Abstract
We explore the intersection of neural dynamics and the effects of psychedelics in light of distinct timescales in a framework integrating concepts from dynamics, complexity, and plasticity. We call this framework neural geometrodynamics for its parallels with general relativity’s description of the interplay of spacetime and matter. The geometry of trajectories within the dynamical landscape of “fast time” dynamics are shaped by the structure of a differential equation and its connectivity parameters, which themselves evolve over “slow time” driven by state-dependent and state-independent plasticity mechanisms. Finally, the adjustment of plasticity processes (metaplasticity) takes place in an “ultraslow” time scale. Psychedelics flatten the neural landscape, leading to heightened entropy and complexity of neural dynamics, as observed in neuroimaging and modeling studies linking increases in complexity with a disruption of functional integration. We highlight the relationship between criticality, the complexity of fast neural dynamics, and synaptic plasticity. Pathological, rigid, or “canalized” neural dynamics result in an ultrastable confined repertoire, allowing slower plastic changes to consolidate them further. However, under the influence of psychedelics, the destabilizing emergence of complex dynamics leads to a more fluid and adaptable neural state in a process that is amplified by the plasticity-enhancing effects of psychedelics. This shift manifests as an acute systemic increase of disorder and a possibly longer-lasting increase in complexity affecting both short-term dynamics and long-term plastic processes. Our framework offers a holistic perspective on the acute effects of these substances and their potential long-term impacts on neural structure and function.
1. Introduction
In this paper, we explore new perspectives to interpret changes in the brain’s landscape and connectivity, focusing on the subtle interplay between structural and dynamical aspects across timescales (fast, slow, and ultraslow). Our primary goal is to present a framework that enhances the understanding of the intricate relationships among brain dynamics, complexity, structure, and plasticity. This framework, which we call “neural geometrodynamics”, draws on principles from non-linear dynamics and is further inspired by conceptual links to general relativity in physics.
In describing neural dynamics, we will refer to the mathematical formalism of neural mass models (NMMs), although other computational neuroscience formulations are equally relevant [1,2]. Neural mass models have been extensively utilized to model various brain activities, from localized brain functions to the coordinated activity observed in different brain regions. By employing mathematical formulations that include essential features like synaptic connectivity and neuronal excitability, NMMs enable the simulation and analysis of complex brain activities in various dynamic regimes [3]. NMMs are particularly useful because they provide a link between the mesoscopic physiological scale and macroscopic brain function, allowing for the connection of effects on neurons at the molecular level, such as those of psychedelics, with those of whole-brain connectivity [4,5].
Analyzing the effects of psychoactive neuroplastogens (psychedelics such as psilocybin or LSD) serves as an illustrative case of the framework, given the immediate and potentially lasting plastic changes these substances can provoke in the brain [6]. By altering neural dynamics and connectivity, psychedelics are thought to induce both transient and sustained shifts in cognition and perception [7]. Several studies underscore the role of psychedelics in inducing neuroplasticity with antidepressant effects, revealing mechanisms at molecular, synaptic, and dendritic levels [8,9], and with significant potential for treating neuropsychiatric disorders [10,11], although the duration and permanence of these effects remain to be fully understood.
Recent conceptual perspectives have enhanced our understanding of the brain’s response to psychedelics, combining biological, dynamical systems, complexity science, and artificial intelligence viewpoints. The REBUS (RElaxed Beliefs Under pSychedelics) framework [12], grounded in the Free Energy Principle (FEP) and the entropic and anarchic brain models, offers a perspective on the effects of psychedelics on the brain whereby psychedelic action results in the collapse of brain functional hierarchies or, in other words, in the “flattening of the landscape” of brain’s dynamics to allow the brain state to escape a deep local minimum. The term annealing is also used in this context in relation to physical annealing in metallurgy and simulated annealing in numerical optimization [13].
Consequently, it has been argued that the observed expansion of the repertoire of functional patterns elicited by hallucinogenic substances can be associated with an increase in entropy in brain dynamics [14,15], with the brain moving to a more disordered state from a relaxation of high-level cognitive priors [12,16]. This may lead to a favorable context for conducting psychotherapy [12,17,18]. Studies on functional neuroimaging regarding psilocybin and LSD effects have shown initial evidence of the mechanistic alterations on brain dynamics at the network level, with the majority of the findings suggesting a relative weakening of usual functional configurations giving place to an increase of brain entropy, global functional integration, and more flexible brain dynamics [14,19,20,21,22,23,24,25,26,27,28]. As mentioned above, these changes are traditionally reflected in the complexity of neural dynamics, which can be evaluated using various techniques such as criticality measures [29,30], complexity measures [31], connectome harmonic decomposition [22,23,24], control theory [25] and Ising (or spinglass) modeling [32,33].
For example, Ising modeling of psychedelics has shown that the increased complexity of brain dynamics under LSD (e.g., increased Ising temperature, Lempel-Ziv, and the Block Decomposition Method complexity) is associated with a decrease of interhemispheric connectivity—especially homotopic links [34], corroborating earlier modeling studies suggesting the central role of long-range connections in controlling phase transitions [35].
The observed push of brain dynamics towards disorder and away from criticality aligns with the REBUS and FEP frameworks, which link the vividness of experience to the entropy of brain activity. At the same time, the notion that a wakeful brain exhibits dimensionality reduction and criticality features that are disrupted by the effect of psychedelics is also predicted by an algorithmic perspective on consciousness [16,36,37], where the psychedelic shift towards disorder is associated with a disruption of the world-modeling/world-tracking circuits in the brain.
Another feature of brain dynamics related to the collapse of higher-order cognitive functions under psychedelics in the REBUS framework is the hierarchical organization along the uni- to trans-modal functional gradient [38]. This asymmetry in neural activity reflects the bottom-up and top-down information flows in cognitive processing [39,40]. This has been suggested to be intimately linked to non-equilibrium dynamics in thermodynamic-inspired frameworks where the level of hierarchy is related to the amount of brain signal irreversibility as well as entropy production [41,42,43]. Indeed it has been demonstrated that the principal functional gradient collapses under the influence of various psychedelics [44,45,46].
A related perspective for this paper is the CANAL framework [11] for describing the pathological plasticity of “being stuck in a rut” in certain mood disorders and the potential therapeutic role of psychedelics through the concept of metaplasticity. In contrast to psychedelics, these changes are reflected in neural dynamics with brain signatures of excessively rigid and highly ordered functional states [47]. The CANAL framework has been further extended by establishing connections with deep artificial neural networks (Deep CANAL [48]) to introduce a distinction between two distinct pathological phenomena—one related to fast brain dynamics and their slow and ultraslow counterparts. These distinctions will be naturally integrated into the presented framework (see the Appendix A Figure A1 relating the concepts in the different frameworks).
While the discussion is centered on the effects of psychedelics, the framework proposed here extends more generally to other phenomena related to plasticity, including neurodevelopment, pathological plasticity in mood disorders [49], and interventions that alter brain dynamics like transcranial brain stimulation (tES) [50], transcranial magnetic stimulation (TMS), or electroconvulsive therapy (ECT).
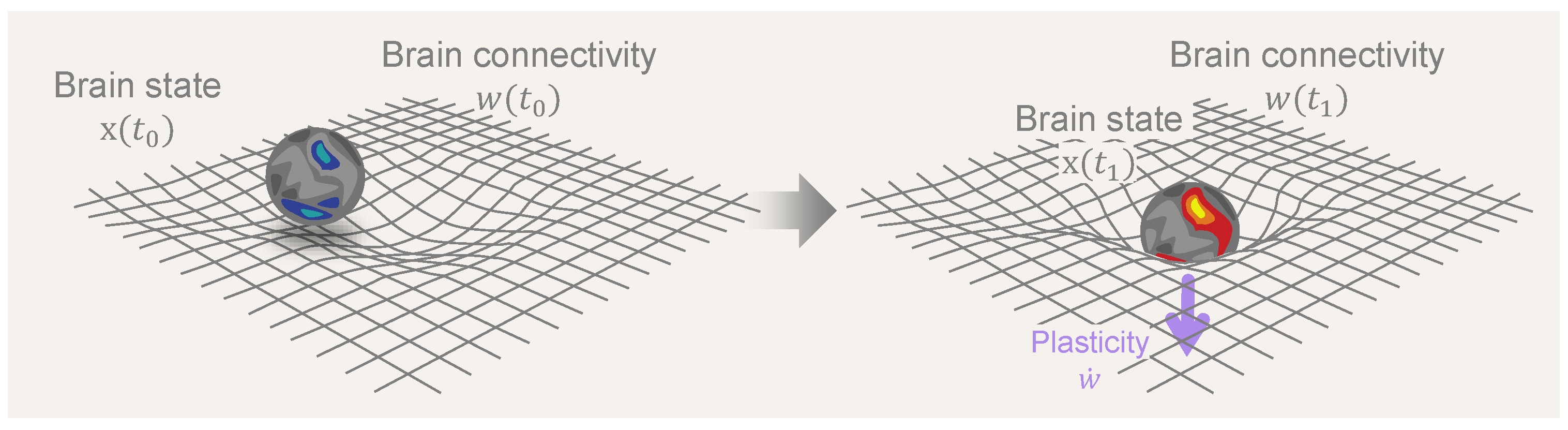
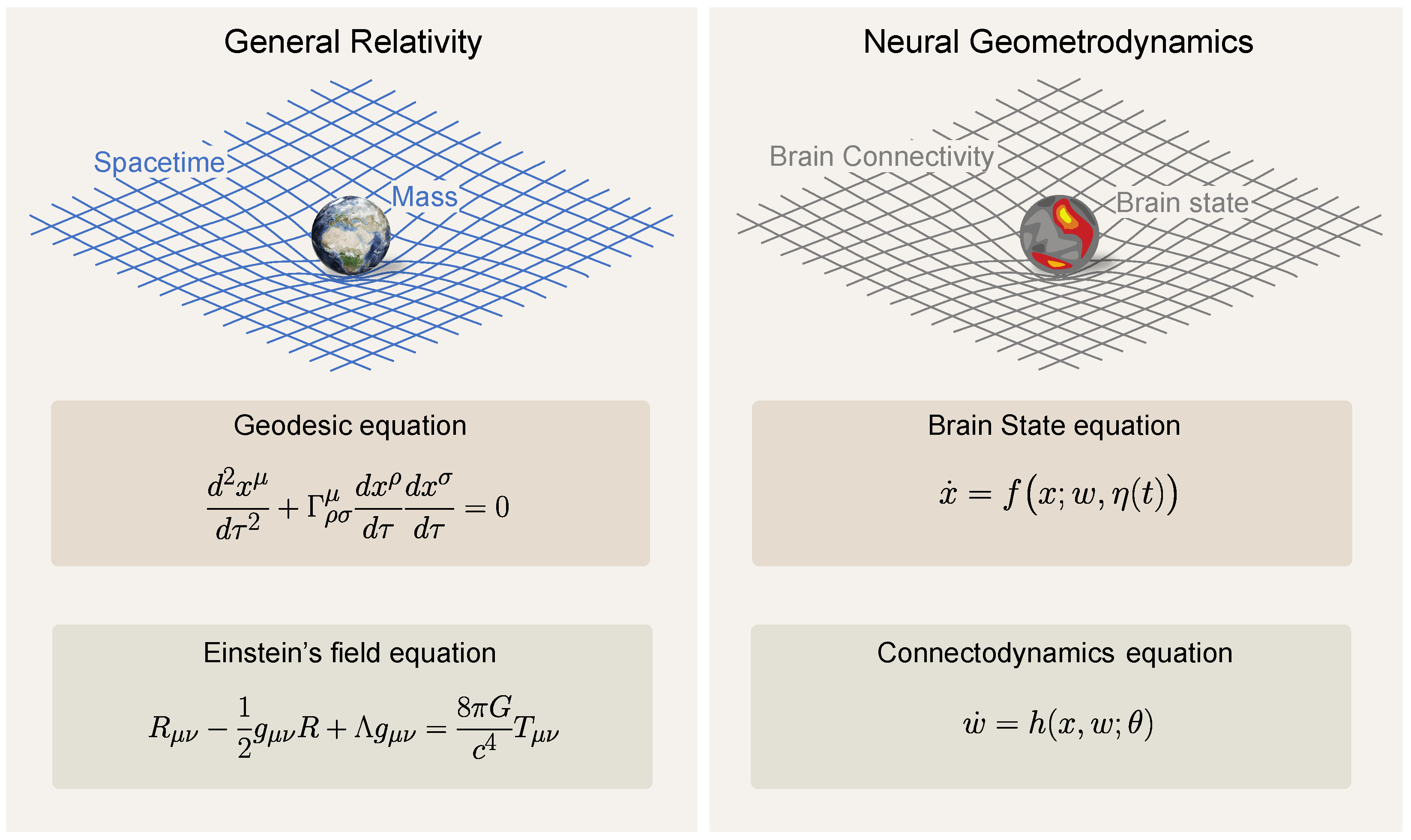
In what follows, we formalize the notions of brain dynamics, plasticity, and their associated timescales and subsequently use them to study the impact of psychedelics on the brain. In the last section, we draw connections between the framework and concepts from general relativity in physics. We hope these parallels will illuminate the complex relationship between the structure and function of brain dynamics. Figure 1 illustrates the reciprocal dynamics between brain states and connectivity as conceptualized in the neural geometrodynamics framework.
