. Thermodynamics of the Dormant States with Much Lower Metabolic Activity
6. Discussion and Remaining Problems for Future Study
6.1. A Hypothesis on the General Evolution
6.2. External Entropy Production at Later Stages of Evolution
6.3. MEPP and Aging–Death Problem
6.4. Predictability of the Present Theoretical Work
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schrödinger, E. What Is Life—The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Klein, M.J. Thermodynamics in Einstein’s thought: Thermodynamics played a special role in Einstein’s early search for a unified foundation of physics. Science 1967, 157, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Boltzmann, L. Über die Beziehung zwischen dem zweiten Hauptsatze der mechanischen Wärmetheorie und der Wahrscheinlichkeitsrechnung respektive den Sätzen über das Wärmegleichgewicht. Wien. Ber. 1877, 76, 373–435. [Google Scholar]
- Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes. II. Phys. Rev. 1931, 38, 2265–2279. [Google Scholar] [CrossRef]
- Ziegler, H. An Introduction to Thermodynamics; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Gibbs, J.W. Elementary Principles of Statistical Mechanics; Ox Bow Press: Woodridge, IL, USA, 1981. [Google Scholar]
- Jains, E.T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Jains, E.T. Information theory and statistical mechanics. II. Phys. Rev. 1957, 108, 171–190. [Google Scholar] [CrossRef]
- Dewar, R.C.; Lineweaver, C.H.; Niven, R.K.; Regenauer-Lieb, K. Beyond the Scond Law. Entropy Production and Non-Equilibrium Systems; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Prigogine, I. Time, Structure and Fluctuations. Science 1978, 201, 777–785. [Google Scholar] [CrossRef]
- Glansdorff, P.; Prigogine, I. Structure, Stability and Fluctuations.; Masson et Cie: Echandens, Switzerland, 1971. [Google Scholar]
- Malkus, W.V.R.; Veronis, G. Finite Amplitude Cellular Convection. J. Fluid Mech. 1958, 4, 225–260. [Google Scholar] [CrossRef]
- Ben-Jacob, E.; Garik, P. The formation of patterns in non-equilibrium growth. Nature 1990, 343, 523–530. [Google Scholar] [CrossRef]
- Hill, A. Entropy production as the selection rule between different growth morphologies. Nature 1990, 348, 426–428. [Google Scholar] [CrossRef]
- Lorenz, R.D.; Lunine, J.I.; Withers, P.G.; McKay, C.P. Titan, Mars and Earth: Entropy production by latitudinal heat transport. Geophys. Res. Lett. 2001, 28, 415–418. [Google Scholar] [CrossRef]
- Ozawa, H.; Shimokawa, S.; Sakuma, H. Thermodynamics of fluid turbulence: A unified approach to the maximum transport properties. Phys. Rev. E 2001, 64, 026303. [Google Scholar] [CrossRef]
- Shimokawa, H.; Ozawa, H. On the thermodynamics of the oceanic general circulation: Irreversible transition to a state with higher rate of entropy production. Q. J. R. Meteorol. Soc. 2002, 128, 2115–2128. [Google Scholar] [CrossRef]
- Martyushev, L.M.; Seleznev, V.D. Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 2006, 426, 1–45. [Google Scholar] [CrossRef]
- Martyushev, L.M. Maximum entropy production principle: History and current status. Phys. Uspekhi 2021, 64, 558–583. [Google Scholar] [CrossRef]
- Haken, H. Synergetics and bifurgation theory. Ann. N. Y. Acad. Sci. 1979, 316, 357–375. [Google Scholar] [CrossRef]
- Vanchurin, V.; Wolf, Y.I.; Koonin, E.V.; Katsnelson, M.I. Thermodynamics of evolution and the origin of life. Proc. Natl. Acad. Sci. USA 2022, 119, e2120042119. [Google Scholar] [CrossRef]
- Pulselli, R.M.; Simoncini, E.; Tiezzi, E. Self-organization in dissipative structures: A thermodynamic theory for the emergence of prebiotic cells and their epigenetic evolution. Biosystems 2009, 96, 237–241. [Google Scholar] [CrossRef]
