Entropy Profiles for Li-Ion Batteries—Effects of Chemistries and Degradation
Abstract
1. Introduction
2. Experimental/Materials and Methods
2.1. Method for Entropy Measurements
- Charge the cell to the maximum voltage limit for the cell (100% SoC) (CC-CV charge with a 0.05C current cut-off).
- Allow cell voltage (OCV) to stabilize to a relaxation rate lower than 1.4 mV/h or a maximum relaxation time of 6 h.
- Cycle the temperature of the water bath from 25 °C to 16 °C, and back to 25 °C via steps of 3 °C increments from 16 °C. At every temperature step, the OCV and temperature were allowed to stabilize before the next temperature step. The temperature was assumed stable when the standard deviation of a five-minute time window was smaller than 0.01 °C. The OCV of the cell was recorded continuously.
- The SoC was changed in steps of approximately 5% through a constant 0.05C current (CC) discharge or charge. (The cell will in steps of 5% SoC eventually reach the lower voltage limit (0% SoC) and then be charged back to 100% SoC in 5% SoC steps)
- Loop back to step 2.
2.2. The Tested Cells
| Name | Manufacturer & Cell Model | Capacity [Ah] | Voltage Range [V] | Form Factor | Chemistry | Energy Density [Wh/kg] | Comments/ References |
|---|---|---|---|---|---|---|---|
| Kokam LTO | Kokam SLPB65205130N | 11 | 1.5–2.8 | pouch | LMO(?)- LTO | 69 | Cathode chemistry unconfirmed |