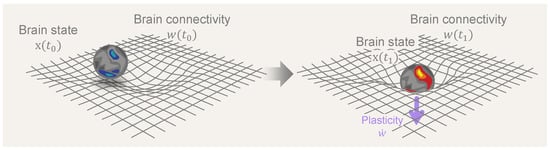
Figure 1.
Neural Geometrodynamics: a dynamic interplay between brain states and connectivity. A central element in the discussion is the dynamic interplay between brain state (x) and connectivity (w), where the dynamics of brain states is driven by neural connectivity while, simultaneously, state dynamics influence and reshape connectivity through neural plasticity mechanisms. The central arrow represents the passage of time and the effects of external forcing (from, e.g., drugs, brain stimulation, or sensory inputs), with plastic effects that alter connectivity (, with the overdot standing for the time derivative).
2. Dynamics across Timescales
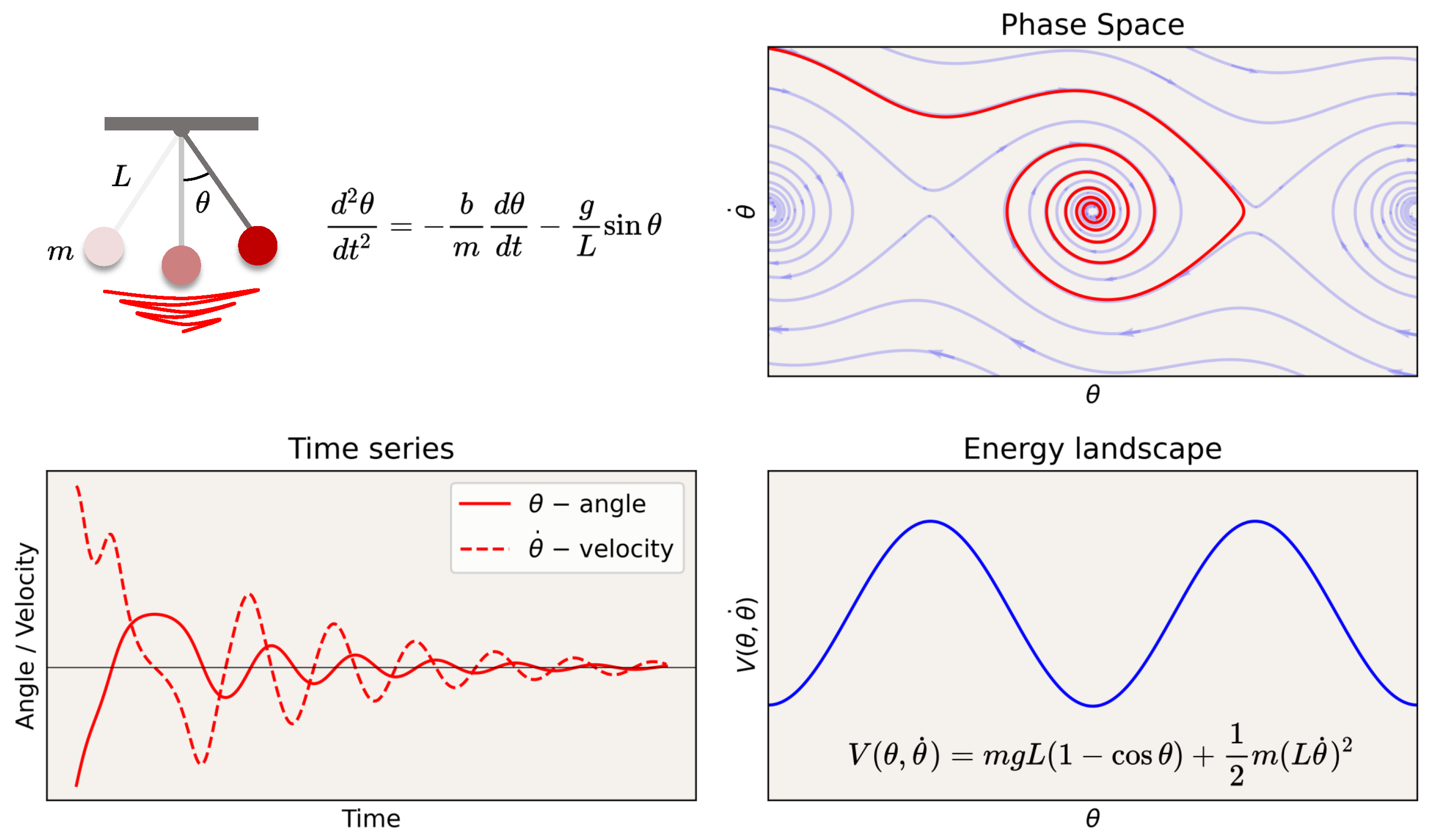
The state of a system can be defined by a set of coordinates in phase space: a multidimensional manifold in which each dimension corresponds to one of the variables. For a single particle moving in one dimension, the phase space is two-dimensional, with one axis representing its position and the other representing its momentum. For example, Figure 2 illustrates the phase space of a pendulum with friction. In phase space, and perhaps after some transient period, the possible trajectories of the states of the system lie in a reduced or invariant manifold (an attractor, see Box 1 for a glossary of terms), which we may refer to as the “geometry” or latent “structure” of the phase space. Together, the structure (geometry and topology) of the phase space with its invariant properties can be referred to as the dynamical landscape, where the depth or shallowness of the “valleys” can, in some cases, be interpreted as the stability of the state in that location given some stochastic forcing. For example, in mechanics, the landscape can be labeled by potential energy isolines, e.g., in a physical system such as in the pendulum example in Figure 2 (bottom right), or their generalization, Lyapunov functions [51]. For definitions and explanations of key terms used in this discussion, see the Glossary in Box 1.
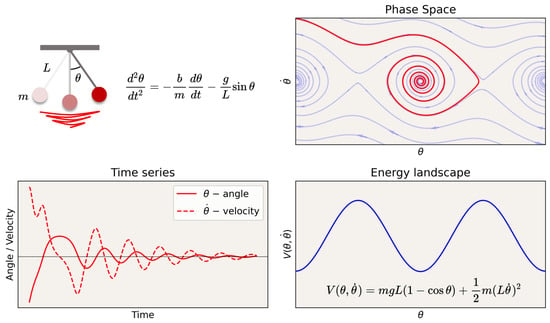
Figure 2.
Dynamics of a pendulum with friction. Time series, phase space, and energy landscape. Attractors in phase space are sets to which the system evolves after a long enough time. In the case of the pendulum with friction, it is a point in the valley in the “energy” landscape (more generally, defined by the level sets of a Lyapunov function).
Box 1. Glossary.
State of the system: Depending on the context, the state of the system is defined by the coordinates x (Equation (1), fast time view) or by the full set of dynamical variables (x, w, )—see Equations (1)–(3).
Entropy: Statistical mechanics: the number of microscopic states corresponding to a given macroscopic state (after coarse-graining), i.e., the information required to specify a specific microstate in the macrostate. Information theory: a property of a probability distribution function quantifying the uncertainty or unpredictability of a system.
Complexity: A multifaceted term associated with systems that exhibit rich, varied behavior and entropy. In algorithmic complexity, this is defined as the length of the shortest program capable of generating a dataset (Kolmogorov complexity). Characteristics of complex systems include nonlinearity, emergence, self-organization, and adaptability.
Critical point: Dynamics: parameter space point where a qualitative change in behavior occurs (bifurcation point, e.g., stability of equilibria, emergence of oscillations, or shift from order to chaos). Statistical mechanics: phase transition where the system exhibits changes in macroscopic properties at certain critical parameters (e.g., temperature), exhibiting scale-invariant behavior and critical phenomena like diverging correlation lengths and susceptibilities. These notions may interconnect, with bifurcation points in large systems leading to phase transitions.
Temperature: In the context of Ising or spinglass models, it represents a parameter controlling the degree of randomness or disorder in the system. It is analogous to thermodynamic temperature and influences the probability of spin configurations. Higher temperatures typically correspond to increased disorder and higher entropy states, facilitating transitions between different spin states.
Effective connectivity (or connectivity for short): In our high-level formulation, this is symbolized by w. It represents the connectivity relevant to state dynamics. It is affected by multiple elements, including the structural connectome, the number of synapses per fiber in the connectome, and the synaptic state (which may be affected by neuromodulatory signals or drugs).
Plasticity: The ability of the system to change its effective connectivity (w), which may vary over time.
Metaplasticity: The ability of the system to change its plasticity over time (dynamics of plasticity).
State or Activity-dependent plasticity: Mechanism for changing the connectivity (w) as a function of the state (fast) dynamics and other parameters (). See Equation (2).
State or Activity-independent plasticity: Mechanism for changing the connectivity (w) independently of state dynamics, as a function of some parameters (). See Equation (2).
Connectodynamics: Equations governing the dynamics of w in slow or ultraslow time.
Fast time: Timescale associated to state dynamics pertaining to x.
Slow time: Timescale associated to connectivity dynamics pertaining to w.
Ultraslow time: Timescale associated to plasticity dynamics pertaining to —v. Equation (3).
Phase space: Mathematical space, also called state space, where each point represents a possible state of a system, characterized by its coordinates or variables.
Geometry and topology of reduced phase space: State trajectories lie in a submanifold of phase space (the reduced or invariant manifold). We call the geometry of this submanifold and its topology the “structure of phase space” or “geometry of dynamical landscape”.
Topology: The study of properties of spaces that remain unchanged under continuous deformation, like stretching or bending, without tearing or gluing. It’s about the ‘shape’ of space in a very broad sense. In contrast, geometry deals with the precise properties of shapes and spaces, like distances, angles, and sizes. While geometry measures and compares exact dimensions, topology is concerned with the fundamental aspects of connectivity and continuity.
Invariant manifold: A submanifold within (embedded into) the phase space that remains preserved or invariant under the dynamics of a system. That is, points within it can move but are constrained to the manifold. Includes stable, unstable, and other invariant manifolds.
Stable manifold or attractor: A type of invariant manifold defined as a subset of the phase space to which trajectories of a dynamical system converge or tend to approach over time.
Unstable Manifold or Repellor: A type of invariant manifold defined as a subset of the phase space from which trajectories diverge over time.
Latent space: A compressed, reduced-dimensional data representation (see Box 2).
Topological tipping point: A sharp transition in the topology of attractors due to changes in system inputs or parameters.
Betti numbers: In algebraic topology, Betti numbers are integral invariants that describe the topological features of a space. In simple terms, the n-th Betti number refers to the number of n-dimensional “holes” in a topological space.
Box 2. The manifold hypothesis and latent spaces.