- Gladyshev, G.P. Thermodynamics of Aging and Heredity. Nat. Sci. 2015, 7, 270–286. [Google Scholar] [CrossRef]
- Ashe, A.; Colot, V.; Oldroyd, B.P. How does epigenetics influence the course of evolution? Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200111. [Google Scholar] [CrossRef]
- Gladyshev, G.P. On the Thermodynamics, Entropy and Evolution of Biological Systems: What Is Life from a Physical Chemist’s Viewpoint. Entropy 1999, 1, 9–20. [Google Scholar] [CrossRef]
- Skene, K.R. Life’s a Gas: A Thermodynamic Theory of Biological Evolution. Entropy 2015, 17, 5522–5548. [Google Scholar] [CrossRef]
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open Biol. 2013, 3, 130156. [Google Scholar] [CrossRef] [PubMed]
- Creighton, S.D.; Stefanelli, G.; Reda, A.; Zovkic, I.B. Epigenetic Mechanisms of Learning and Memory: Implications for Aging. Int. J. Mol. Sci. 2020, 21, 6918. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.D.; Kay, J.J. Life as a Manifestation of the Second Law of Thermodynamics. Math. Comput. Model. 1994, 19, 25–48. [Google Scholar] [CrossRef]
- Sawada, Y.A. Thermodynamic Variational Principle in Nonlinear Non-Equilibrium Phenomena. Prog. Theor. Phys. 1981, 66, 68–76. [Google Scholar] [CrossRef]
- Sawada, Y.A. Thermodynamics variational principle in nonlinear systems far from equilibrium. J. Stat. Phys. 1984, 34, 1039–1045. [Google Scholar] [CrossRef]
- Singh, M.S.; O’Neill, M.E. Thermodynamics of the climate system. Phys. Today 2022, 75, 30–37. [Google Scholar] [CrossRef]
- Kleidon, A.; Malhi, Y.; Cox, P.M. Maximum entropy production in environmental and ecological systems. Phil. Trans. R. Soc. B 2010, 365, 1297–1302. [Google Scholar] [CrossRef]
- Kleidon, A.; Lorenz, R.D. Entropy production by earth system processes. In Non-Equilibrium Thermodynamics and the Production of Entropy: Life, Earth, and Beyond; Kleidon, A., Lorenz, R.D., Eds.; Springer: Berlin, Germany, 2004; pp. 1–20. [Google Scholar]
- Endres, R.G. Entropy production selects nonequilibrium states in multistable systems. Sci. Rep. 2017, 7, 14437. [Google Scholar] [CrossRef]
- Boltzmann, L. Populäre Schriften; J. A. Barth: Leipzig, Germany, 1905. [Google Scholar]
- Lotka, A.J. Contribution to the energetics of evolution. Proc. Natl. Acad. Sci. USA 1922, 8, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lotka, A.J. Natural selection as a physical principle. Proc. Natl. Acad. Sci. USA 1922, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Kirkaldy, J.S. Thermodynamics of terrestrial evolution. Biophys. J. 1965, 5, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Dol’nik, V.R. Energy metabolism and animal evolution. Usp. Sovr. Biol. 1968, 66, 276–293. [Google Scholar]
- Aoki, I. Entropy Principle for the Development of Complex Biotic Systems: Organisms, Ecosystems, the Earth; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kitano, H. Computational systems biology. Nature 2002, 420, 206–210. [Google Scholar] [CrossRef]
- Juretic, D. Bioenergetics: A Bridge Across Life and Universe; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Cech, T.R. A model for the RNA-catalyzed replication of RNA. Proc. Natl. Acad. Sci. USA 1986, 83, 4360–4363. [Google Scholar] [CrossRef]
- Unrau, P.J.; Bartel, D. RNA-catalysed nucleotide synthesis. Nature 1998, 395, 260–263. [Google Scholar] [CrossRef]
- Szostak, J.W. On the Origin of Life. Medicina (B Aires) 2016, 76, 199–203. [Google Scholar]
- Johnston, W.K.; Unrau, P.J.; Lawrence, M.S.; Glasner, M.E.; Bartel, D.P. RNA-Catalyzed RNA Polymerization: Accurate and General RNA-Templated Primer Extension. Science 2001, 292, 1319–1325. [Google Scholar] [CrossRef]
- Lincoln, T.A.; Joyce, G.F. Self-sustained Replication of an RNA Enzyme. Science 2009, 323, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F. The antiquity of RNA-based evolution. Nature 2002, 418, 214–221. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2011, 4, a003608. [Google Scholar] [CrossRef]
