On the First Quantum Correction to the Second Virial Coefficient of a Generalized Lennard-Jones Fluid
Abstract
1. Introduction
2. First Quantum Correction to the Second Virial Coefficient
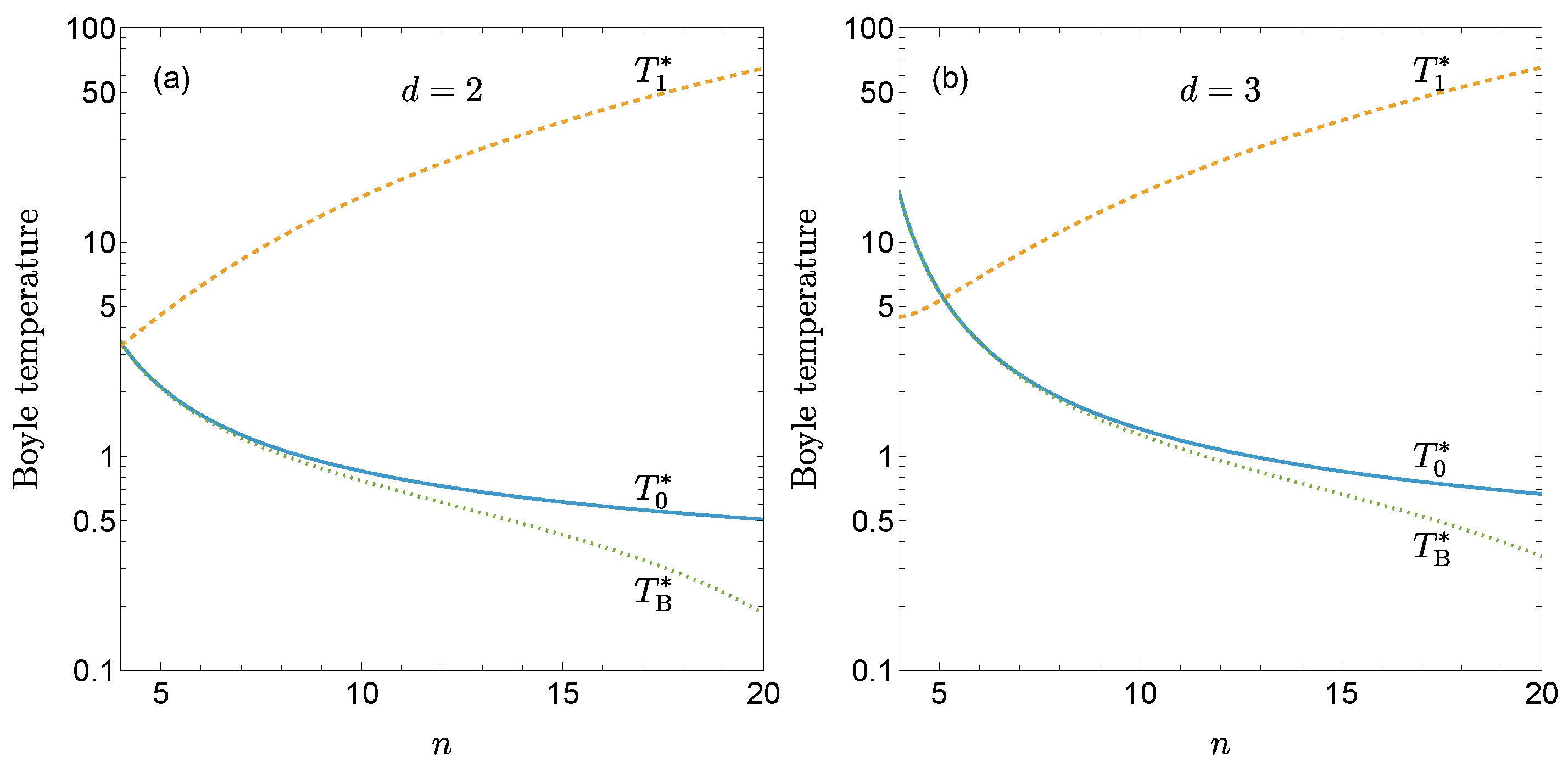
3. First Quantum Correction to the Boyle Temperature
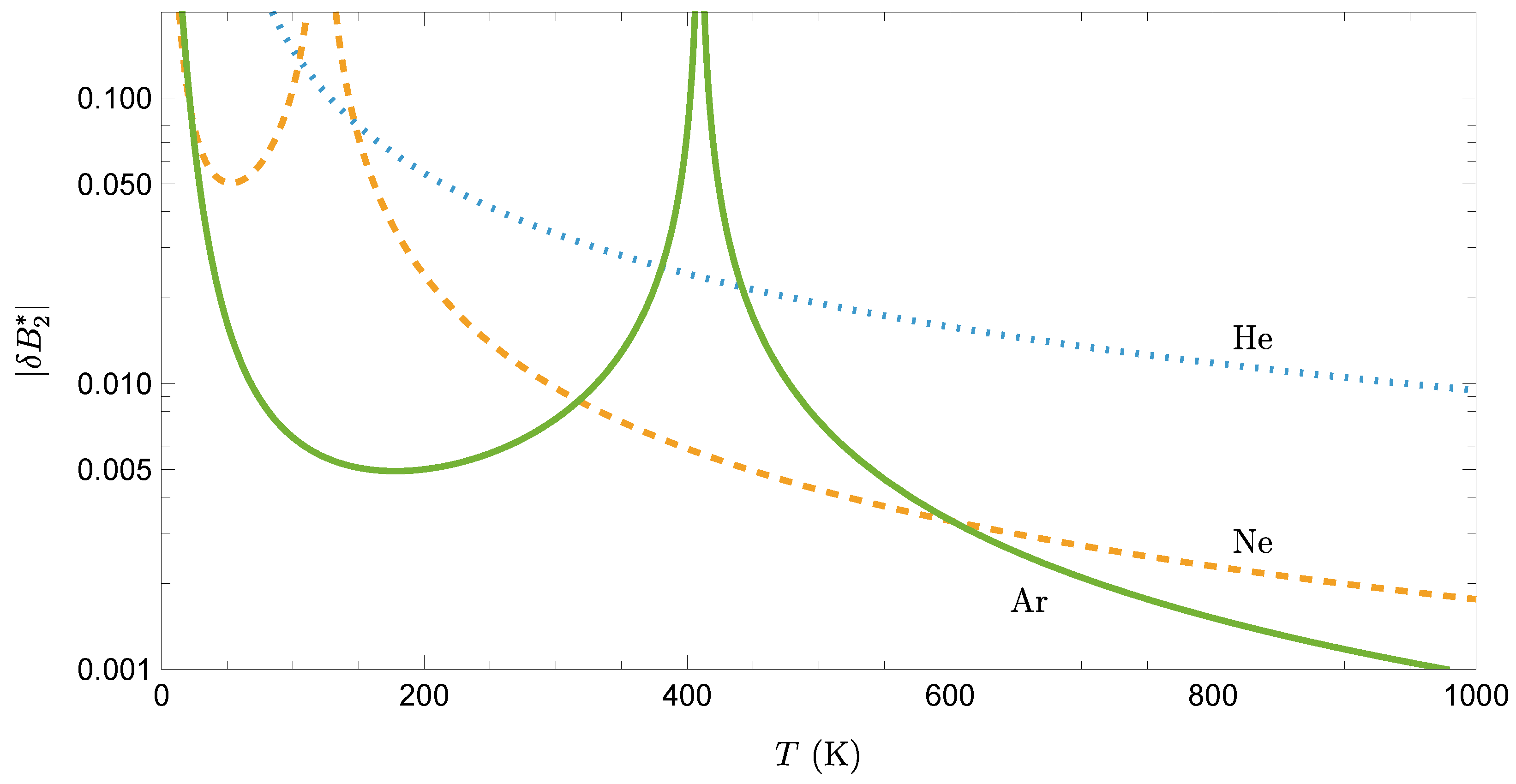
4. Application to Noble Gases
5. Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| gLJ | generalized Lennard-Jones |
| LJ | Lennard-Jones |
| sLJ | standard Lennard-Jones |
References
- Kihara, T. Virial Coefficients and Models of Molecules in Gases. B. Rev. Mod. Phys. 1955, 27, 412–423. [Google Scholar] [CrossRef]
- Kihara, T.; Midzuno, Y.; Shizume, T. Virial Coefficients and Intermolecular Potential of Helium. J. Phys. Soc. Jpn. 1955, 10, 249–255. [Google Scholar] [CrossRef]