| Toshiba SciB | Toshiba SciB 20 | 20 | 1.5–2.7 | prismatic | LMO(?)/- LTO | 89 | Cathode possibly LMO [36,37] |
| LG JP3 | LGV JP3 | 64 | 3.0–4.2 | pouch | NMC532- graphite | 210 | [38,39] |
| LG E58C | LG E58C | 58 | 3.0–4.2 | pouch | NMC622- graphite | 242 | Cell harvested from a Jaguar I-pace battery |
| Xalt 31 HE | Xalt 31 HE | 31 | 2.7–4.2 | pouch | NMC111- graphite | 180 | [34,38] |
| Panasonic UR | Panasonic UR18650W | 1.5 | 2.75–4.2 | cylinder | NMC- graphite | 120 | [40,41] |
| Enerdel | Enerdel 151105203A 102401 | 17.5 | 2.5–4.1 | pouch | NMC- hard carbon | 147 | [42] |
| Panasonic NCR | Panasonic NCR18650B | 3.25 | 2.5–4.2 | cylinder | NCA- graphite | 243 | [41] |
| SAFT VL6P | SAFT VL6P | 6 | 2.7–4.0 | cylinder | NCA- graphite | 74 | [40] |
| SAFT VL30PFe | SAFT VL30PFe | 30 | 2.5–3.8 | cylinder | LFP- graphite | 91 | [40] |
| Melasta 8C | LPB042126-8C | 7 | 3.0–42 | pouch | LCO- graphite | 190 | [35,39] |
| Melasta 10C | SLPBB042126-10C | 6.55 | 3.0–42 | pouch | LCO- graphite | 188 | [35,39] |
| Samsung SDI | ICP103450A | 2 | 2.75–4.2 | prismatic | LCO- graphite | - | |
| GPB ICR10440 | Great Power Battery Co Ltd. ICR10440 | 0.36 | 3.0–4.2 | cylinder | NMC532/ LCO/LMO-graphite | 69 | [43] |
2.3. The Cycle Life Study
2.4. Data Handling
3. Results and Discussion
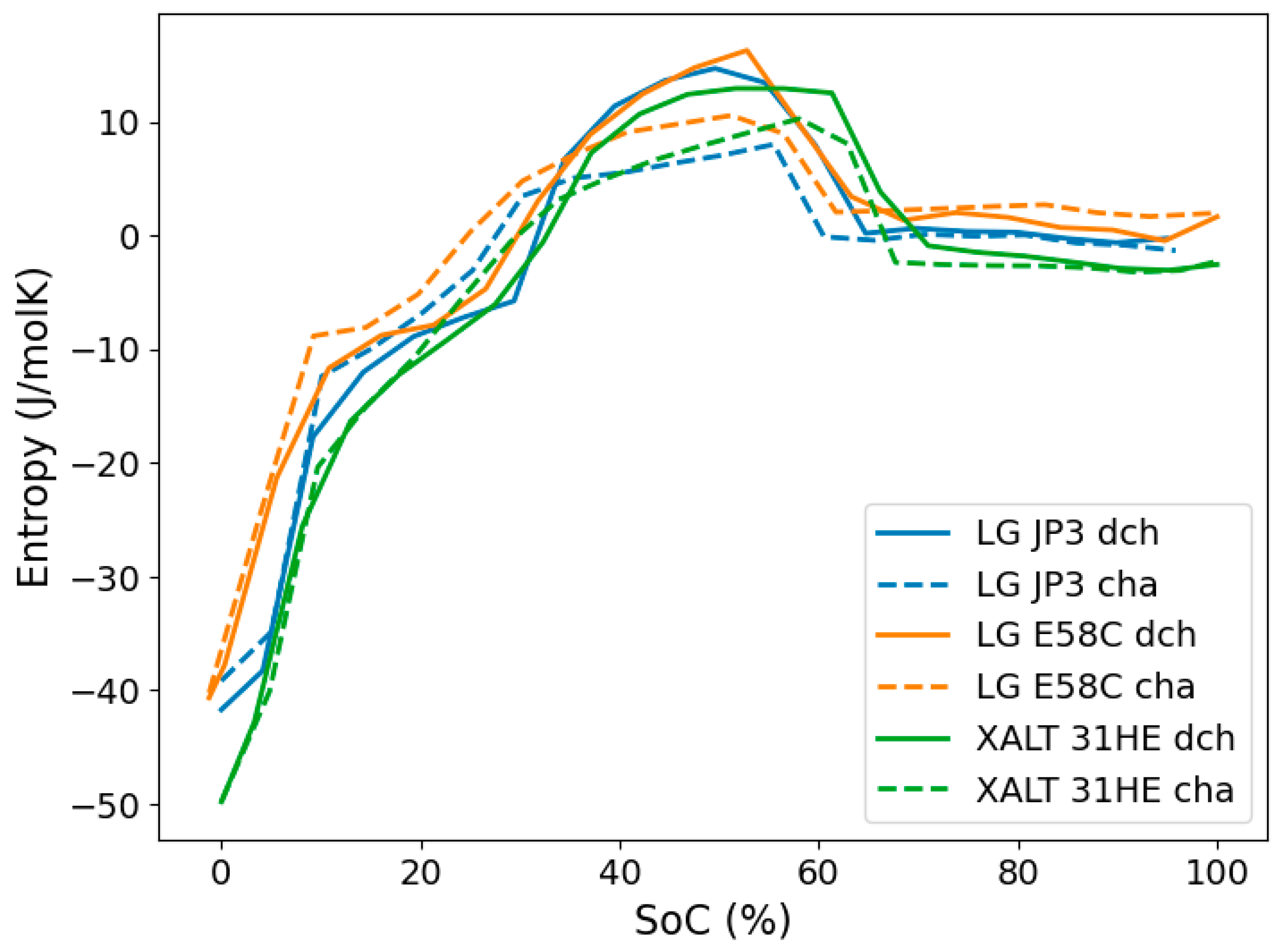
3.1. Differences in Entropy Profiles by Electrode Chemistry