- Zhou, L.; Kim, S.C.; Ho, K.H.; O’Flaherty, D.K.; Giurgiu, C.; Wright, T.H.; Szostak, J.W. Non-enzymatic primer extension with strand displacement. eLife 2019, 8, e51888. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Daigaku, Y.; Toma, K. Onset model of mutually catalytic self-replicative systems formed by an assembly of polynucleotides. Phys. Rev. E 2023, 107, 05440423. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S.A. Autocatalytic sets of proteins. J. Theor. Biol. 1986, 119, 1–24. [Google Scholar] [CrossRef]
- Mossel, E.; Steel, M. Random biochemical networks: The probability of self-sustaining autocatalysis. J. Theor. Biol. 2005, 233, 327–336. [Google Scholar] [CrossRef]
- Hordijk, W.; Steel, M. Autocatalytic Networks at the Basis of Life’s Origin and Organization. Life 2018, 8, 62. [Google Scholar] [CrossRef]
- Eigen, M. Self-organization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The Hypercycle: A Principle of Natural Self-Organization; Springer: Berlin, Germany, 1979. [Google Scholar]
- Szostak, N.; Wasik, S.; Blazewicz, J. Hypercycle. PLoS Comput. Biol. 2016, 12, e1004853. [Google Scholar] [CrossRef]
- Boerlijst, M.C.; Hogeweb, P. Spiral wave structure in pre-biotic evolution: Hypercycles stable against parasites. Physica D 1991, 48, 17–28. [Google Scholar] [CrossRef]
- Sardanyés, J.; Solé, R.V. Bifurcations and phase transitions in spatially-extended two-member hypercycles. J. Theor. Biol. 2006, 243, 468–482. [Google Scholar] [CrossRef]
- Higgs, P.G. Chemical Evolution and the Evolutionary Definition of Life. J. Mol. Evol. 2017, 84, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tupper, A.S.; Higgs, P.S. Rolling-circle and strand-displacement mechanisms for non-enzymatic RNA replication at the time of the origin of life. J. Theor. Biol. 2021, 527, 110822. [Google Scholar] [CrossRef]
- Toyabe, S.; Braun, D. Cooperative Ligation Breaks Sequence Symmetry and Stabilizes Early Molecular Replication. Phys. Rev. X 2019, 9, 011056. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Szostak, J.W. The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 2012, 3, 2. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; NIH-National Library of Medicine: Bethesda, MD, USA, 2000.
- Alberts, B.; Heald, R.; Johnson, A.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Publishing: New York, NY, USA, 1983. [Google Scholar]
- Lubarsky, B.; Krasnow, M.A. Tube Morphogenesis: Making and Shaping Biological Tubes. Cell 2003, 112, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Łuczka, J.; Hänggi, P.; Gadomski, A. Diffusion of clusters with randomly growing masses. Phys. Rev. E 1995, 51, 5762. [Google Scholar] [CrossRef]
- Gadomski, A. Stretched Exponential Kinetics of the Pressure Induced Hydration of Model Lipid Membranes. A Possible Scenario. J. Phys. II Fr. 1996, 6, 1537–1546. [Google Scholar] [CrossRef][Green Version]
- Kopelman, R. Fractal Reaction Kinetics. Science 1988, 241, 1620–1626. [Google Scholar] [CrossRef]
- Arango-Restrepo, A.; Torrenegra-Rico, J.D.; Rubi, J.M. Entropy Production in a System of Janus Particles. Entropy 2025, 27, 112. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.; Dodd, M. Aggregation territories in the cellular slime molds. Biol. Bull. 1962, 122, 13–24. [Google Scholar] [CrossRef]
- Jang, W.; Gomer, R.H. Combining experiments and modelling to understand size regulation in Dictyostelium discoideum. R. Soc. Interface 2008, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.R.; Bode, P.M. Formation of pattern in regenerating tissue pieces of Hydra attenuata. I. Head-body proportion regulation. Dev. Biol. 1980, 78, 484–496. [Google Scholar] [CrossRef]