- DeWitt, H.E. Analytic Properties of the Quantum Corrections to the Second Virial Coefficient. J. Math. Phys. 1962, 3, 1003–1016. [Google Scholar] [CrossRef]
- Michels, H.H. Low-Temperature Quantum Corrections to the Second Virial Coefficient. Phys. Fluids 1966, 9, 1352–1358. [Google Scholar] [CrossRef]
- Boyd, M.E.; Larsen, S.Y.; Kilpatrick, J.E. Quantum Mechanical Second Virial Coefficient of a Lennard-Jones Gas. Helium. J. Chem. Phys. 1969, 50, 4034–4055. [Google Scholar] [CrossRef]
- Boyd, M.E. Quantum Corrections to the Second Virial Coefficientfor the Lennard-Jones (m-6) Potential. J. Res. Natl. Bur. Stand. A Phys. Chem. 1971, 75A, 57–95. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, B.A.; Somuncu, E. Evaluation of Quantum Corrections to Second Virial Coefficient with Lennard-Jones (12-6) Potential. J. Phys. Conf. Ser. 2016, 766, 012010. [Google Scholar] [CrossRef]
- Zhao, Z.; González-Calderón, A.; Perera-Burgos, J.A.; Estrada, A.; Hernández-Anguiano, H.; Martínez-Lázaro, C.; Li, Y. Exact ODE Framework for Classical and Quantum Correctionsfor the Lennard-Jones Second Virial Coefficient. Entropy 2025, 27, 1059. [Google Scholar] [CrossRef] [PubMed]
- Santos, A. A Concise Course on the Theory of Classical Liquids. Basics and Selected Topics; Lecture Notes in Physics; Springer: New York, NY, USA, 2016; Volume 923. [Google Scholar]
- Olver, F.W.J.; Daalhuis, A.B.O.; Lozier, D.W.; Schneider, B.I.; Boisvert, R.F.; Clark, C.W.; Miller, B.R.; Saunders, B.V.; Cohl, H.S.; McClain, M.A. (Eds.) NIST Digital Library of Mathematical Functions; NIST: Gaithersburg, MD, USA, 2025. Available online: https://dlmf.nist.gov/ (accessed on 16 November 2025).