3.2. Quantitative Comparison of Entropy Variation
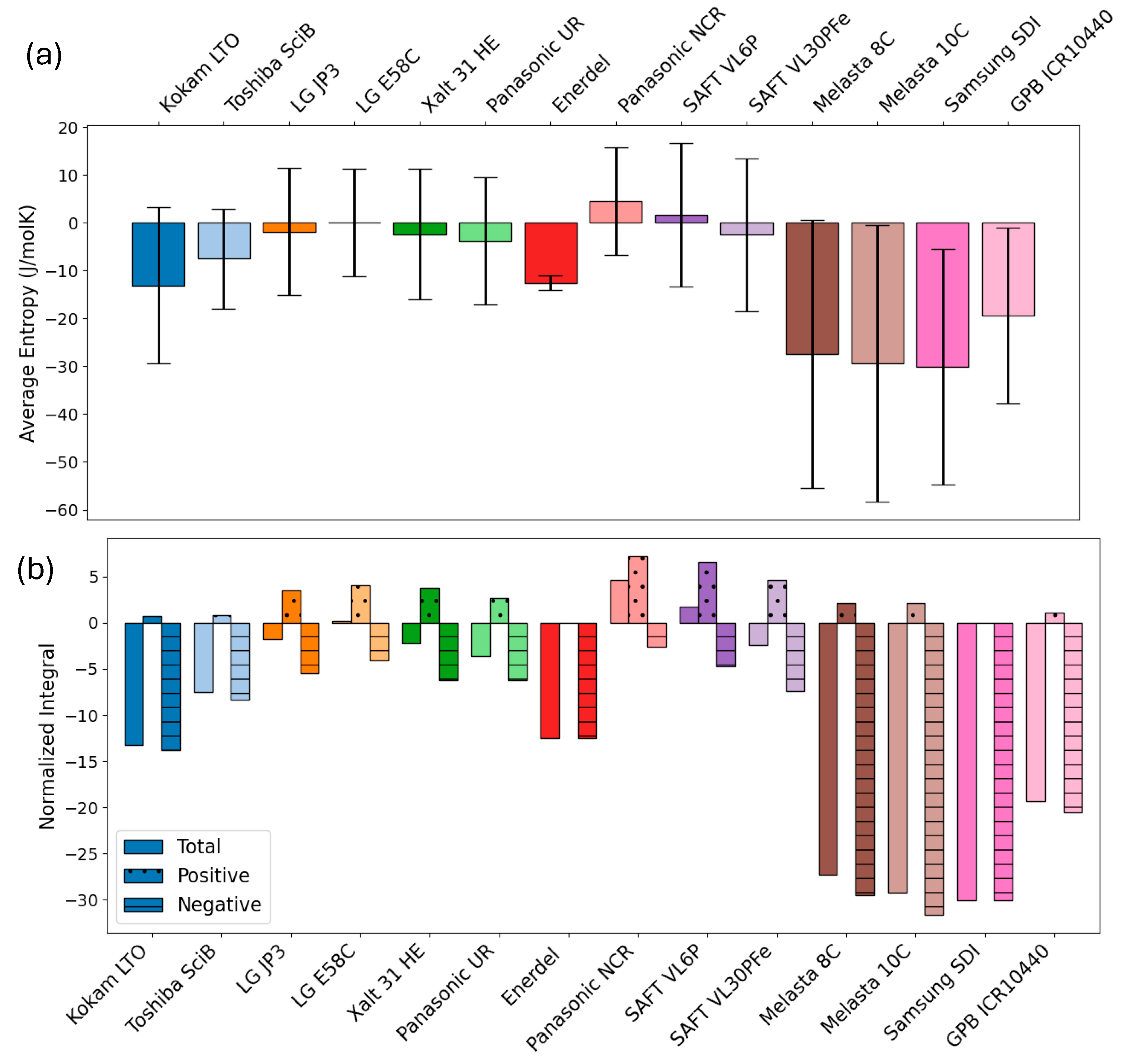
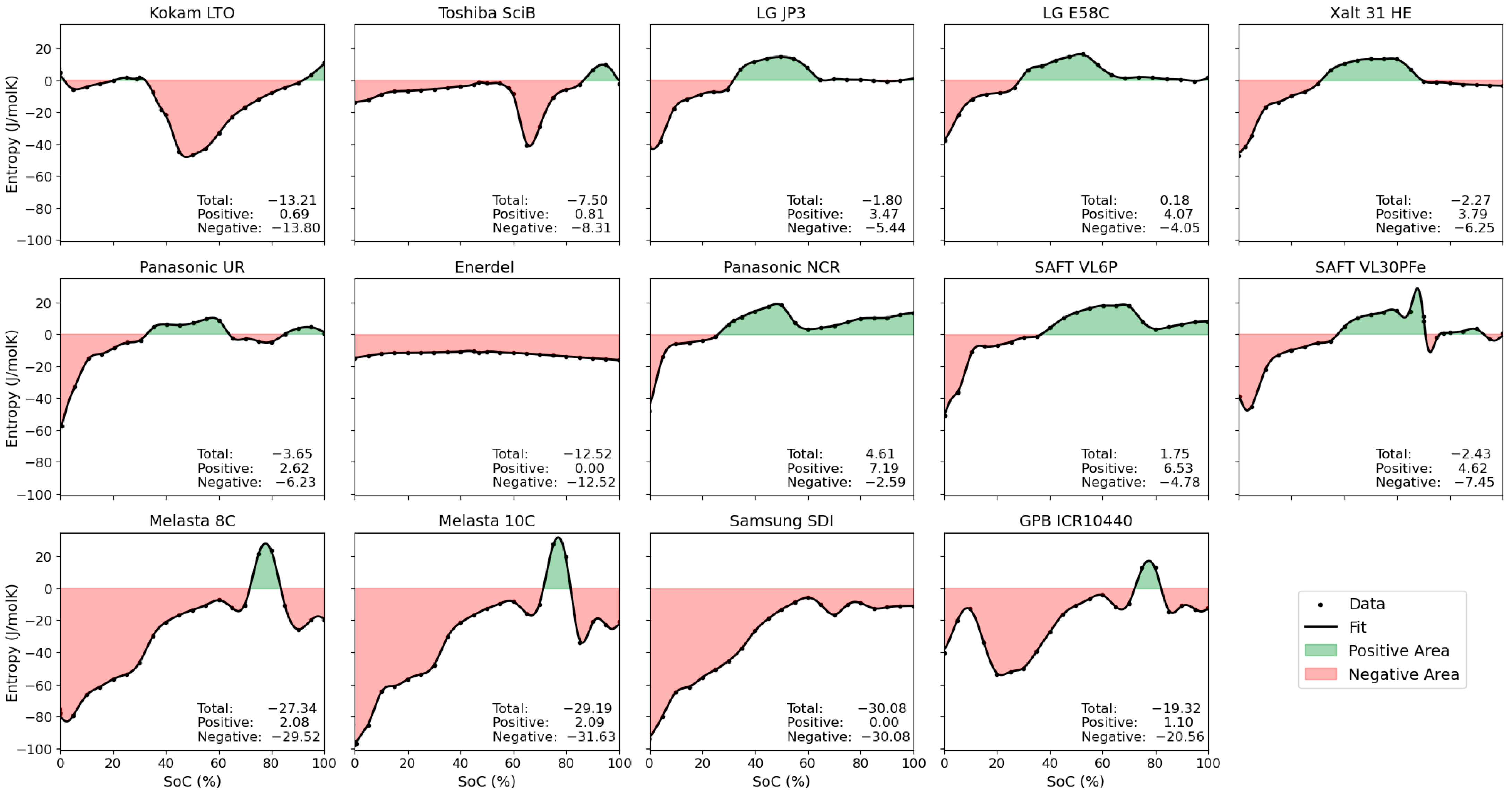
| Cell Label | Chemistry | Integral * | Positive Integral * | Negative Integral * | Mean ** | Stdv ** |
|---|---|---|---|---|---|---|
| Kokam LTO | LMO(?)- LTO | −13.21 | 0.69 | −13.80 | −13 | 16 |
| Toshiba SciB | LMO(?)- LTO | −7.50 | 0.81 | −8.31 | −7 | 11 |
| LG JP3 | NMC532- graphite | −1.80 | 3.47 | −5.44 | −2 | 13 |
| LG E58C | NMC622- graphite | 0.18 | 4.07 | −4.05 | 0 | 11 |
| Xalt 31 HE | NMC111- graphite | −2.27 | 3.79 | −6.25 | −2 | 14 |
| Panasonic UR | NMC- graphite | −3.65 | 2.62 | −6.23 | −4 | 13 |
| Enerdel | NMC- hard carbon | −12.52 | 0 | −12.52 | −13 | 2 |
| Panasonic NCR | NCA- graphite | 4.61 | 7.19 | −2.59 | 5 | 11 |
| SAFT VL6P | NCA- graphite | 1.75 | 6.53 | −4.78 | 2 | 15 |
| SAFT VL30PFe | LFP- graphite | −2.43 | 4.62 | −7.45 | −3 | 16 |
| Melasta 8C | LCO- graphite | −27.34 | 2.08 | −29.52 | −27 | 28 |
| Melasta 10C | LCO- graphite | −29.19 | 2.09 | −31.63 | −29 | 29 |
| Samsung SDI | LCO- graphite | −30.08 | 0 | −30.08 | −30 | 25 |
| GPB ICR10440 | NMC532/ LCO/LMO-graphite | −19.32 | 1.10 | −20.56 | −19 | 19 |
3.3. Difference in Charge and Discharge Entropy
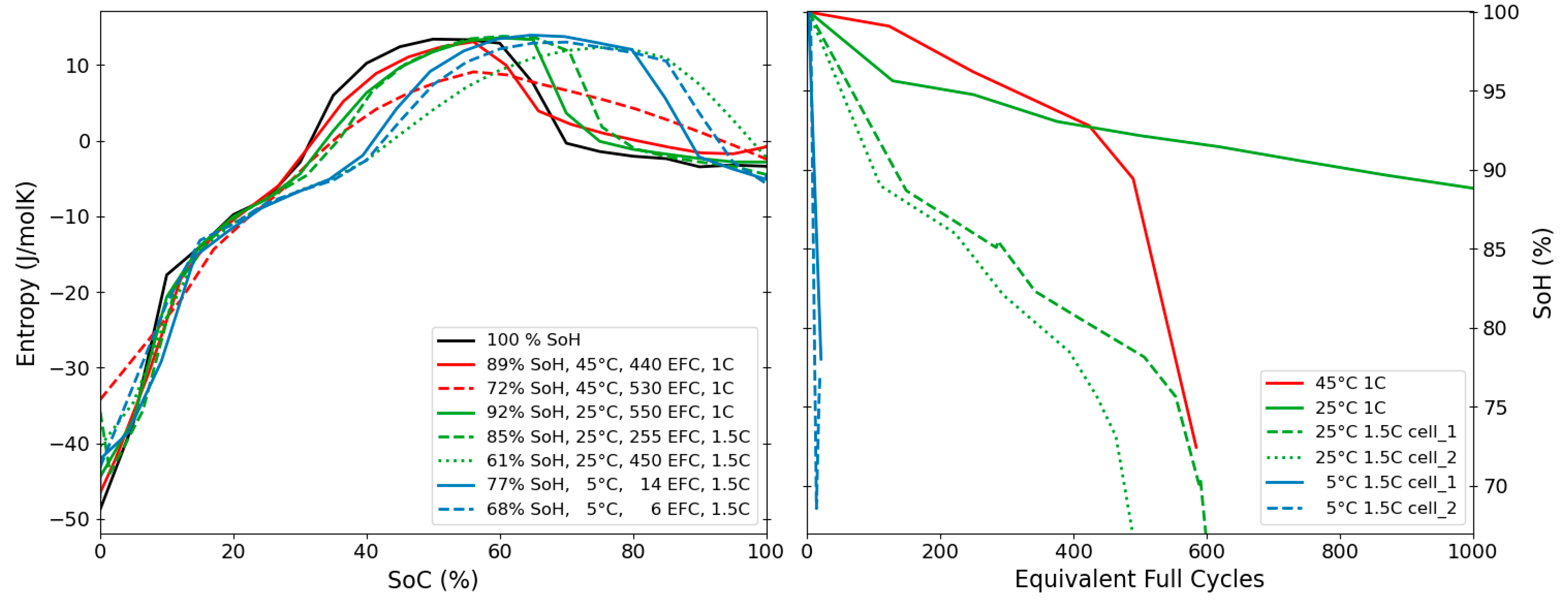
