Continuous Monitoring of Entropy Production and Entropy Flow in Humans Exercising under Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subjects and Experimental Design
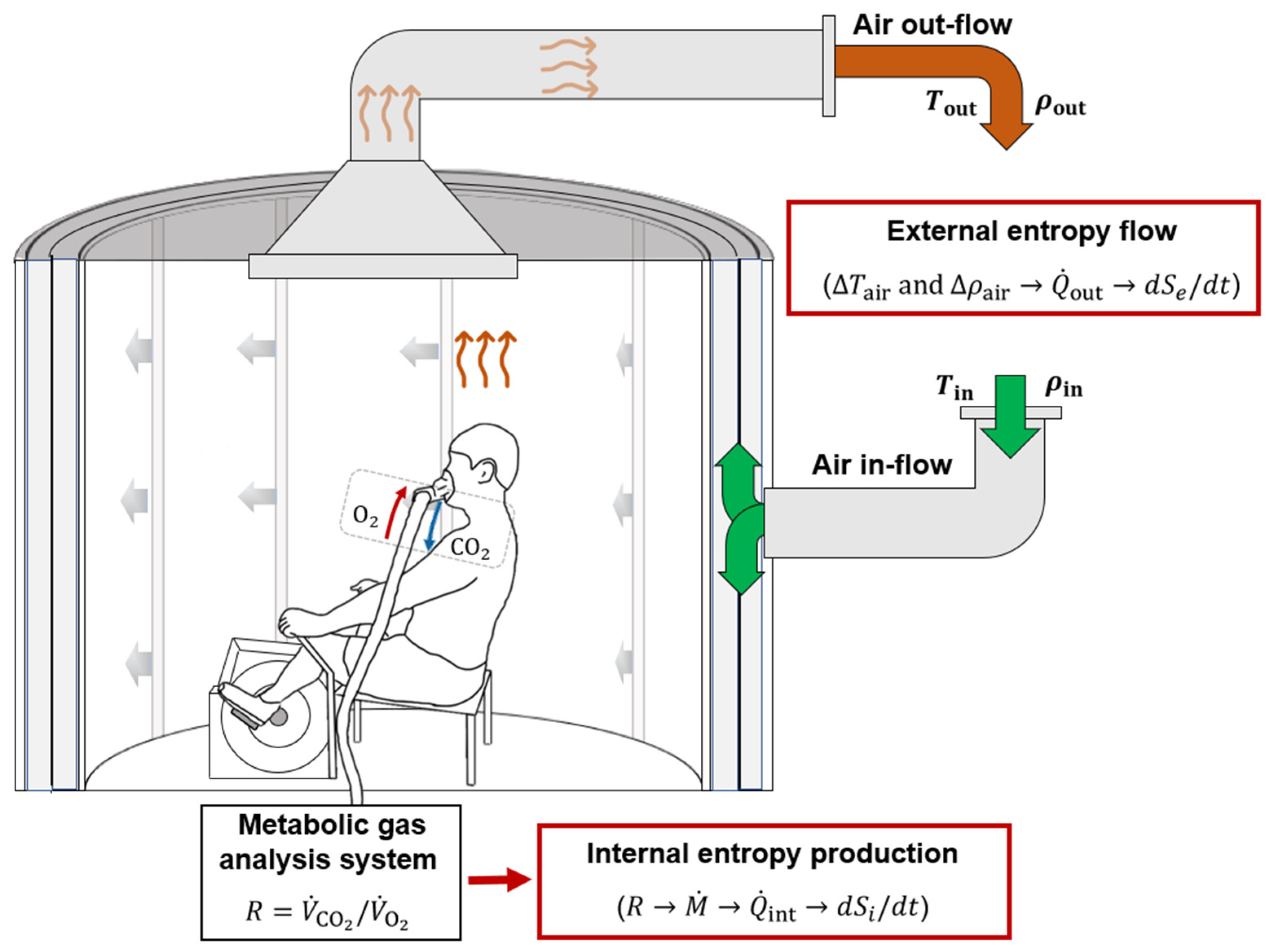