- Hirschfelder, J.O.; Curtiss, C.F.; Bird, R.B. Molecular Theory of Gases and Liquids; Wiley: New York, NY, USA, 1964. [Google Scholar]
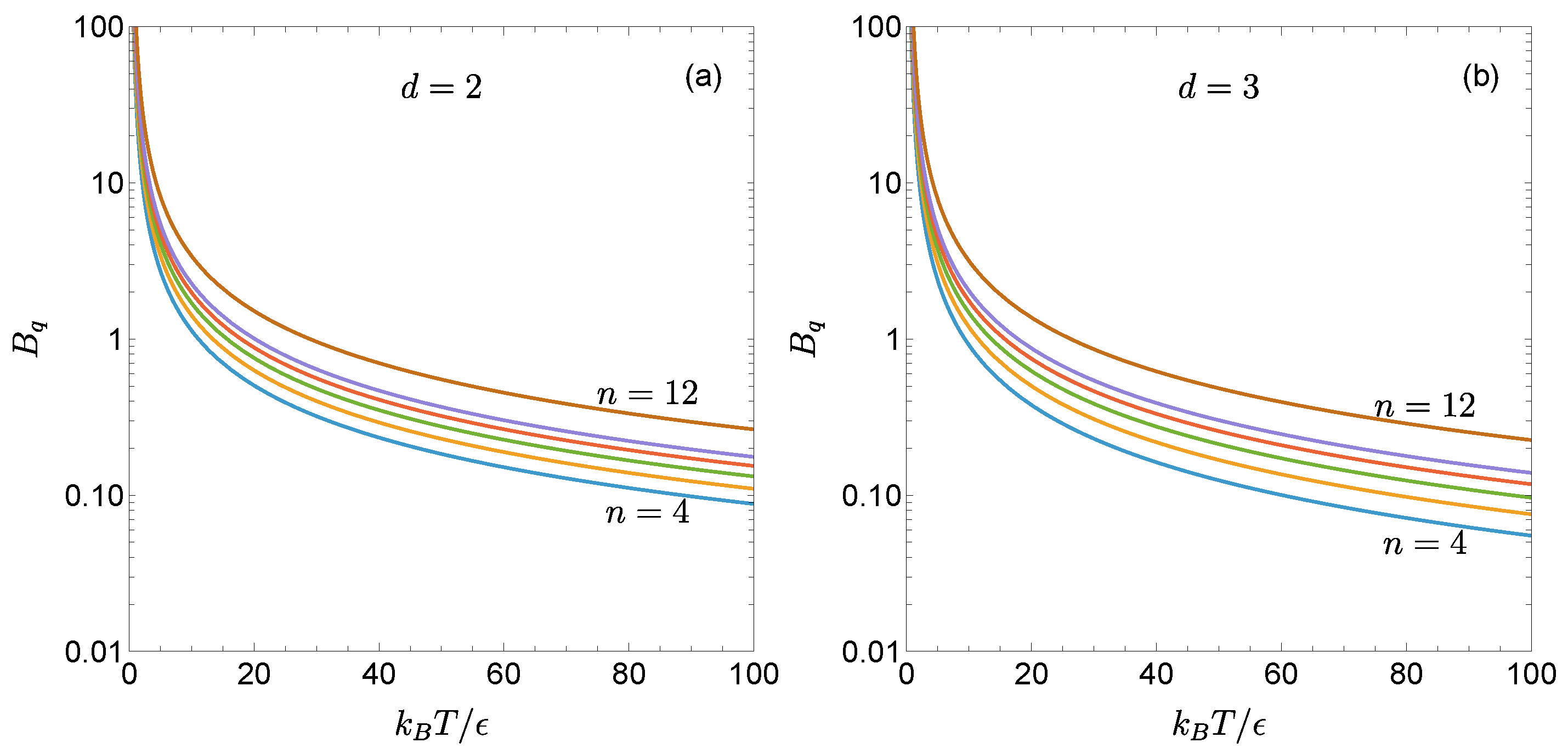
| Gas | m (kg) | (K) | (m) | q |
|---|---|---|---|---|
| He | ||||
| Ne | ||||
| Ar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parejo, D.; Santos, A. On the First Quantum Correction to the Second Virial Coefficient of a Generalized Lennard-Jones Fluid. Entropy 2025, 27, 1251. https://doi.org/10.3390/e27121251
Parejo D, Santos A. On the First Quantum Correction to the Second Virial Coefficient of a Generalized Lennard-Jones Fluid. Entropy. 2025; 27(12):1251. https://doi.org/10.3390/e27121251
Chicago/Turabian StyleParejo, Daniel, and Andrés Santos. 2025. "On the First Quantum Correction to the Second Virial Coefficient of a Generalized Lennard-Jones Fluid" Entropy 27, no. 12: 1251. https://doi.org/10.3390/e27121251
APA StyleParejo, D., & Santos, A. (2025). On the First Quantum Correction to the Second Virial Coefficient of a Generalized Lennard-Jones Fluid. Entropy, 27(12), 1251. https://doi.org/10.3390/e27121251