3.4. Ageing & Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sæther, S.R.; Moe, E. A green maritime shift: Lessons from the electrification of ferries in Norway. Energy Res. Soc. Sci. 2021, 81, 102282. [Google Scholar] [CrossRef]
- Chen, T.M.; Jin, Y.; Lv, H.Y.; Yang, A.T.; Liu, M.Y.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Williard, N.; He, W.; Hendricks, C.; Pecht, M. Lessons Learned from the 787 Dreamliner Issue on Lithium-Ion Battery Reliability. Energies 2013, 6, 4682–4695. [Google Scholar] [CrossRef]
- BBC. Samsung Confirms Battery Faults as Cause of Note 7 Fires. BBC, 23 January 2017. [Google Scholar]
- Warren, J. E-Bike Ignites Every Two Days, Says Fire Brigade. BBC News, 28 June 2023. [Google Scholar]
- Stensvold, T. Fergebrann: Brann- og Eksplosjonsårsak Fortsatt en Dåte, in Teknisk Ukeblad. 2019. Available online: https://www.tu.no/artikler/fergebrann-brann-og-eksplosjonsarsak-fortsatt-en-gate/476597 (accessed on 18 March 2025).
- Norwegian Safety Investigation Authority. Report: Fire on Board ‘MS Brim’ in the Outer Oslofjord on 11 March 2021. 2022. Available online: https://nsia.no/Marine/Published-reports/2022-08?pid=SHT-Report-ReportFile&attach=1 (accessed on 18 March 2025).
- Maritime Battery Forum. Fire on Board the Car-Ferry MF Ytterøyningen. 2019. Available online: https://www.maritimebatteryforum.com/reports-documents-1/fire-on-board-the-car-ferry-mf-ytteryningen-2019 (accessed on 18 March 2025).