The dimension of the phase (or state) space is determined by the number of independent variables required to specify the complete state of the system and the future evolution of the system. The Manifold hypothesis posits that high-dimensional data, such as neuroimaging data, can be compressed into a reduced number of parameters due to the presence of a low-dimensional invariant manifold within the high-dimensional phase space [52,53]. Invariant manifolds can take various forms, such as stable manifolds or attractors and unstable manifolds. In attractors, small perturbations or deviations from the manifold are typically damped out, and trajectories converge towards it. They can be thought of as lower-dimensional submanifolds within the phase space that capture the system’s long-term behavior or steady state. Such attractors are sometimes loosely referred to as the “latent space” of the dynamical system, although the term is also used in other related ways. In the related context of deep learning with variational autoencoders, latent space is the compressive projection or embedding of the original high-dimensional data or some data derivatives (e.g., functional connectivity [54,55]) into a lower-dimensional space. This mapping, which exploits the underlying invariant manifold structure, can help reveal patterns, similarities, or relationships that may be obscured or difficult to discern in the original high-dimensional space. If the latent space is designed to capture the full dynamics of the data (i.e., is constructed directly from time series) across different states and topological tipping points, it can be interpreted as a representation of the invariant manifolds underlying system.
2.1. Fast Time: Neural Dynamics
Here, we discuss the first equation in neural geometrodynamics in the context of neural mass models, though the ideas are applicable more extensively in computational neuroscience. The standard equation we use in neural mass modeling is a multidimensional ODE of the form
with and where w denotes connectivity parameters and where, as usual, a dot over a variable denotes its time derivative. The above equation governs dynamics at short time scales (seconds or less) when the connectivity parameters w are assumed to be constant.
The external input term makes the equations non-autonomous (an autonomous ODE does not explicitly depend on time). This term can refer to external forces providing random kicks to the trajectory or to a more steady and purposeful forcing from unspecified internal systems, external inputs from sensory systems, or external electric fields, for example.
We may think of this equation describing phenomena in fast time scales as providing the “structure” for the dynamics of the neuronal population state. The fast timescale is set by synaptic transmission (milliseconds) and by ephaptic coupling (electromagnetic waves) [56,57,58,59] on a nanosecond or subnanosecond scale [58].
Equation (1) characterizes the dynamical landscape, which is established through the geometric structure of the phase space, where trajectories are shaped by the given set of ordinary differential equations. The landscape is determined by the functional form of and by the parameters w, and is analogous to the Neural Activation Landscape proposed in [48]. In the REBUS model [12], w would correspond to the weights or precision assigned to priors/beliefs from the Free Energy perspective, while from the Entropic Brain perspective w would correspond to the weights of the effective connectivity between neuronal populations on the macroscopic scale.
More specifically, we talk about the landscape as defined by the manifold generated by the motion of trajectories with coordinates . Typically, trajectories lie in a reduced manifold of dimensionality lower than . The fact that such a reduced space exists means that it can be generated by coordinates in a reduced latent space. The geometry and topology of this reduced space in different states provide a synthetic description of the dynamics and are of special interest [37].
2.2. Slow Time: Connectodynamics
The landscape, like that on planet Earth, may appear to be static; however, in reality it is not fixed. It flows in slow time; thus, we can consider changes in connectivity in the system, which is now . We refer to the potential for such changes as the plasticity of the system. The general form of this equation is , with standing for the set of parameters controlling the plasticity.
To be more concrete, we can think of two types of process: one that modifies the connectivity parameters independently of the system’s state (), and another that is a function of the state (e.g., Hebbian plasticity [60], h). We express this by writing
(with the second term understood as not separable into parts where any part is a function of only w). This decomposition separates out state-dependent (via the term ) and state-independent plasticity (with ) processes. The set of parameters is similarly decomposed as : we separate out the plasticity-controlling parameters in order to differentiate the state-dependent (α) and state-independent (γ) plasticity control parameters (e.g., Hebbian vs. drug-enhanced structural plasticity [11]). The parameters may vary in time to reflect, for example, the effects of drugs. The dynamics of these parameters are formalized in the next section.
Hebbian plasticity is the most prominent example of state-dependent plasticity [60]. State dependence implies that state-related concepts such as system temperature, phase transitions, and critical phenomena are relevant for the study of the dynamics of plasticity. In particular, within the scope of slower “slow time” (taking place over many hours), we include homeostatic plasticity [61,62,63,64], which may itself target desired complexity states as a homeostatic goal [65,66]. In the case of state-independent plasticity, there are numerous candidates for these plastic processes, such as heterosynaptic plasticity [67] or critical-period plasticity [68].
In summary, the functions h and with parameters and regulate connectodynamics, defining where and how fast the effective connectivity will change in a state-dependent or state-independent way.
These connectodynamics differential equations define a new dynamical landscape, which we can call the plasticity landscape (analogous to the Synaptic Weight Landscape in [48]). The state w in this plasticity landscape will determine the shape of the neural dynamics landscape.
2.3. Ultraslow Time: Metaplasticity
Plasticity is required to adapt to a changing environment [69], and the environment may change at different rates at different times. Plasticity in the healthy brain should match this variation in the character of dynamics accordingly. This is analogous to the situation in biology, where optimal mutation rates ensure successful adaptation in a tradeoff with genetic integrity [70]. More specifically, the plasticity-regulating parameters and in Equation (2) should adapt to changes in the environmental conditions.
In pathological cases, plasticity levels can either become overly exuberant, reflecting the notion of catastrophic forgetting in artificial neural networks, or impoverished and rigid, reflecting general plasticity loss [48]. These scenarios can be tentatively related to certain neurological and psychiatric conditions. For example, reduced plasticity could underlie conditions such as major depressive disorder, obsessive-compulsive disorder, anxiety, or substance abuse [48,71].
To account for the dynamics of plasticity, we allow the plasticity parameters to be dynamic, i.e.,
This equation is again state-dependent, allowing the system to respond to changes in the neural dynamics (with state dynamics as drivers of plasticity parameter regulation [72]), including critical phenomena (changes in criticality regime [65]) and complexity. Plasticity dynamics reflect changes in the parameters regulating state-dependent (Hebbian) plasticity (changes in ) during neurodevelopment, and state-independent plasticity, such as the ones induced by psychedelics in the acute or post-acute phases (changes in ). Finally, this equation is a function of other parameters and non-autonomous terms (), reflecting external perturbations of the system, such as those from drugs. We provide analogies in the context of sailing and electrodynamics in Appendix A to further clarify these concepts.
The dynamics of plasticity presented above reflect a physiological principle well described by Abraham et al. in the definition of metaplasticity [73]:
Metaplasticity […] is manifested as a change in the ability to induce subsequent synaptic plasticity, such as long-term potentiation or depression. Thus, metaplasticity is a higher-order form of synaptic plasticity.[73]
Thus, metaplasticity and its counterparts are terms used in neuroscience to refer to the plasticity of synaptic plasticity. That is, the idea that the ability of synapses to strengthen or weaken in response to increases or decreases in their activity (which is called synaptic plasticity) can be modulated based on the history of the synaptic activity or other factors (e.g., age, neuromodulatory systems, drugs, or lifestyle [74]). Metaplasticity has important implications for the learning and memory of an organism, as it can regulate the ability of synaptic plasticity to change and adapt over time as required by its environmental context.
We call the set of Equations (1)–(3)—somewhat whimsically—the equations for neural geometrodynamics in reference to the equations of general relativity in physics. We recall that general relativity provides equations defining the dynamics of spacetime geometry (via the “metric”) coupled with matter [75]. Section 4 elaborates further on this parallel.
3. Dynamics under Psychedelics
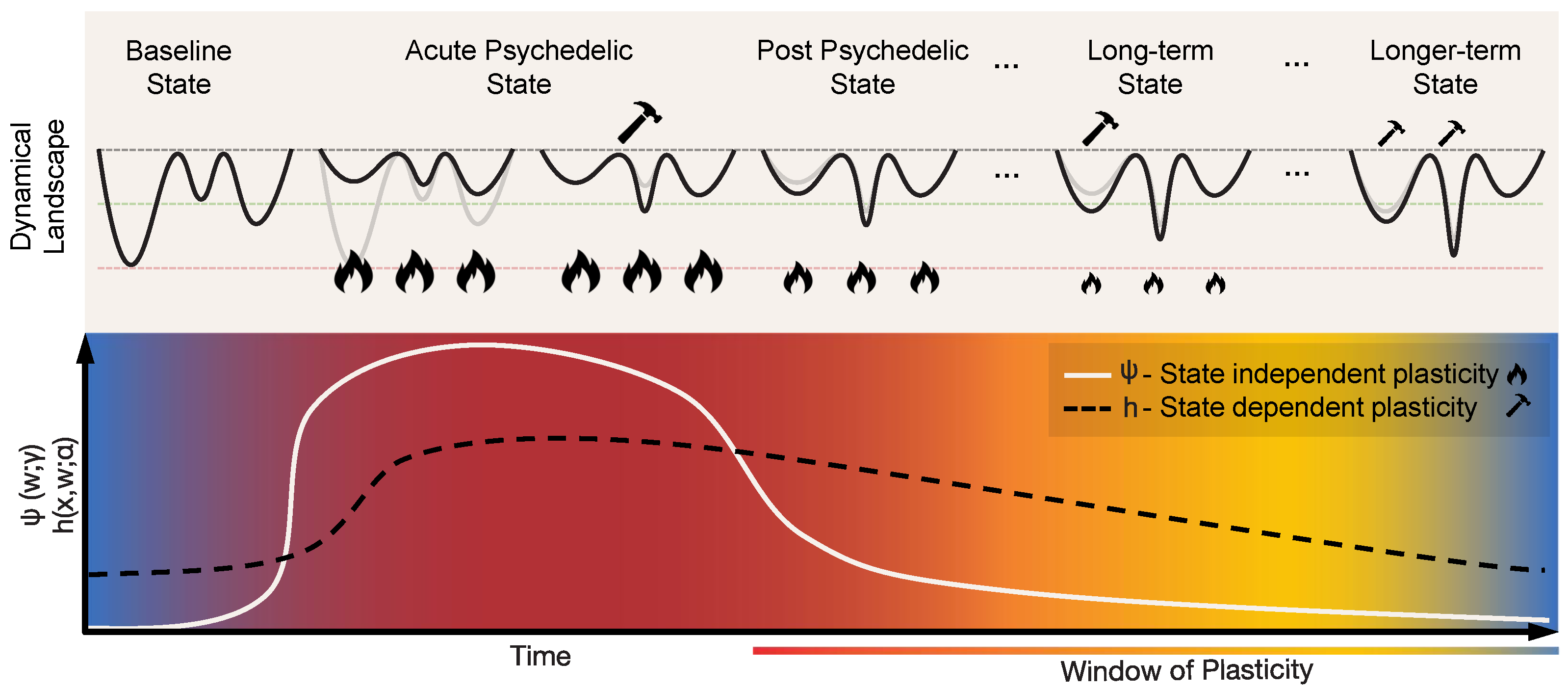
Psychedelics like psilocybin and LSD act as agonists or partial agonists for serotonin 5-hydroxytryptamine 2A (5-HT2A) receptors, specifically targeting Layer V cortical pyramidal neurons [11,12,14,76,77], leading to increased neuronal excitability through an increase in excitatory postsynaptic currents and discharge rates in pyramidal neurons [12]. The highest expression of 5-HT2ARs is found on the apical dendrites of Layer 5 pyramidal cells in both cortical and subcortical structures [12,78]. In the cortex, 5-HT2A receptors are strongly expressed along a steep anteroposterior gradient [79]. When psychedelics bind to these receptors, they can lead to a gradual increase of the excitability of these pyramidal neurons—depolarizing them and making them more susceptible to excitatory inputs such as those associated with glutamate receptors [79]—much as the gain knob in an amplifier. This increased excitability and susceptibility to inputs can lead to changes in the firing patterns of these neurons and alterations in the overall neural circuit activity. Recognized for their potent and immediate impact on the brain, these drugs cause a swift reconfiguration of neural dynamics. As we explain, this immediate effect is represented in our model by state-independent alterations in the connectivity parameters (w) (see Figure 3).
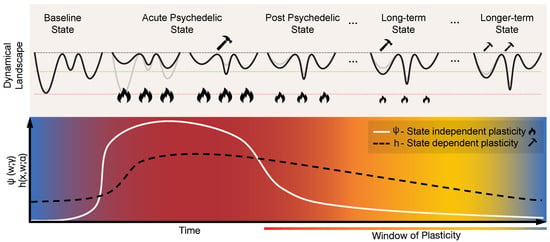
Figure 3.