- Bode, H.R. Head regeneration in Hydra. Dev. Dyn. 2003, 226, 225–236. [Google Scholar] [CrossRef]
- Shimizu, H.; Sawada, Y.; Sugiyama, T. Minimum tissue size required for hydra regeneration. Dev. Biol. 1993, 155, 287–296. [Google Scholar] [CrossRef]
- Van Cauwelaert, E.M.; Arias Del Angel, J.A.; Benites, M.; Azpeitia, E.M. Development of cell differentiation in the transition to multicellularity: A dynamical modeling approach. Front. Microbiol. 2015, 6, 603. [Google Scholar] [CrossRef]
- Furusawa, C.; Kaneko, K. Origin of multicellular organisms as an inevitable consequence of dynamical systems. Anat. Rec. 2002, 268, 327–342. [Google Scholar] [CrossRef]
- Shimizu, H.; Sawada, Y. Relative stability among metastable steady state structures in chemical reaction system. J. Chem. Phys. 1983, 79, 3828–3835. [Google Scholar] [CrossRef]
- Prigogine, I.; Lefever, R. Symmetry Breaking Instabilities in Biological Systems. II. J. Chem. Phys. 1968, 48, 1695–1700. [Google Scholar] [CrossRef]
- Shanks, N. Modeling Biological Systems: The Belousov–Zhabotinsky Reaction. Found. Chem. 2001, 3, 33–53. [Google Scholar] [CrossRef]
- Blaser, H.; Reichman-Fried, M.; Castanon, I.; Dumstrei, K.; Marlow, F.L.; Kawakami, K.; Solnica-Krezel, L.; Heisenberg, C.P.; Raz, E. Migration of zebrafish primordial germ cells: A role for myosin contraction and cytoplasmic flow. Dev. Cell 2006, 11, 613–627. [Google Scholar] [CrossRef]
- Tiezzi, E.B.P.; Pulselli, R.M.; Marchettini, N.; Tiezzi, E. Dissipative Structures In Nature And Human Systems. Des. Nat. IV 2008, 114, 293–299. [Google Scholar]
- Berna, F.; Goldberg, P.; Horwitz, L.K.; Brink, J.; Holt, S.; Bamford, M.; Chazan, M. Microstratigraphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. Proc. Natl. Acad. Sci. USA 2012, 109, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Total Energy Consumption. Available online: https://yearbook.enerdata.net/total-energy/world-consumption-statistics.html (accessed on 11 October 2024).
- Rafols, I.; Amagai, A.; Maeda, Y.; Macwilliams, H.K.; Sawada, Y. Cell type proportioning in Dictyostelium slugs: Lack of regulation within a 2.5-fold tolerance range. Differentiation 2001, 67, 107–116. [Google Scholar] [CrossRef]
- Oga, I. The Oldest Flower: Buried 2000 years, lotus seed finally gets chance to bloom. Life 1952, 3, 60. [Google Scholar]
- Møbjerg, N.; Halberg, K.A.; Jørgensen, A.; Persson, D.; Bjørn, M.; Ramløv, H.; Kristensen, R.M. Survival in extreme environments—On the current knowledge of adaptations in tardigrades. Acta Physiol. 2011, 202, 409–420. [Google Scholar] [CrossRef]
- Dragicevic, V. Thermodynamics of Seed and Plant Growth. In Thermodynamics—Systems in Equilibrium and Non-Equilibrium; Piraján, J.C.M., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Noble, D. Conrad Waddington and the origin of epigenetics. J. Exp. Biol. 2015, 218, 816–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, Y.; Daigaku, Y.; Toma, K. Maximum Entropy Production Principle of Thermodynamics for the Birth and Evolution of Life. Entropy 2025, 27, 449. https://doi.org/10.3390/e27040449
Sawada Y, Daigaku Y, Toma K. Maximum Entropy Production Principle of Thermodynamics for the Birth and Evolution of Life. Entropy. 2025; 27(4):449. https://doi.org/10.3390/e27040449
Chicago/Turabian StyleSawada, Yasuji, Yasukazu Daigaku, and Kenji Toma. 2025. "Maximum Entropy Production Principle of Thermodynamics for the Birth and Evolution of Life" Entropy 27, no. 4: 449. https://doi.org/10.3390/e27040449
APA StyleSawada, Y., Daigaku, Y., & Toma, K. (2025). Maximum Entropy Production Principle of Thermodynamics for the Birth and Evolution of Life. Entropy, 27(4), 449. https://doi.org/10.3390/e27040449