- McKinnon, M.B.; DeCrance, S.; Kerber, S. Four Firefigheters Injured In Lithium-Ion Battery Energy Storage System Explosion—Arizona; Underwriters Laboratories: Columbia, MD, USA, 2020; p. 66. [Google Scholar]
- Na, Y.U.; Jeon, J.W. Unraveling the Characteristics of ESS Fires in South Korea: An In-Depth Analysis of ESS Fire Investigation Outcomes. Fire 2023, 6, 389. [Google Scholar] [CrossRef]
- Smith, A.J.; Fang, Y.; Mikheenkova, A.; Ekstrom, H.; Svens, P.; Ahmed, I.; Lacey, M.J.; Lindbergh, G.; Furo, I.; Lindstrom, R.W. Localized lithium plating under mild cycling conditions in high-energy lithium-ion batteries. J. Power Source 2023, 573, 233118. [Google Scholar] [CrossRef]
- Xie, Y.C.; Wang, S.; Li, R.H.; Ren, D.S.; Yi, M.C.; Xu, C.S.; Han, X.B.; Lu, L.G.; Friess, B.; Offer, G.; et al. Inhomogeneous degradation induced by lithium plating in a large-format lithium-ion battery. J. Power Source 2022, 542, 231753. [Google Scholar] [CrossRef]
- Anseán, D.; Dubarry, M.; Devie, A.; Liaw, B.Y.; García, V.M.; Viera, J.C.; González, M. Operando lithium plating quantification and early detection of a commercial LiFePO4 cell cycled under dynamic driving schedule. J. Power Source 2017, 356, 36–46. [Google Scholar] [CrossRef]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-Situ Detection of Lithium Plating Using High Precision Coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Source 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; Smart, M.C. Lithium Plating Behavior in Lithium-ion Cells. In Rechargeable Lithium-Ion Batteries; Winter, M., Doughty, D.H., Zaghib, K., Abraham, K.M., Ogumi, Z., Dudney, N.J., Eds.; Electrochemical Society Inc.: Pennington, NJ, USA, 2010; Volume 25, pp. 241–252. [Google Scholar]
- Dubarry, M.; Ansean, D. Best practices for incremental capacity analysis. Front. Energy Res. 2022, 10, 1023555. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, H.; Yang, Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim. Acta 2008, 53, 3539–3546. [Google Scholar] [CrossRef]
- Dubarry, M.; Svoboda, V.; Hwu, R.; Liaw, B.Y. Incremental capacity analysis and close-to-equilibrium OCV measurements to quantify capacity fade in commercial rechargeable lithium batteries. Electrochem. Solid State Lett. 2006, 9, A454–A457. [Google Scholar] [CrossRef]
- Lewerenz, M.; Marongiu, A.; Warnecke, A.; Sauer, D.U. Differential voltage analysis as a tool for analyzing inhomogeneous aging: A case study for LiFePO4|Graphite cylindrical cells. J. Power Source 2017, 368, 57–67. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy-Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5–12. [Google Scholar] [CrossRef]
- Thomas, K.E.; Newman, J. Thermal modeling of porous insertion electrodes. J. Electrochem. Soc. 2003, 150, A176–A192. [Google Scholar] [CrossRef]
- Hua, J.S.; Vie, P.J.S.; Wind, J. Methods for faster estimation of the entropy profile of a lithium-ion battery: A comparison of accelerated potentiometry and the estimation of entropy through thermal signatures. Electrochim. Acta 2025, 509, 145289. [Google Scholar] [CrossRef]
- Reynier, Y.; Yazami, R.; Fultz, B. The entropy and enthalpy of lithium intercalation into graphite. J. Power Source 2003, 119, 850–855. [Google Scholar] [CrossRef]
- Takano, K.; Saito, Y.; Kanari, K.; Nozaki, K.; Kato, K.; Negishi, A.; Kato, T. Entropy change in lithium ion cells on charge and discharge. J. Appl. Electrochem. 2002, 32, 251–258. [Google Scholar] [CrossRef]
- Viswanathan, V.V.; Choi, D.; Wang, D.H.; Xu, W.; Towne, S.; Williford, R.E.; Zhang, J.G.; Liu, J.; Yang, Z.G. Effect of entropy change of lithium intercalation in cathodes and anodes on Li-ion battery thermal management. J. Power Source 2010, 195, 3720–3729. [Google Scholar] [CrossRef]
- Prosser, R.; Offer, G.; Patel, Y. Lithium-Ion Diagnostics: The First Quantitative In-Operando Technique for Diagnosing Lithium Ion Battery Degradation Modes under Load with Realistic Thermal Boundary Conditions. J. Electrochem. Soc. 2021, 168, 030532. [Google Scholar] [CrossRef]
- Thomas, K.E.; Bogatu, C.; Newman, J. Measurement of the entropy of reaction as a function of state of charge in doped and undoped lithium manganese oxide. J. Electrochem. Soc. 2001, 148, A570–A575. [Google Scholar] [CrossRef]
- Geng, Z.Y.; Groot, J.; Thiringer, T. A Time- and Cost-Effective Method for Entropic Coefficient Determination of a Large Commercial Battery Cell. IEEE Trans. Transp. Electrif. 2020, 6, 257–266. [Google Scholar] [CrossRef]
- Hu, Y.; Choe, S.Y. Simultaneous and continuous characterization of reversible and irreversible heat of lithium-ion battery using wavelet transform technique. Electrochim. Acta 2021, 375, 137973. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Vinassa, J.M. Thermal characterization of a high-power lithium-ion battery: Potentiometric and calorimetric measurement of entropy changes. Energy 2013, 61, 432–439. [Google Scholar] [CrossRef]
- Murashko, K.A.; Mityakov, A.V.; Mityakov, V.Y.; Sapozhnikov, S.Z.; Jokiniemi, J.; Pyrhonen, J. Determination of the entropy change profile of a cylindrical lithium-ion battery by heat flux measurements. J. Power Source 2016, 330, 61–69. [Google Scholar] [CrossRef]
- Damay, N.; Forgez, C.; Bichat, M.P.; Friedrich, G. A method for the fast estimation of a battery entropy-variation high-resolution curve—Application on a commercial LiFePO4/graphite cell. J. Power Source 2016, 332, 149–153. [Google Scholar] [CrossRef]
- Hua, J.; Vie, P.J.S.; Wind, J. Extending an Empirical Degradation Model for Li-Ion Batteries into Second-Life Applications. In Proceedings of the 245th ECS Meeting, San Fransicso, CA, USA, 26–30 May 2024. [Google Scholar]