Geometrodynamics of the acute and post-acute plastic effects of psychedelics. The acute plastic effects can be represented by rapid state-independent changes in connectivity parameters, i.e., the term in Equation (3). This results in the flattening or de-weighting of the dynamical landscape. Such flattening allows for the exploration of a wider range of states, eventually creating new minima through state-dependent plasticity, represented by the term in Equation (3). As the psychedelic action fades out, the landscape gradually transitions towards its initial state, though with lasting changes due to the creation of new attractors during the acute state. The post-acute plastic effects can be described as a “window of enhanced plasticity”. These transitions are brought about by changes of the parameters and , each controlling the behavior of state-independent and state-dependent plasticity, respectively. In this post-acute phase, the landscape is more malleable to internal and external influences.
How are these effects represented in Equations (1) and (2)? If we include the neuromodulatory nodes in our model—the dorsal raphe and median raphe nuclei in the brainstem are the source of most serotonergic neurons projecting throughout the brain [79]—, the modulation of serotonin receptors could be represented by changes in neuromodulatory connectivity (the subset of w parameters in the model connecting the raphe nuclei to other nodes). Alternatively, if neuromodulatory nodes are not explicitly included in the model, for the purposes at hand, we can think of the changes in the excitability of the nodes affected by neuromodulatory inputs as leading to changes in their effective connectivity (w) to other nodes (e.g., through an increase of the connectivity of glutamatergic synapses into Layer 5 pyramidal cells).
The abrupt shift induced by psychedelics can be thought of as a transformation of the phase space geometry that allows the neural state to explore new trajectories. This process manifests in an increase of complexity and disorder, which can be measured using various tools in different modalities, e.g., EEG or fMRI BOLD, and with measures such as entropy, fractal dimension, algorithmic complexity, etc. [29,31,34,80]). The decrease in effective connectivity under LSD (especially in interhemispheric homotopic connections), as inferred using Ising modeling of BOLD signals measured using fMRI imaging, is associated with a subsequent increase in algorithmic complexity [34].
Psychedelic-induced changes in connectivity can correspond to a flattening of the dynamical landscape [12] or a destabilization of it [48].In our framework, the alteration of effective connectivity results in an immediate and state-independent remodeling of the dynamical landscape during the acute phase of psychedelics, which is represented by the term in the connectodynamics equation (Equation (2)). Notably, in the REBUS model and the Entropic Brain perspective [12], the weights of the effective connectivity during the psychedelic-induced state are “flattened” or “de-weighted”, representing a more symmetrical and non-hierarchical connectivity profile.
The instantaneous modification of the landscape is, however, ephemeral, gradually fading as the acute effects of the psychedelic wears off. The system returns to near its original geometrical configuration, though with lasting influences brought about by the plastic changes resulting from the exploration of new trajectories in the acute phase. These residual changes are captured by the state-dependent plasticity term , which reflects changes in connectivity due to Hebbian plasticity that arise from the co-activation of neurons during the acute psychedelic stage.
In the literature, there is an increasing body of evidence suggesting a post-acute phase following psychedelic exposure characterized by a period of enhanced plasticity [7,11,12,81,82]. This phase can be interpreted as an extended window of malleability of the landscape, which could have profound implications for learning and therapy. Such a window of plasticity has been related to increased neurogenesis and upregulation of Brain-Derived Neurotropic Factor (BDNF) in humans and mice [8]. The activity-dependent release of BDNF plays a crucial role in selectively strengthening active synapses while weakening inactive ones, a critical process for Hebbian-type plasticity. Intriguingly, recent studies with mice have found psychedelic-induced changes in plasticity and antidepressant-like behavior dependent on the increase of endogenous BDNF and TrkB binding (the receptor of BDNF) that are independent of the activation of 5-HT2A [9,10].
In terms of our model, these two pathways correspond to changes in connectivity through Equation (2) due to a temporary modulation of the parameters and (i.e., metaplasticity; see Equation (3)) that upregulates state-independent and state-dependent plasticity processes, respectively. The strong acute-phase increase of state-independent plasticity () would be directly associated with the activation of serotonergic receptors, as discussed above, with a possible gradual decrease during the post-acute phase (the solid white line in Figure 3). The sustained increase of state-dependent plasticity (h) in the post-acute phase (the dashed black line in Figure 3) would be linked to dendritic growth, neurogenesis, upregulation of BDNF, and other related changes. This means that in the post-acute period the landscape would be more responsive to state changes (itself being influenced by external factors), offering a potential mechanism for the long-lasting changes reported after psychedelic experiences. Such external influences are modeled by the external input term in the state equation (Equation (1)), and can represent environmental/sensory inputs, psychotherapy, or neuromodulatory brain stimulation techniques such as transcranial electrical current stimulation (tES).
Dynamics of Psychedelics and Psychopathology
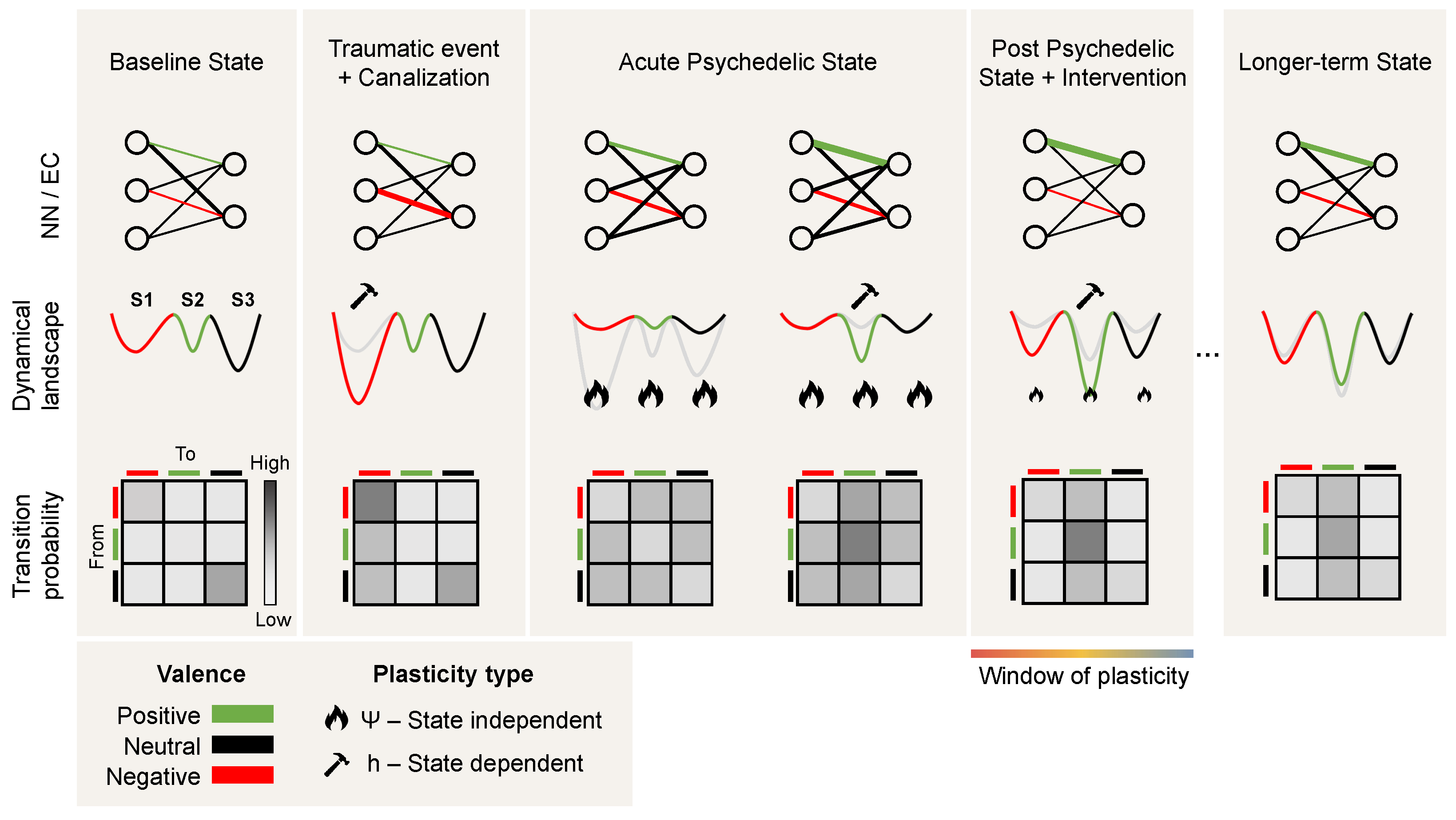
Recently, psychedelic medicine has emerged as a promising direction for treating mental disorders such as depression and addiction [83]. The nuanced interaction between the brain’s neurophysiology and emergent brain activity underlies the pathophysiology of mood disorders, often resulting in a persistent and maladaptive rigidity in cognitive and emotional processes [84]. Such changes to the brain’s neurophysiology can be explained through the CANAL framework, whereby pathological plasticity, often caused by a traumatic event asserts itself and dominates brain activity, driving the brain state to become “stuck in a rut” [11], i.e., a deepening minimum in the dynamical landscape (see Figure 4).
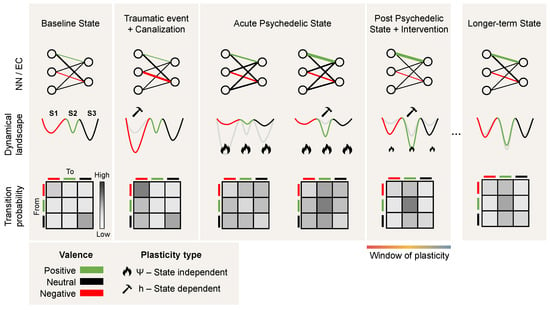
Figure 4.
Psychedelics and psychopathology: a dynamical systems perspective. From left to right, we provide three views of the transition from health to canalization following a traumatic event and back to a healthy state following the acute effects and post-acute effects of psychedelics and psychotherapy. The top row provides the neural network (NN) and effective connectivity (EC) view. The circles represent nodes in the network and the edge connectivity between them, with the edge thickness representing the connectivity strength between the nodes. The middle row provides the landscape view, with three schematic minima and colors depicting the valence of each corresponding state (positive, neutral, or negative). The bottom row represents the transition probabilities across states and how they change across the different phases. Due to traumatic events, excessive canalization may result in a pathological landscape, reflected as deepening of a negative valence minimum in which the state may become trapped. During the acute psychedelic state, this landscape becomes deformed, enabling the state to escape. Moreover, plasticity is enhanced during the acute and post-acute phases, benefiting interventions such as psychotherapy and brain stimulation (i.e., changes in effective connectivity). Not shown here is the possibility that a deeper transformation of the landscape may take place during the acute phase (see the discussion on the wormhole analogy in Section 4).
The interplay between external inputs, neural (fast time) dynamics, and connectivity (slow time) dynamics can drive the system into a joint stable and canalized state of lower complexity. Under the influence of psychedelics, more diverse and complex dynamics destabilize the plasticity equilibrium point, leading to a more fluid and adaptable neural state in a process that is amplified by the plasticity-enhancing effects of psychedelics. This shift manifests as an acute systemic increase of disorder, and possibly as a longer-lasting increase in complexity (Ising temperature, Lempel-Ziv complexity, etc.) that affects both short-term dynamics and long-term plastic processes.
The CANAL framework offers insight into the neural mechanisms underlying the persistence of various brain disorders. In particular, psychedelics may mediate their effects by altering the balance between stability and plasticity in neural networks through metaplasticity, and thereby act as potential therapeutic treatments. By acting on the serotonergic receptors, they trigger a cascade of neurochemical events that subsequently facilitate the reorganization of entrenched neural patterns. As discussed above, this alteration of the neural network during the acute phase (connectodynamics) can be interpreted as a rapid deformation or flattening of the landscape that allows the trapped state to escape and access more adaptive cognitive and emotional patterns. The rapid increase in complexity (a change in the dynamics) is itself a likely driver of metaplasticity. The acute phase is believed to be followed by an extended window of malleability of the landscape, otherwise known as a “window of plasticity”, where treatments such as psychotherapy and transcranial electrical stimulation can further alter the pathological rigidity characteristic of various brain disorders (see Figure 4).
4. Neural Geometrodynamics and General Relativity
A parallel can be drawn between neural geometrodynamics and Einstein’s equations of general relativity—the original geometrodynamics. Both frameworks involve the dynamical interaction between structure and resulting activity, each influencing and being influenced by the other. The Einstein field equations, including the cosmological constant , are
Here, is the metric tensor, is the Ricci curvature tensor and a function of , is the Ricci scalar (or curvature scalar) and a function of , and is the stress–energy tensor, a function of the mass and energy distribution (all the indices refer to spacetime dimensions), which is sometimes called the energy–momentum tensor. This is a central concept in general relativity that encapsulates the distribution and flow of energy and momentum in spacetime; its components include the energy density, momentum density, and stress (pressure and shear stress) within a given region. In addition, G is the gravitational constant, c is the speed of light, and is the cosmological constant. These equations describe the fundamental interaction of gravitation as a result of spacetime being curved by matter and energy. Specifically, they equate local spacetime curvature (on the left-hand side) with the local energy and momentum within that spacetime (on the right-hand side).
To complete these equations, the geodesic equation portrays how particles (matter) move in this curved spacetime, encapsulated by the notion that particles follow the straightest possible paths (geodesics) in curved spacetime:
where are the coordinates of the particle, is the proper time along the particle’s path, and are the Christoffel symbols, which are a function of and encode the connection (a mathematical object that describes how vectors change as they are parallel transported along curves in spacetime). The stress–energy tensor can be computed from the state of the particles to close the system of equations. For example, for N particles it is provided by , where and are the mass and velocity of the ith particle. More generally, the stress–energy tensor represents the state of matter and energy, which corresponds to x in our neural model. The metric , which specifies the geometry of spacetime, is akin to the connectivity w, which shapes the structure of the space where fast dynamics occur.
In the context of neural mass models, the state equation is analogous to the geodesic equation: “the state of the system evolves according to the landscape geometry specified by the parameters w”. On the other hand, the connectodynamics equation (with standing for plasticity parameters) is analogous to Einstein’s field equations; the parameters w, which describe the “structure” of the space where dynamics take place, evolve according to the current state of the system x and its ‘readiness’ for plasticity (parametrized by ).
The analogy to psychedelic effects in general relativity can be clarified further. The neural effects of psychedelics, as we understand them, start with a disruption of connectivity in a spatially dependent manner. Since the analog of w is g (the metric), in cosmological terms, we would first see a dynamic deformation of spacetime independent of the mass distribution (state-independent plasticity). Spacetime would “flatten”. This would cause the mass in the universe to escape from gravitational wells following new geodesics (just as the state in the brain will explore new regions of phase space), in turn creating further deformations of spacetime (state-dependent plasticity).
We emphasize that this comparison is largely metaphorical and therefore limited: the mutual influence between particles and spacetime in general relativity is akin to the state of the neural system and its underlying connectivity parameters. In both cases, dynamics and structure are intertwined (see Figure 5). However, as an example of the limitations of the analogy, the slow and fast nature of the different variables is interchanged in the two formulations, with spacetime responding faster (at the speed of light) to changes in the distribution of energy than the stress-energy tensor itself.
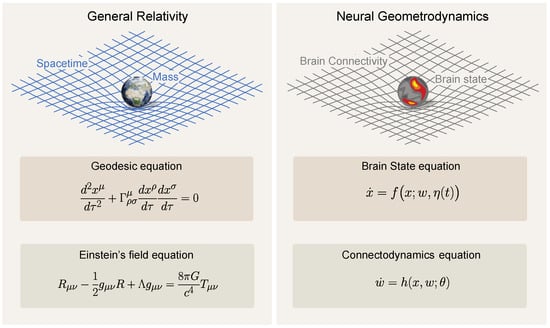
Figure 5.
General Relativity and Neural Geometrodynamics. Left: Equations for general relativity (the original geometrodynamics), coupling the dynamics of matter with those of spacetime. Right: Equations for neural geometrodynamics, coupling neural state and connectivity. Only the fast time and slow time equations are shown (ultraslow time endows the “constants” appearing in these equations with dynamics).
4.1. Metaplasticity and Variable Constants in Cosmology
In our neural mass model framework, the concept of metaplasticity is introduced to refer to dynamic variations in the plasticity control constants, in the connectodynamics equation, namely, . This set of constants can be represented as evolving over time as a function of the state of the system or other relevant variables:
where defines the evolution of the plasticity control constants with parameters .
Analogously, in the realm of general relativity and cosmology, it has been speculated that the fundamental constants such as the speed of light c, gravitational constant G, or cosmological constant , may in fact be dynamic. Although not part of the mainstream cosmological model, theories proposing variable constants such as the “Variable Speed of Light” (VSL) or “Variable Cosmological Constant” provide an intriguing parallel. For instance, within VSL theories the speed of light c is postulated to vary over cosmological time scales. Certain hypothetical dynamical equations could dictate the dynamical evolution of these constants. Although these theories are quite speculative and do not form a part of mainstream physics, they offer an interesting perspective on the concept of metaplasticity and its potential implications for the dynamical evolution of neural mass models and the structure of their landscapes.
4.2. Psychedelics as Wormholes in the Neural Landscape
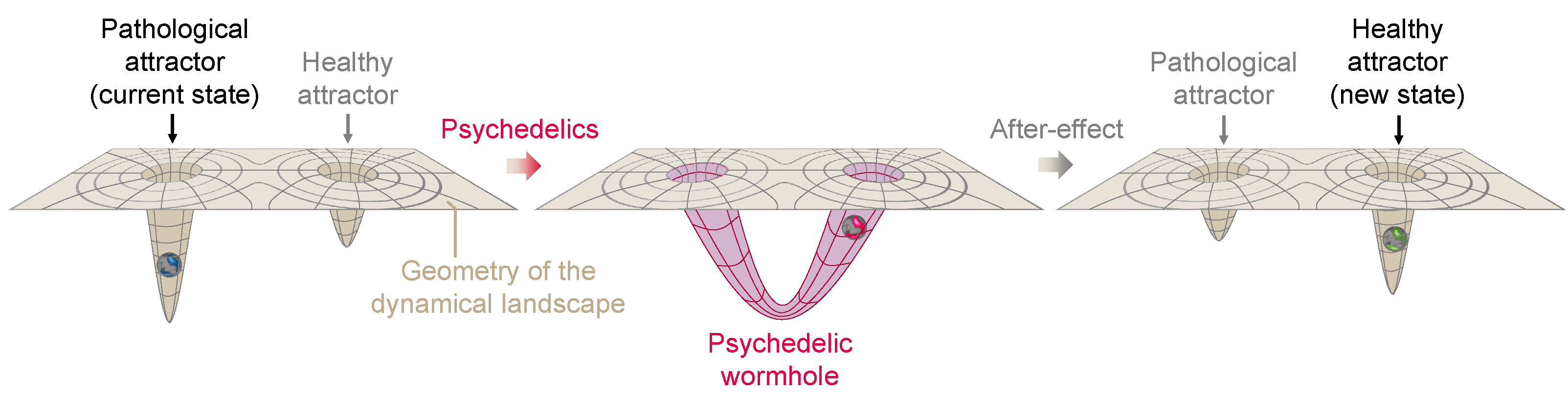
In the parallel between general relativity and neural geometrodynamics, we see the effects of psychedelics as a deformation of the neural landscape (spacetime) that allows the brain state (of a particle or set of particles) to escape from a local minimum and transition to another location in the landscape (spacetime). Although transitions may be smooth and respect the topology of the landscape [85,86], deformations of the landscape may be more extreme as well, i.e., sharp transitions through a topological tipping point of the dynamical landscape. The creation of a wormhole (in 4D or higher dimensional spaces) would alter the topological structure of the manifold it inhabits and the associated Betti numbers. This may be due to external inputs () when the system is non-autonomous [87], e.g., due to sensory or brain stimulatory effects. As we have discussed, this may be due to connectivity dynamics.
Wormholes, a term coined by John A. Wheeler [88] (sometimes called Einstein–Rosen bridges), are solutions to the Einstein field equations of general relativity which certain models suggest could exist under specific conditions. The creation of a wormhole in general relativity can be viewed as a profound deformation of spacetime, bending and connecting distant parts of the universe in such a way that matter/energy (such as an astronaut) can travel vast distances in an instant. This change in the geometry and topology of spacetime can be likened to the effect of psychedelics on the human mind. Just as a wormhole alters the structure of spacetime, psychedelics may radically deform the dynamical landscape of neural dynamics, creating connections across distant landscape locations. In the same way that the astronaut would use a wormhole to bypass vast stretches of space, the deformation caused by psychedelics may allow the state of the brain to “tunnel out” and escape from a local minimum or stuck pattern of thought, providing access to new areas of the landscape, new perspectives, and potentially unexplored territories of consciousness. Both phenomena are characterized by a fundamental transformation that enables traversal into otherwise inaccessible regions, whether in physical space or the brain’s dynamical landscape; see Figure 6 for a sketch of this concept.
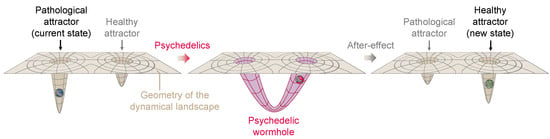
Figure 6.
A hypothetical psychedelic wormhole. On the left, the landscape is characterized by a deep pathological attractor which leads the neural state to become trapped. After ingestion of psychedelics (middle) a radical transformation of the neural landscape takes place, with the formation of a wormhole connecting the pathological attractor to another healthier attractor location and allowing the neural state to tunnel out. After the acute effects wear off (right panel), the landscape returns near to its original topology and geometry, but the activity-dependent plasticity reshapes it into a less pathological geometry.
4.3. Characterizing the Landscape
An important challenge in the program of neural geometrodynamics is to explore practical methods with which to characterize the landscape. Here, we can again draw inspiration from physics and mathematics.
The roots of this approach can be traced back to the 19th century, when Carl Friedrich Gauss pioneered the field of differential geometry. Gauss’s Theorema Egregium demonstrated that the curvature of a surface could be determined entirely by measurements within the surface, without any reference to the surrounding space [89]. This seminal insight has laid the groundwork for understanding manifolds in various contexts, including the theory of relativity. In the era of general relativity, the interplay between geometry and physics was further enriched. Differential geometry and algebraic topology, which comes into play when one is interested in the global properties of the manifold, such as its shape, connectedness, and the presence of holes [90,91], became essential in describing the fabric of spacetime itself. It enabled physicists to conceptualize how mass and energy warp the geometry of spacetime, thereby influencing the motion of objects.
In our current endeavor, these ideas find application in characterizing the complex dynamical landscapes of neural data. Modern tools from deep learning, such as variational autoencoders, can be used to unravel the reduced spaces underlying neuroimaging or neurophysiological data [54,55], while dynamical systems theory in concert with differential geometry, group theory, and algebraic topology data analysis [92] offer robust frameworks to understand and characterize them [87,93,94,95,96,97]. Topological data analysis can also be used to explore the graphs associated with model space, for example, the structural (connectome) or effective connectivity between regions in the brain (see [98] for a recent review). Topological methods have already been successfully employed to analyze detailed microscopic models [95], to study the relationship of criticality and topology in models [99], and to characterize functional brain networks derived from neuroimaging data [85,86,98].
World-tracking constraints force the brain as a dynamical system to mirror the symmetry in the data [37], a requirement that translates into constraints on structural and dynamical aspects of the system (and which can be analyzed using Lie group theory). This suggests leveraging the known links between topology and Lie groups [100]. The convergence of these mathematical techniques extends to neuroscience the fruitful exercise in physics of linking geometry and topology.
Finally, it would be interesting to explore if hierarchical data processing systems such as the brain display dynamical manifolds with hierarchical structure, including topology. This possibility is intuitive given the connections between the notions of criticality, information processing, and hierarchical organization [34,101]. In this sense, the effects of psychedelics, which are seen to increase the temperature of the system [34] and the complexity of dynamics, should be reflected as an increase in the topological complexity of the associated dynamical attractors, as we discussed above with the analogy to wormholes.
The relationship between hierarchy and topological complexity could be analyzed, for example, by exploring artificial neural networks carrying out hierarchical processing (any generative deep network trained on real-world data would do, in principle). Such networks could then be used to generate neural activation data and analyze, for instance, whether the depth of the network (the number of layers in its hierarchical architecture) is reflected in the topology (e.g., in Betti numbers) associated with the data or its latent space.