- Spitthoff, L.; Wahl, M.S.; Lamb, J.J.; Shearing, P.R.; Vie, P.J.S.; Burheim, O.S. On the Relations between Lithium-Ion Battery Reaction Entropy, Surface Temperatures and Degradation. Batteries 2023, 9, 249. [Google Scholar] [CrossRef]
- Appiah, W.A.; Park, J.; Byun, S.; Ryou, M.-H.; Lee, Y.M. A Mathematical Model for Cyclic Aging of Spinel LiMn2O4/Graphite Lithium-Ion Cells. J. Electrochem. Soc. 2016, 163, A2757. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Rumyantsev, A. Lithium-Ion Cells: Materials and Applications; MDPI: Basel, Switzerland, 2024. [Google Scholar] [CrossRef]
- Spitthoff, L.; Vie, P.J.S.; Wahl, M.S.; Wind, J.; Burheim, O.S. Incremental capacity analysis (dQ/dV) as a tool for analysing the effect of ambient temperature and mechanical clamping on degradation. J. Electroanal. Chem. 2023, 944, 117627. [Google Scholar] [CrossRef]
- Spitthoff, L.; Wahl, M.S.; Vie, P.J.S.; Burheim, O.S. Thermal transport in lithium-ion batteries: The effect of degradation. J. Power Source 2023, 577, 233149. [Google Scholar] [CrossRef]
- Lian, T.; Vie, P.J.S.; Gilljam, M.; Forseth, S. (Invited) Changes in Thermal Stability of Cyclic Aged Commercial Lithium-Ion Cells. ECS Trans. 2019, 89, 73–81. [Google Scholar] [CrossRef]
- Lian, T. Sikkerhetsaspekter i Litiumionbatterier. Master Thesis, Universitetet i Oslo, Oslo, Norway, 2017. [Google Scholar]
- Richter, F.; Vie, P.J.S.; Kjelstrup, S.H.; Burheim, O.S. Measurements of ageing and thermal conductivity in a secondary NMC-hard carbon Li-ion battery and the impact on internal temperature profiles. Electrochim. Acta 2017, 250, 228–237. [Google Scholar] [CrossRef]
- Nazer, N.; Yartys, V.; Azib, T.; Latroche, M.; Cuevas, F.; Forseth, S.; Vie, P.; Denys, R.; Sørby, M.; Hauback, B. In operando neutron diffraction study of a commercial graphite/(Ni, Mn, Co) oxide-based multi-component lithium ion battery. J. Power Source 2016, 326, 93–103. [Google Scholar]
- Reynier, Y.F.; Yazami, R.; Fultz, B. Thermodynamics of lithium intercalation into graphites and disordered carbons. J. Electrochem. Soc. 2004, 151, A422–A426. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Yun, F.L.; Jin, W.R.; Tang, L.; Li, W.C.; Pang, J.; Lu, S.G. Analysis of Capacity Fade from Entropic Heat Coefficient of Li[NixCoyMnz]O-2/Graphite Lithium Ion Battery. J. Electrochem. Soc. 2016, 163, A639–A643. [Google Scholar] [CrossRef]
- Gunnarshaug, A.F.; Vie, P.J.S.; Kjelstrup, S. Review-Reversible Heat Effects in Cells Relevant for Lithium-ion Batteries. J. Electrochem. Soc. 2021, 168, 050522. [Google Scholar] [CrossRef]
- Jalkanen, K.; Vuorilehto, K. Entropy change characteristics of LiMn0.67Fe0.33PO4 and Li4Ti5O12 electrode materials. J. Power Source 2015, 273, 351–359. [Google Scholar] [CrossRef]
- Lu, W.; Belharouak, I.; Liu, J.; Amine, K. Electrochemical and thermal investigation of Li4/3Ti5/3O4 spinel. J. Electrochem. Soc. 2007, 154, A114–A118. [Google Scholar] [CrossRef]
- Hudak, N.S.; Davis, L.E.; Nagasubramanian, G. Cycling-Induced Changes in the Entropy Profiles of Lithium Cobalt Oxide Electrodes. J. Electrochem. Soc. 2015, 162, A315–A321. [Google Scholar] [CrossRef]
- Williford, R.E.; Viswanathan, V.V.; Zhang, J.G. Effects of entropy changes in anodes and cathodes on the thermal behavior of lithium ion batteries. J. Power Source 2009, 189, 101–107. [Google Scholar] [CrossRef]
- Sturm, J.; Rheinfeld, A.; Zilberman, I.; Spingler, F.B.; Kosch, S.; Frie, F.; Jossen, A. Modeling and simulation of inhomogeneities in a 18650 nickel-rich, silicon-graphite lithium-ion cell during fast charging. J. Power Source 2019, 412, 204–223. [Google Scholar] [CrossRef]
- Marcicki, J.; Yang, X.G. Model-Based Estimation of Reversible Heat Generation in Lithium-Ion Cells. J. Electrochem. Soc. 2014, 161, A1794–A1800. [Google Scholar] [CrossRef]
- Schlueter, S.; Genieser, R.; Richards, D.; Hoster, H.E.; Mercer, M.P. Quantifying structure dependent responses in Li-ion cells with excess Li spinel cathodes: Matching voltage and entropy profiles through mean field models. Phys. Chem. Chem. Phys. 2018, 20, 21417–21429. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Weber, A.; Ivers-Tiffee, E. A novel and precise measuring method for the entropy of lithium-ion cells: Delta S via electrothermal impedance spectroscopy. Electrochim. Acta 2014, 137, 311–319. [Google Scholar] [CrossRef]
- Jalkanen, K.; Aho, T.; Vuorilehto, K. Entropy change effects on the thermal behavior of a LiFePO4/graphite lithium-ion cell at different states of charge. J. Power Source 2013, 243, 354–360. [Google Scholar] [CrossRef]
- Wojtala, M.E.; Zulke, A.A.; Burrell, R.; Nagarathinam, M.; Li, G.C.; Hoster, H.E.; Howey, D.A.; Mercer, M.P. Entropy Profiling for the Diagnosis of NCA/Gr-SiOx Li-Ion Battery Health. J. Electrochem. Soc. 2022, 169, 100527. [Google Scholar] [CrossRef]
- Osswald, P.J.; del Rosario, M.; Garche, J.; Jossen, A.; Hoster, H.E. Fast and Accurate Measurement of Entropy Profiles of Commercial Lithium-Ion Cells. Electrochim. Acta 2015, 177, 270–276. [Google Scholar] [CrossRef]
- Yazami, R.; Reynier, Y.; Fultz, B. Entropymetry of Lithium Intercalation in Spinel Manganese Oxide: Effect of Lithium Stoichiometry. ECS Trans. 2006, 1, 87. [Google Scholar] [CrossRef]
- Reiter, A.; Schütte, M.F.; Rosenmüller, C.; Stroebl, F.; Lehner, S.; Sauer, D.U.; Bohlen, O. Exploring the Effects of Aging, Temperature and Hysteresis on the Entropy Variation of Lithium-Ion Batteries. 2025. Available online: https://ssrn.com/abstract=5142673 (accessed on 18 March 2025).