5. Conclusions
In this paper, we have defined the umbrella of neural geometrodynamics to study the coupling of state dynamics, their complexity, geometry, and topology with plastic phenomena. We have enriched the discussion by framing it in the context of the acute and longer-lasting effects of psychedelics.
As a source of inspiration, we have established a parallel with other mathematical theories of nature, specifically, general relativity, where dynamics and the “kinematic theater” are intertwined.
Although we can think of the “geometry” in neural geometrodynamics as referring to the structure imposed by connectivity on the state dynamics (paralleling the role of the metric in general relativity), it is more appropriate to think of it as the geometry of the reduced phase space (or invariant manifold) where state trajectories ultimately lie, which is where the term reaches its fuller meaning. Because the fluid geometry and topology of the invariant manifolds underlying apparently complex neural dynamics may be strongly related to brain function and first-person (structured) experience [16], further research should focus on creating and characterizing these fascinating mathematical structures.
Author Contributions
Conceptualization, G.R., E.L.-S., J.V. and R.S.-T.; methodology, G.R., E.L.-S. and J.V; writing—original draft preparation, G.R.; writing—review and editing, G.R., E.L.-S., J.V. and R.S.-T.; visualization, G.R., E.L.-S., J.V. and R.S.-T.; supervision, G.R.; project administration, G.R.; funding acquisition, G.R. and R.S.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Commission under European Union’s Horizon 2020 research and innovation programme Grant Number 101017716 (Neurotwin) and European Research Council (ERC Synergy Galvani) under the European Union’s Horizon 2020 research and innovation programme Grant Number 855109.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
E.L.-S. and R.S.-T. work at Neuroelectrics, a company developing brain stimulation solutions. G.R. works at and is a co-founder of Neuroelectrics. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A
Appendix A.1. A Nautical Analogy
To illustrate the interconnected dynamics of neural states, connectodynamics, and metaplasticity, consider a toy sailing boat navigating a circular pond. The boat moves through the pond, creating ripples that propagate across the water’s surface, eventually reflecting off the pond’s boundaries. These reflected ripples, in turn, influence the boat’s trajectory. This mirrors the dynamics of brain states, analogous to neural dynamics expressed by the equation , where the boat’s position represents the state x and the water surface’s geometry reflects the effective connectivity w. The term may be associated with an external force such as the wind.
The changes in the geometry of the water surface caused by the boat’s movement symbolize connectodynamics. This is captured by the plasticity equation , where the evolving connectivity parameters w depend on the boat’s position x and other factors. The boat’s position and the water’s surface geometry are intrinsically linked, akin to brain state and effective connectivity.
Further, imagine that other external factors, such as temperature fluctuations or changes in water viscosity, modify the water’s molecular structure over time. For example, a temperature decrease nearing freezing could alter the water structure (density and viscosity [102]) in the pond and how the boat’s movement affects the water geometry. This change in the water’s properties symbolizes the dynamics of plasticity, or metaplasticity, as described by .
Appendix A.2. Classical Dynamics of Particles and Fields
Here, we provide the equations for other systems; one can think of part of the equation describing the geometry of a space-providing subsystem (“kinematic theater”) and another describing the subsystem moving in this space, influenced by the structure and affecting its geometry in return. Several such examples can be found in physics.
Appendix A.2.1. Non-Relativistic Electrodynamics
The non-relativistic dynamics of N-charged particles and the associated electromagnetic field are described by the coupling of Newton’s second law and Maxwell’s equations. The charged particles are influenced by the electromagnetic field while generating it at the same time. This can lead to problematic scenarios, such as the self-interaction problem, in which charged particles generate an electromagnetic field; if one considers a particle’s interaction with its own field, paradoxical or unphysical results can arise. This self-interaction leads to divergences in calculations, and until recently has been a longstanding challenge in classical electrodynamics [103].
The motion of the ith electron is provided by , where is the Lorentz force. The electromagnetic field obeys Maxwell’s equations:
where and are the charge and current densities, respectively.
Appendix A.2.2. Relativistic Equations
While Maxwell’s equations are relativistic (i.e., they transform properly under Lorentz transformations), Newton’s equation is not. For the relativistic version, the field strength tensor is defined in terms of the four-potential [104]:
The dual tensor is defined in terms of , and the Levi-Civita symbol :
The homogeneous Maxwell’s equations are provided by
and the inhomogeneous Maxwell’s equations by
where is the four-current vector, which we now define for a particular case. Given a distribution of N particles, each with charge and four-velocity , the four-current at position and time t is provided by
where is the position of the i-th particle at time t and is the three-dimensional Dirac delta function.
In its relativistic form, the equation of motion for a charged particle in an electromagnetic field (commonly known as the Lorentz force equation) is
Here, is the four-momentum of the particle, is the four-velocity of the particle, is the electromagnetic field tensor, and q is the charge of the particle. The equation describes how the four-momentum of the particle changes with the proper time under the influence of the electromagnetic field.
Appendix A.3. Modeling Plasticity in Neural Mass Models
In this section, we provide a brief overview of plasticity mechanisms and how they relate to the terms in the formalism, namely, the functions h and ; see Table A1 for a summary. Including plasticity in Neuronal Mass Models (NMMs) allows for the modeling of time-varying connectivity strengths that reflect the learning and adaptation processes observed in biological neuronal networks.

Table A1.
Summary of Different Types of Neural Plasticity Phenomena. State-dependent Plasticity (h) refers to changes in neural connections that depend on the current state or activity of the neurons involved. For example, functional plasticity often relies on specific patterns of neural activity to induce changes in synaptic strength. State-independent Plasticity (ψ) refers to changes that are not directly dependent on the specific activity state of the neurons; for example, acute psychedelic-induced plasticity acts on the serotonergic neuroreceptors, thereby acting on brain networks regardless of specific activity patterns. Certain forms of plasticity, such as structural plasticity and metaplasticity, may exhibit characteristics of both state-dependent and state-independent plasticity depending on the context and specific mechanisms involved. Finally, metaplasticity refers to the adaptability or dynamics of plasticity mechanisms.
Table A1.
Summary of Different Types of Neural Plasticity Phenomena. State-dependent Plasticity (h) refers to changes in neural connections that depend on the current state or activity of the neurons involved. For example, functional plasticity often relies on specific patterns of neural activity to induce changes in synaptic strength. State-independent Plasticity (ψ) refers to changes that are not directly dependent on the specific activity state of the neurons; for example, acute psychedelic-induced plasticity acts on the serotonergic neuroreceptors, thereby acting on brain networks regardless of specific activity patterns. Certain forms of plasticity, such as structural plasticity and metaplasticity, may exhibit characteristics of both state-dependent and state-independent plasticity depending on the context and specific mechanisms involved. Finally, metaplasticity refers to the adaptability or dynamics of plasticity mechanisms.
| Type | Time Scale | Mechanism | Effects | State-Dependence |
|---|---|---|---|---|
| Functional or dynamical Plasticity | Milliseconds to minutes | Changes in strength/efficiency of synapses (e.g., Hebbian plasticity, LTP, LTD) | Short and long-term memory, fine-tuning of connections | State-dependent (h) |
| Acute Psychedelic-induced Plasticity | Minutes to Hours | Targeting of serotonergic neuroreceptors especially the 5- with a result in overall excitability [7,11,12,81] | Flattens or de-weights the dynamical landscape | State-independent () |
| Homeostatic Plasticity | Hours to days | Regulation of overall excitability to maintain stability (e.g., adjusting synapse strength for E/I balance) | Balances and stabilizes network | State-dependent (h) |
| Structural Plasticity (e.g., post-acute psychedelic-induced plasticity) | Hours to years | Larger physical changes in neurons (e.g., dendrite growth, synapse formation) through an increase of endogenous BDNF and via TrkB binding (the receptor of BDNF) [82] | Long-term memory, development | Depends on context |
| Metaplasticity | Various, often longer-term | Changes in mechanisms governing synaptic plasticity (e.g., modulation of thresholds/rules) | Regulates other forms of plasticity, “plasticity of plasticity” [73] | Depends on context |
Appendix A.3.1. Functional Plasticity
The simplest and most common way to include synaptic plasticity is through Hebbian learning rules. Hebbian plasticity, a type of functional plasticity, follows the principle that “neurons that fire together wire together” [60]. It can be included in NMM using the equation
where is the synaptic strength from neuron j to neuron i, and are the neuronal activities, and and are parameters controlling the learning and decay rates.
Appendix A.3.2. Homeostatic Plasticity
Homeostatic plasticity, a form of plasticity that adjusts synaptic strengths to keep the overall activity of a neuron or network within a certain range, can be included in an NMM using the equation [61,62,63,64,65]
where is the target activity level.
Appendix A.3.3. Structural Plasticity
Structural plasticity, where the actual number, dendrites, and arrangement of synapses change over time, can be represented in NMMs by modifying or even adding rows and columns from the w adjacency matrix to represent the formation or elimination of synapses or even fibers.
Appendix A.3.4. Empirically-Derived Structural Plasticity
NMMs can be used to infer structural changes to plasticity without explicitly describing the plastic mechanism per se. For example, in the post-acute psychedelic state, NMMs can be used to infer the plastic changes to by optimizing the modeled functional connectivity to approximate the empirical functional connectivity with a certain learning rate , as in the following equation:
Such optimization can be computed through, for example, gradient descent methods with priors on the topology of structural connectivity between brain regions [105]. Recent methods have further extended this framework by adding time-shifted correlation [42] to the optimization as a better description of the overall brain state (in this case, the post-acute psychedelic state).
Including these forms of plasticity in NMMs allows for more realistic modeling of neural systems that can better capture their adaptive nature along with the impact of learning and experience on synaptic connections.
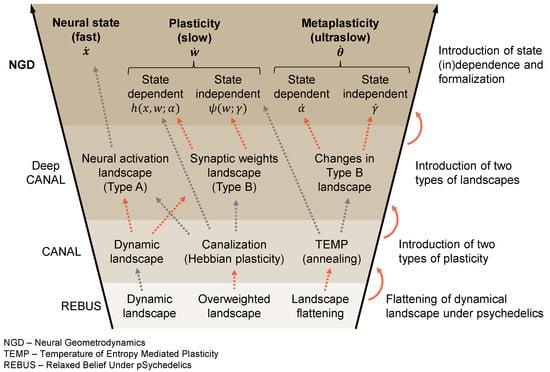
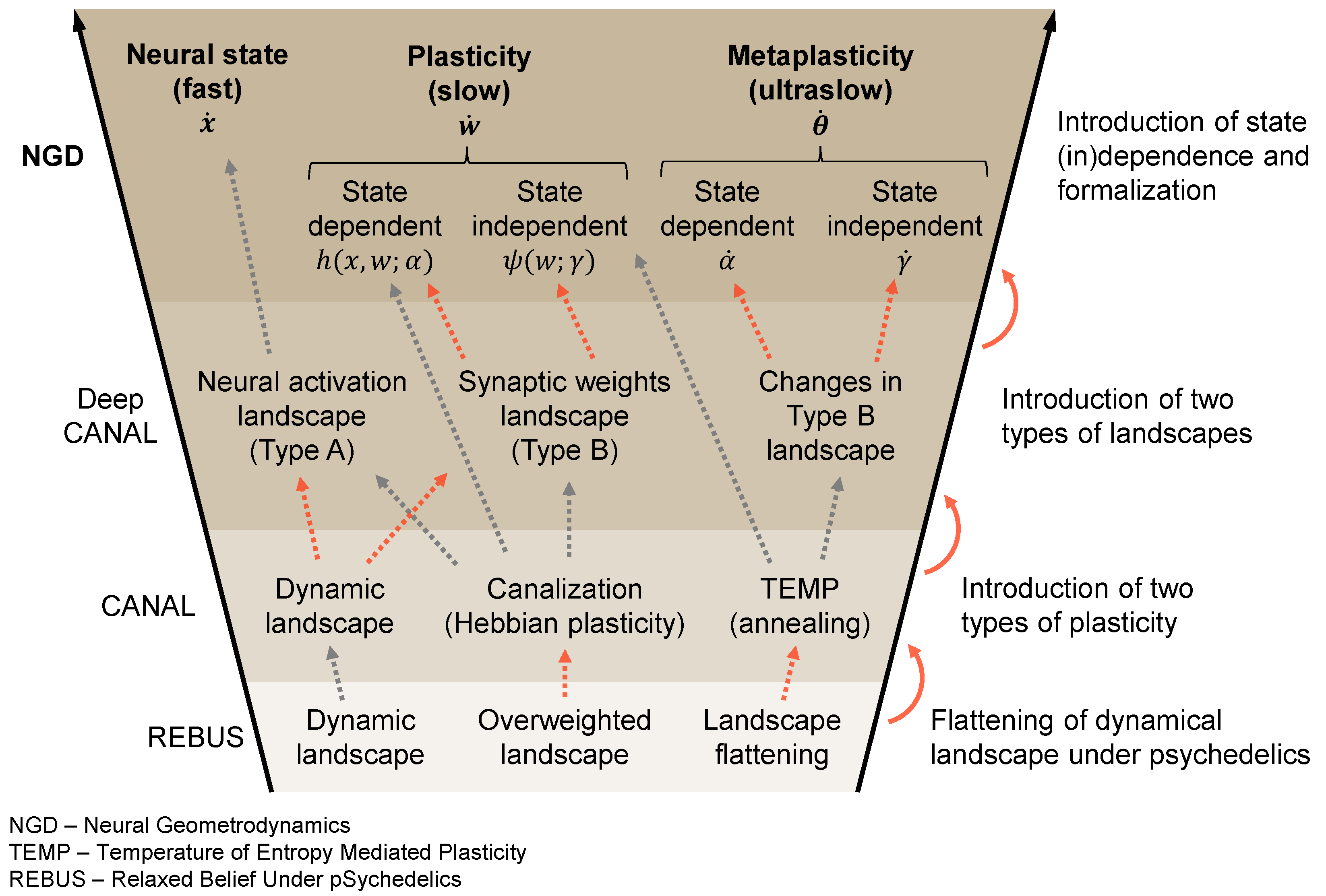
Figure A1.