- Bedurftig, B.; Oldenburger, M.; Hufner, T.; Richter, E.; Braatz, R.D.; Gruhle, A.; Findeisen, R. Measuring the reversible heat of lithium-ion cells via current pulses for modeling of temperature dynamics. J. Power Source 2021, 506, 230110. [Google Scholar] [CrossRef]
- Dreyer, W.; Jamnik, J.; Guhlke, C.; Huth, R.; Moskon, J.; Gaberscek, M. The thermodynamic origin of hysteresis in insertion batteries. Nat. Mater. 2010, 9, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Petzl, M.; Danzer, M.A. Advancements in OCV Measurement and Analysis for Lithium-Ion Batteries. IEEE Trans. Energy Convers. 2013, 28, 675–681. [Google Scholar] [CrossRef]
- Wind, J.; Vie, P.J.S. Revisiting Pulse-Based OCV Incremental Capacity Analysis for Diagnostics of Li-Ion Batteries. Batteries 2024, 10, 277. [Google Scholar] [CrossRef]
- Tardy, E.; Thivel, P.X.; Druart, F.; Kuntz, P.; Devaux, D.; Bultel, Y. Internal temperature distribution in lithium-ion battery cell and module based on a 3D electrothermal model: An investigation of real geometry, entropy change and thermal process. J. Energy Storage 2023, 64, 107090. [Google Scholar] [CrossRef]
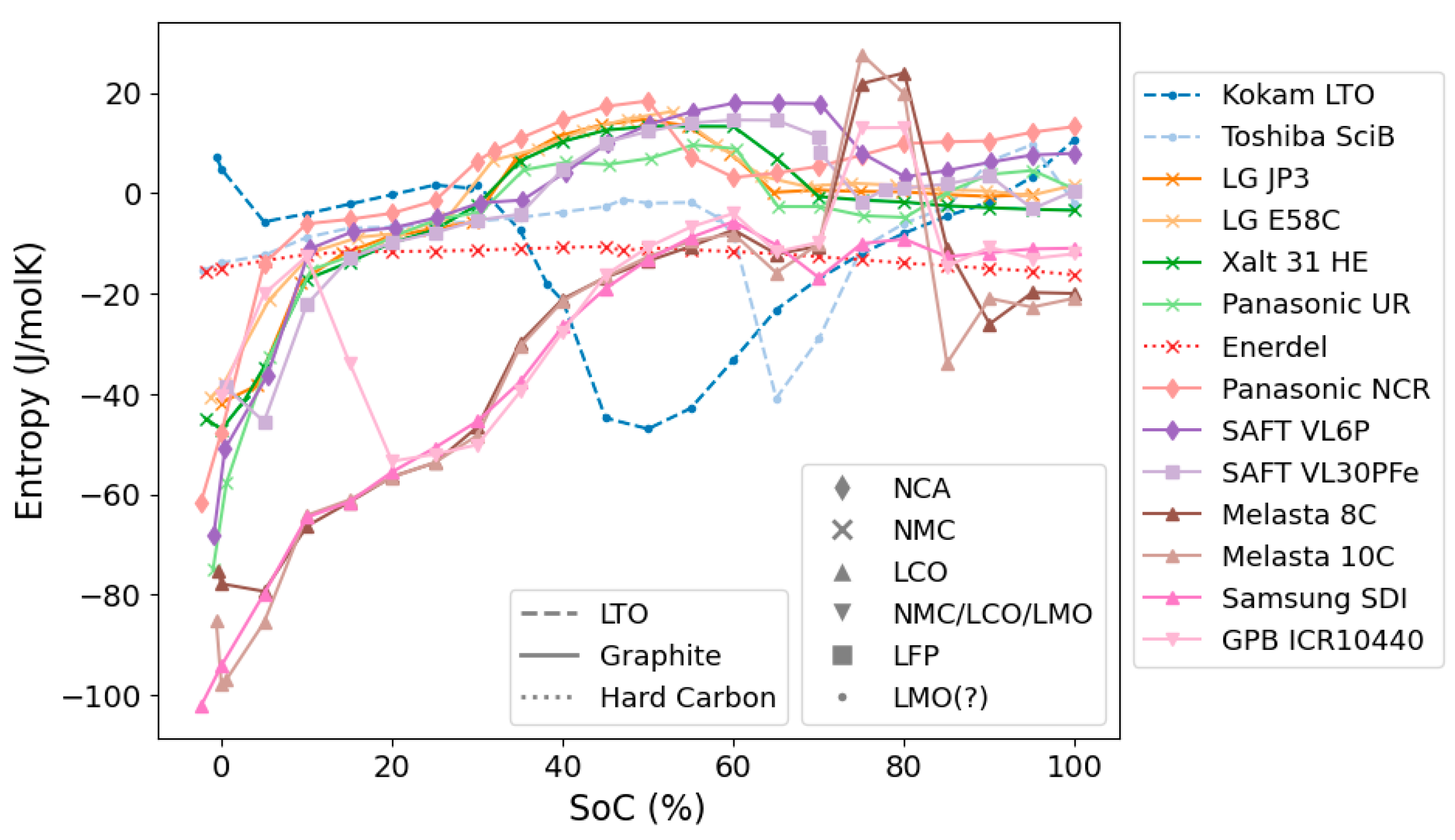
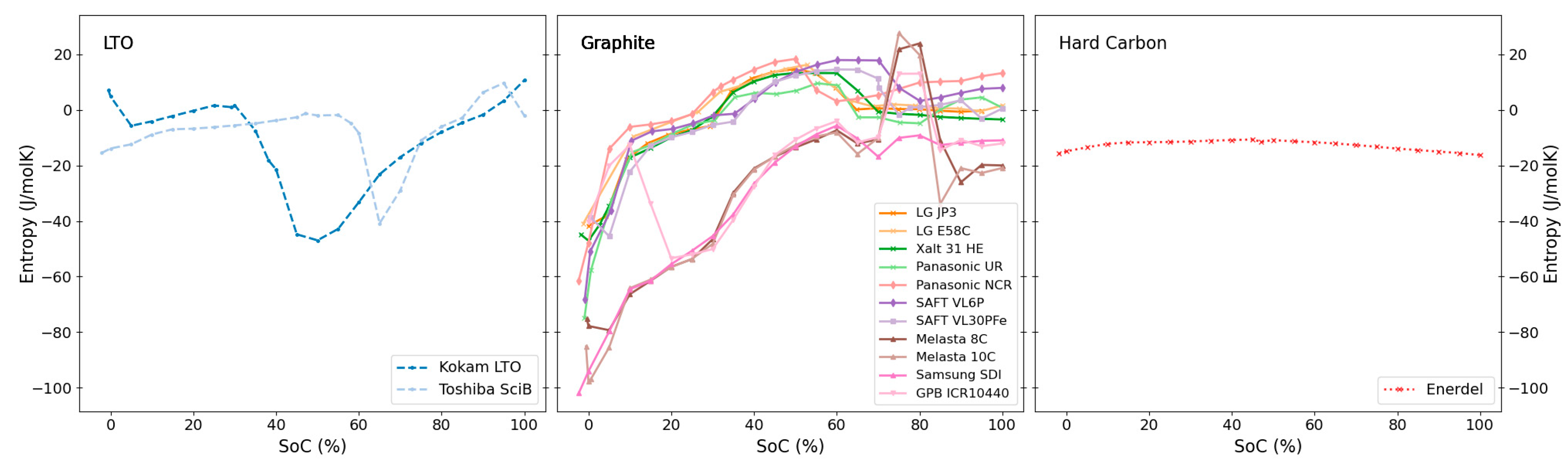
| Chemistry | Cell Labels (This Work) | Features and Comments | References |
|---|---|---|---|
| LTO | Kokam LTO, Toshiba SciB | Small overall contribution and little SoC variation | Half-cell: [48,49] Full cell: [26] |
| Graphite | LG JP3, LG E58C, XALT 31 HE, Panasonic UR, Panasonic NCR, SAFT VL6P, SAFT VL30PFe, Melasta 8C, Melasta 10C, Samsung SDI, GPB ICR10440 | Characteristic entropy profile following intercalation stages of Li into graphite (see text) | Full cell: [25,46,55,60,61] Half cell: [24,26,44,46,47] |