Conceptual funnel of terms between the NGD (neural geometrodynamics), Deep CANAL [48], CANAL [11], and REBUS [12] frameworks. The figure provides an overview of the different frameworks discussed in the paper and how the concepts in each relate to each other, including their chronological evolution. We wish to stress that there is no one-to-one mapping between the concepts as different frameworks build and expand on the previous work in a non-trivial way. In red, we highlight the main conceptual leaps between the frameworks. See the main text or the references for a definition of all the terms, variables, and acronyms used.
Figure A1.
Conceptual funnel of terms between the NGD (neural geometrodynamics), Deep CANAL [48], CANAL [11], and REBUS [12] frameworks. The figure provides an overview of the different frameworks discussed in the paper and how the concepts in each relate to each other, including their chronological evolution. We wish to stress that there is no one-to-one mapping between the concepts as different frameworks build and expand on the previous work in a non-trivial way. In red, we highlight the main conceptual leaps between the frameworks. See the main text or the references for a definition of all the terms, variables, and acronyms used.
References
- Deco, G.; Jirsa, V.K.; Robinson, P.A.; Breakspear, M.; Friston, K. The dynamic brain: From spiking neurons to neural masses and cortical fields. PLoS Comput. Biol. 2008, 4, e1000092. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 2017, 20, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Kringelbach, M.L.; Deco, G. Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. NeuroImage 2017, 160, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Cruzat, J.; Cabral, J.; Knudsen, G.M.; Carhart-Harris, R.L.; Whybrow, P.C.; Logothetis, N.K.; Kringelbach, M.L. Whole-brain multimodal neuroimaging model using serotonin receptor maps explains non-linear functional effects of LSD. Curr. Biol. 2018, 28, 3065–3074. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Cruzat, J.; Cabral, J.; Knudsen, G.M.; Carhart-Harris, R.; Whybrow, P.C.; Logothetis, N.K.; Deco, G. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc. Natl. Acad. Sci. USA 2020, 117, 9566–9576. [Google Scholar] [CrossRef] [PubMed]
- Aday, J.S.; Mitzkovitz, C.M.; Bloesch, E.K.; Davoli, C.C.; Davis, A.K. Long-term effects of psychedelic drugs: A systematic review. Neurosci. Biobehav. Rev. 2020, 113, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- de Vos, C.M.H.; Mason, N.L.; Kuypers, K.P.C. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 724606. [Google Scholar] [CrossRef]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef]
- Nardou, R.; Sawyer, E.; Song, Y.J.; Wilkinson, M.; Padovan-Hernandez, Y.; de Deus, J.L.; Wright, N.; Lama, C.; Faltin, S.; Goff, L.A.; et al. Psychedelics reopen the social reward learning critical period. Nature 2023, 618, 790–798. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Chandaria, S.; Erritzoe, D.E.; Gazzaley, A.; Girn, M.; Kettner, H.; Mediano, P.A.M.; Nutt, D.J.; Rosas, F.E.; Roseman, L.; et al. Canalization and plasticity in psychopathology. Neuropharmacology 2023, 226, 109398. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.; Gelatt, C.D., Jr.; Vecchi, M.P. Optimization by simulated annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L. The entropic brain—Revisited. Neuropharmacology 2018, 142, 167–178. [Google Scholar] [CrossRef]
- Girn, M.; Rosas, F.E.; Daws, R.E.; Gallen, C.L.; Gazzaley, A.; Carhart-Harris, R.L. A complex systems perspective on psychedelic brain action. Trends Cogn. Sci. 2023, 27, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, G.; Lopez-Sola, E. AIT foundations of structured experience. J. AI Consci. 2022, 9, 153–191. [Google Scholar] [CrossRef]
- Geyer, M.A.; Vollenweider, F.X. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol. Sci. 2008, 29, 445–453. [Google Scholar] [CrossRef]
- Hipólito, I.; Mago, J.; Rosas, F.E.; Carhart-Harris, R. Pattern breaking: A complex systems approach to psychedelic medicine. Neurosci. Conscious. 2023, 2023, niad017. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858. [Google Scholar] [CrossRef]
- Atasoy, S.; Roseman, L.; Kaelen, M.; Kringelbach, M.L.; Deco, G.; Carhart-Harris, R.L. Connectome-harmonic decomposition of human brain activity reveals dynamical repertoire re-organization under LSD. Sci. Rep. 2017, 7, 17661. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, S.; Vohryzek, J.; Deco, G.; Carhart-Harris, R.L.; Kringelbach, M.L. Common neural signatures of psychedelics: Frequency-specific energy changes and repertoire expansion revealed using connectome-harmonic decomposition. Prog. Brain Res. 2018, 242, 97–120. [Google Scholar]
- Luppi, A.I.; Vohryzek, J.; Kringelbach, M.L.; Mediano, P.A.; Craig, M.M.; Adapa, R.; Carhart-Harris, R.L.; Roseman, L.; Pappas, I.; Finoia, P.; et al. Distributed harmonic patterns of structure-function dependence orchestrate human consciousness. Commun. Biol. 2023, 6, 117:1–117:19. [Google Scholar] [CrossRef] [PubMed]
- Singleton, S.P.; Luppi, A.I.; Carhart-Harris, R.L.; Cruzat, J.; Roseman, L.; Nutt, D.J.; Deco, G.; Kringelbach, M.L.; Stamatakis, E.A.; Kuceyeski, A. LSD and psilocybin flatten the brain’s energy landscape: Insights from receptor-informed network control theory. bioRxiv 2022. [Google Scholar] [CrossRef]
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D.; et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife 2018, 7, e35082. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, E.; Roseman, L.; Kaelen, M.; Orban, C.; Muthukumaraswamy, S.D.; Murphy, K.; Laufs, H.; Leech, R.; McGonigle, J.; Crossley, N.; et al. Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr. Biol. 2016, 26, 1043–1050. [Google Scholar] [CrossRef]
- McCulloch, D.E.W.; Knudsen, G.M.; Barrett, F.S.; Doss, M.K.; Carhart-Harris, R.L.; Rosas, F.E.; Deco, G.; Kringelbach, M.L.; Preller, K.H.; Ramaekers, J.G.; et al. Psychedelic resting-state neuroimaging: A review and perspective on balancing replication and novel analyses. Neurosci. Biobehav. Rev. 2022, 138, 104689. [Google Scholar] [CrossRef]
- Varley, T.F.; Carhart-Harris, R.; Roseman, L.; Menon, D.K.; Stamatakis, E.A. Serotonergic Psychedelics LSD & Psilocybin Increase the Fractal Dimension of Cortical Brain Activity in Spatial and Temporal Domains. NeuroImage 2020, 220, 117049. [Google Scholar] [CrossRef]
- Toker, D.; Pappas, I.; Lendner, J.D.; Frohlich, J.; Mateos, D.M.; Muthukumaraswamy, S.; Carhart-Harris, R.; Paff, M.; Vespa, P.M.; Monti, M.M.; et al. Consciousness is supported by near-critical slow cortical electrodynamics. Proc. Natl. Acad. Sci. USA 2022, 119, e2024455119. [Google Scholar] [CrossRef]
- Schartner, M.M.; Carhart-Harris, R.L.; Barrett, A.B.; Seth, A.K.; Muthukumaraswamy, S.D. Increased Spontaneous MEG Signal Diversity for Psychoactive Doses of Ketamine, LSD and Psilocybin. Sci. Rep. 2017, 7, 46421. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, T.; Watanabe, T.; Ohzeki, M.; Masuda, N. Energy landscape analysis of neuroimaging data. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20160287. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, T.; Fonseca Dos Reis, E.; Watanabe, T.; Sakaki, M.; Masuda, N. Closer to critical resting-state neural dynamics in individuals with higher fluid intelligence. Commun. Biol. 2020, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, G.; Damiani, G.; Lozano-Soldevilla, D.; Deco, N.; Rosas, F.E.; Kiani, N.A.; Ponce-Alvarez, A.; Kringelbach, M.L.; Carhart-Harris, R.; Deco, G. LSD-induced increase of Ising temperature and algorithmic complexity of brain dynamics. PLoS Comput. Biol. 2023, 19, e1010811. [Google Scholar] [CrossRef]
- Ruffini, G.; Deco, G. The 2D Ising model, criticality and AIT. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ruffini, G. An algorithmic information theory of consciousness. Neurosci. Conscious. 2017, 3, nix019. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, G. Structured dynamics in the algorithmic agent. bioRxiv 2023. [Google Scholar] [CrossRef]
- Margulies, D.S.; Ghosh, S.S.; Goulas, A.; Falkiewicz, M.; Huntenburg, J.M.; Langs, G.; Bezgin, G.; Eickhoff, S.B.; Castellanos, F.X.; Petrides, M.; et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. USA 2016, 113, 44. [Google Scholar] [CrossRef]
- Xu, Y.; Long, X.; Feng, J.; Gong, P. Interacting spiral wave patterns underlie complex brain dynamics and are related to cognitive processing. Nat. Hum. Behav. 2023, 7, 1196–1215. [Google Scholar] [CrossRef]
- Vézquez-Rodríguez, B.; Liu, Z.Q.; Hagmann, P.; Misic, B. Signal propagation via cortical hierarchies. Netw. Neurosci. 2020, 4, 1072–1090. [Google Scholar] [CrossRef]
- Deco, G.; Sanz Perl, Y.; Bocaccio, H.; Tagliazucchi, E.; Kringelbach, M.L. The INSIDEOUT framework provides precise signatures of the balance of intrinsic and extrinsic dynamics in brain states. Commun. Biol. 2022, 5, 572. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L.; Perl, Y.S.; Tagliazucchi, E.; Deco, G. Toward naturalistic neuroscience: Mechanisms underlying the flattening of brain hierarchy in movie-watching compared to rest and task. Sci. Adv. 2023, 9, eade6049. [Google Scholar] [CrossRef] [PubMed]
- Lynn, C.W.; Cornblath, E.J.; Papadopoulos, L.; Bertolero, M.A.; Bassett, D.S. Broken detailed balance and entropy production in the human brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2109889118. [Google Scholar] [CrossRef]
- Girn, M.; Roseman, L.; Bernhardt, B.; Smallwood, J.; Carhart-Harris, R.; Spreng, R.N. Serotonergic psychedelic drugs LSD and psilocybin reduce the hierarchical differentiation of unimodal and transmodal cortex. NeuroImage 2022, 256, 119220. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, H.; Lei, X.; He, Y.; Wu, Q.; Yan, Y.; Zhou, X.; Tian, X.; Peng, Y.; Huang, S.; et al. Hierarchical fluctuation shapes a dynamic flow linked to states of consciousness. Nat. Commun. 2023, 14, 3238. [Google Scholar] [CrossRef]
- Vohryzek, J.; Cabral, J.; Timmermann, C.; Atasoy, S.; Roseman, L.; Nutt, D.; Carhart-Harris, R.; Deco, G.; Kringelbach, M.L. Harmonic decomposition of spacetime (HADES) framework characterises the spacetime hierarchy of the DMT brain state. bioRxiv 2023. [Google Scholar] [CrossRef]
- Vohryzek, J.; Cabral, J.; Vuust, P.; Deco, G.; Kringelbach, M.L. Understanding brain states across spacetime informed by whole-brain modelling. Philos. Trans. R. Soc. A 2022, 380, 20210247. [Google Scholar] [CrossRef]
- Juliani, A.; Safron, A.; Kanai, R. Deep CANALs: A Deep Learning Approach to Refining the Canalization Theory of Psychopathology. Psyarxiv Prepr. 2023. [Google Scholar] [CrossRef]
- Nichols, D.E.; Johnson, M.W.; Nichols, C.D. Psychedelics as medicines: An emerging new paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. [Google Scholar] [CrossRef]
- Kuo, M.F.; Nitsche, M.A. Effects of transcranial electrical stimulation on cognition. Clin. EEG Neurosci. 2012, 43, 192–199. [Google Scholar] [CrossRef]
- Strogatz, S.H. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering, 1st ed.; Westview Press: Boulder, CO, USA, 2001. [Google Scholar]
- Cayton, L. Algorithms for Manifold Learning; Technical Report; Department of Computer Science & Engineering: San Diego, CA, USA, 2008. [Google Scholar]
- Konz, N.; Gu, H.; Dong, H.; Mazurowski, M.A. Correction to: The intrinsic manifolds of radiological images and their role in deep learning. In Lecture Notes in Computer Science; Springer Nature Switzerland: Cham, Swizerland, 2022; p. C1. [Google Scholar]
- Perl, Y.S.; Bocaccio, H.; Pérez-Ipiña, I.; Zamberlán, F.; Piccinini, J.; Laufs, H.; Kringelbach, M.; Deco, G.; Tagliazucchi, E. Generative Embeddings of Brain Collective Dynamics Using Variational Autoencoders. Phys. Rev. Lett. 2020, 125, 238101. [Google Scholar] [CrossRef]
- Sanz Perl, Y.; Fittipaldi, S.; Gonzalez Campo, C.; Moguilner, S.; Cruzat, J.; Fraile-Vazquez, M.E.; Herzog, R.; Kringelbach, M.L.; Deco, G.; Prado, P.; et al. Model-based whole-brain perturbational landscape of neurodegenerative diseases. eLife 2023, 12, e83970. [Google Scholar] [CrossRef]
- Jefferys, J.G. Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Physiol. Rev. 1995, 75, 689–723. [Google Scholar] [CrossRef]
- Anastassiou, C.A.; Perin, R.; Markram, H.; Koch, C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 2011, 14, 217–223. [Google Scholar] [CrossRef]
- Ruffini, G.; Salvador, R.; Tadayon, E.; Sanchez-Todo, R.; Pascual-Leone, A.; Santarnecchi, E. Realistic modeling of mesoscopic ephaptic coupling in the human brain. PLoS Comput. Biol. 2020, 16, e1007923. [Google Scholar] [CrossRef]
- Pinotsis, D.A.; Miller, E.K. In vivo ephaptic coupling allows memory network formation. Cereb. Cortex 2023, 33, 9877–9895. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; John Wiley and Sons: New York, NY, USA, 1949. [Google Scholar]
- Turrigiano, G.G.; Leslie, K.R.; Desai, N.S.; Rutherford, L.C.; Nelson, S.B. Activity-Dependent Scaling of Quantal Amplitude in Neocortical Neurons. Nature 1998, 391, 892–896. [Google Scholar] [CrossRef]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef]
- Maffei, A.; Nelson, S.B.; Turrigiano, G.G. Selective Reconfiguration of Layer 4 Visual Cortical Circuitry by Visual Deprivation. Nat. Neurosci. 2004, 7, 1353–1359. [Google Scholar] [CrossRef]
- Hengen, K.B.; Lambo, M.E.; Van Hooser, S.D.; Katz, D.B.; Turrigiano, G.G. Firing Rate Homeostasis in Visual Cortex of Freely Behaving Rodents. Neuron 2013, 80, 335–342. [Google Scholar] [CrossRef]
- Hellyer, P.J.; Jachs, B.; Clopath, C.; Leech, R. Local Inhibitory Plasticity Tunes Macroscopic Brain Dynamics and Allows the Emergence of Functional Brain Networks. NeuroImage 2016, 124, 85–95. [Google Scholar] [CrossRef]
- Ma, Z.; Turrigiano, G.G.; Wessel, R.; Hengen, K.B. Cortical Circuit Dynamics Are Homeostatically Tuned to Criticality In Vivo. Neuron 2019, 104, 655–664.e4. [Google Scholar] [CrossRef]
- Chistiakova, M.; Bannon, N.M.; Bazhenov, M.; Volgushev, M. Heterosynaptic plasticity: Multiple mechanisms and multiple roles. Neuroscientist 2014, 20, 483–498. [Google Scholar] [CrossRef]
- Lepow, L.; Morishita, H.; Yehuda, R. Critical period plasticity as a framework for psychedelic-assisted psychotherapy. Front. Neurosci. 2021, 15, 710004. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2013; ISBN 978-1-259-64223-4. [Google Scholar]
- Rosenberg, S.M.; Hastings, P.J. Stress-Induced Mutagenesis in Bacteria. Science 2003, 300, 1404–1409. [Google Scholar] [CrossRef]
- Vose, L.R.; Stanton, P.K. Synaptic plasticity, metaplasticity and depression. Curr. Neuropharmacol. 2017, 15, 71–86. [Google Scholar] [CrossRef]
- Voss, P.; Thomas, M.E.; Cisneros-Franco, J.M.; de Villers-Sidani, É. Dynamic brains and the changing rules of neuroplasticity: Implications for learning and recovery. Front. Psychol. 2017, 8, 1657. [Google Scholar] [CrossRef]
- Abraham, W.C.; Bear, M.F. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996, 19, 126–130. [Google Scholar] [CrossRef]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The combined influences of exercise, diet and sleep on neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef]
- Misner, C.W.; Thorne, K.S.; Wheeler, J.A. Gravitation; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Carhart-Harris, R.L.; Nutt, D. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Burstein, E.S. Relevance of 5-HT2A receptor modulation of pyramidal cell excitability for dementia-related psychosis: Implications for pharmacotherapy. CNS Drugs 2021, 35, 727–741. [Google Scholar] [CrossRef]
- Andrade, R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology 2011, 61, 382–386. [Google Scholar] [CrossRef]
- Lau, Z.J.; Pham, T.; Chen, S.H.A.; Makowski, D. Brain Entropy, Fractal Dimensions and Predictability: A Review of Complexity Measures for EEG in Healthy and Neuropsychiatric Populations. Eur. J. Neurosci. 2022, 56, 5047–5069. [Google Scholar] [CrossRef]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef]
- Calder, A.E.; Hasler, G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology 2023, 48, 104–112. [Google Scholar] [CrossRef]
- Rucker, J.J.; Iliff, J.; Nutt, D.J. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 2018, 142, 200–218. [Google Scholar]
- Calvey, T.; Howells, F.M. An introduction to psychedelic neuroscience. Prog. Brain Res. 2018, 242, 1–23. [Google Scholar]
- Petri, G.; Expert, P.; Turkheimer, F.; Carhart-Harris, R.; Nutt, D.; Hellyer, P.J.; Vaccarino, F. Homological scaffolds of brain functional networks. J. R. Soc. Interface 2014, 11, 20140873. [Google Scholar] [CrossRef]
- Santos, F.A.N.; Raposo, E.P.; Coutinho-Filho, M.D.; Copelli, M.; Stam, C.J.; Douw, L. Topological phase transitions in functional brain networks. Phys. Rev. E 2019, 100, 032414. [Google Scholar] [CrossRef]
- Charó, G.D.; Chekroun, M.D.; Sciamarella, D.; Ghil, M. Noise-driven topological changes in chaotic dynamics. Chaos 2021, 31, 103115. [Google Scholar] [CrossRef]
- Misner, C.W.; Thorne, K.S.; Zurek, W.H. John Wheeler, relativity, and quantum information. Phys. Today 2009, 62, 40–46. [Google Scholar] [CrossRef]
- do Carmo, M.P. Differential Geometry of Curves and Surfaces; Prentice-Hall: Upper Saddle River, NJ, USA, 1976. [Google Scholar]
- Hawking, S.W.; Ellis, G.F.R. The Large Scale Structure of Space-Time; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Luminet, J.P. The Status of Cosmic Topology after Planck Data. Universe 2016, 2, 1. [Google Scholar] [CrossRef]
- Gilmore, R. Topological analysis of chaotic dynamical systems. Rev. Mod. Phys. 1998, 70, 1455–1529. [Google Scholar] [CrossRef]
- Carlsson, G. Topological pattern recognition for point cloud data. Acta Numer. 2014, 23, 289–368. [Google Scholar] [CrossRef]
- Giusti, C.; Ghrist, R.; Bassett, D.S. Two’s company, three (or more) is a simplex: Algebraic-topological tools for understanding higher-order structure in neural data. J. Comput. Neurosci. 2016, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reimann, M.W.; Nolte, M.; Scolamiero, M.; Turner, K.; Perin, R.; Chindemi, G.; Dłotko, P.; Levi, R.; Hess, K.; Markram, H. Cliques of neurons bound into cavities provide a missing link between structure and function. Front. Comput. Neurosci. 2017, 11, 48. [Google Scholar] [CrossRef]
- Perea, J.A. Topological time series analysis. Not. Am. Math. Soc. 2019, 66, 686–694. [Google Scholar] [CrossRef]
- Sciamarella, D.; Mindlin, G.B. Topological structure of chaotic flows from human speech data. Phys. Rev. Lett. 1999, 82, 1450–1453. [Google Scholar] [CrossRef]
- Billings, J.; Saggar, M.; Hlinka, J.; Keilholz, S.; Petri, G. Simplicial and topological descriptions of human brain dynamics. Netw. Neurosci. 2021, 5, 549–568. [Google Scholar] [CrossRef]
- Bai, X.; Yu, C.; Zhai, J. Topological data analysis of the firings of a network of stochastic spiking neurons. Front. Neural Circuits 2024, 17, 1308629. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.J. The Betti numbers of the simple Lie groups. Canad. J. Math. 1958, 10, 349–356. [Google Scholar] [CrossRef]
- Meunier, D.; Lambiotte, R.; Bullmore, E.T. Modular and hierarchically modular organization of brain networks. Front. Neurosci. 2010, 4, 200. [Google Scholar] [CrossRef] [PubMed]
- Nayar, K.G.; Sharqawy, M.H.; Banchik, L.D.; Lienhard, V.J.H. Thermophysical properties of seawater: A review and new correlations that include pressure dependence. Desalination 2016, 390, 1–24. [Google Scholar] [CrossRef]
- Gralla, S.E.; Harte, A.I.; Wald, R.M. Rigorous derivation of electromagnetic self-force. Phys. Rev. 2009, 80, 024031. [Google Scholar] [CrossRef]
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; John Wiley & Sons: Nashville, TN, USA, 1998. [Google Scholar]
- Deco, G.; Cruzat, J.; Cabral, J.; Tagliazucchi, E.; Laufs, H.; Logothetis, N.K.; Kringelbach, M.L. Awakening: Predicting external stimulation to force transitions between different brain states. Proc. Natl. Acad. Sci. USA 2019, 116, 18088–18097. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).