| Hard Carbon | Enerdel | Rather featureless, overall low magnitude values with very little variation with SoC | Full cell: [25] Half cell: [44] |
| NMC | LG JP3, LG E58C, XALT 31 HE, Panasonic UR, Enerdel | Low magnitude entropy, little SoC variation; detailed half-cell entropy measurements necessary to establish differences between different NMC compositions | Full cell: [26,46,51,52,53,60] Half cell: [46,52] |
| NCA | Panasonic NCR, SAFT VL6P | Low magnitude entropy, little SoC variation | Full cell: [57,58] |
| LFP | SAFT VL30PFe | Low magnitude entropy, little SoC variation | Full cell: [55,56,61] Half cell: [56] |
| LCO | Melasta 8C, Melasta 10C, Samsung SDI | Large negative entropy across almost the entire SoC window, except for pronounced peak at around 70–90% SoC (monoclinic-hexagonal phase transition) | Full cell: [26,44] Half cell: [25] |
| LMO | GPB ICR10440 (part of cathode mix in), Kokam LTO, Toshiba SciB | Low magnitude entropy with characteristic features: maximum around x = 0.65, minimum around x = 0.55 (x in LixMn2O4) | Half or full cell: [26,49,54,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wind, J.; Vie, P.J.S. Entropy Profiles for Li-Ion Batteries—Effects of Chemistries and Degradation. Entropy 2025, 27, 364. https://doi.org/10.3390/e27040364
Wind J, Vie PJS. Entropy Profiles for Li-Ion Batteries—Effects of Chemistries and Degradation. Entropy. 2025; 27(4):364. https://doi.org/10.3390/e27040364
Chicago/Turabian StyleWind, Julia, and Preben J. S. Vie. 2025. "Entropy Profiles for Li-Ion Batteries—Effects of Chemistries and Degradation" Entropy 27, no. 4: 364. https://doi.org/10.3390/e27040364
APA StyleWind, J., & Vie, P. J. S. (2025). Entropy Profiles for Li-Ion Batteries—Effects of Chemistries and Degradation. Entropy, 27(4), 364. https://doi.org/10.3390/e27040364