2.2. Internal Heat Production Measured with Indirect Calorimetry
2.3. External Heat Dissipation Measured with Direct Calorimetry
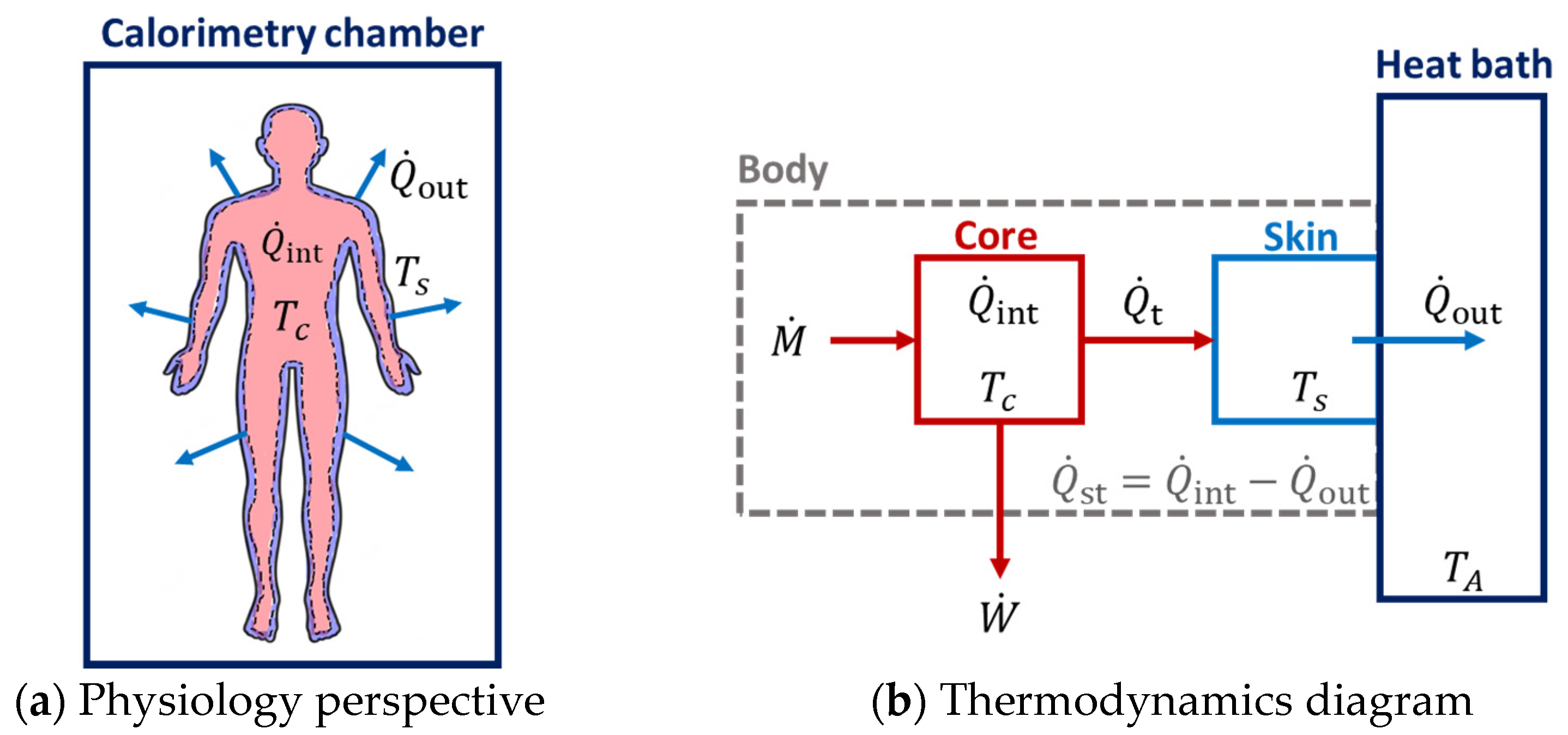
2.4. Rate of Heat Storage in the Body
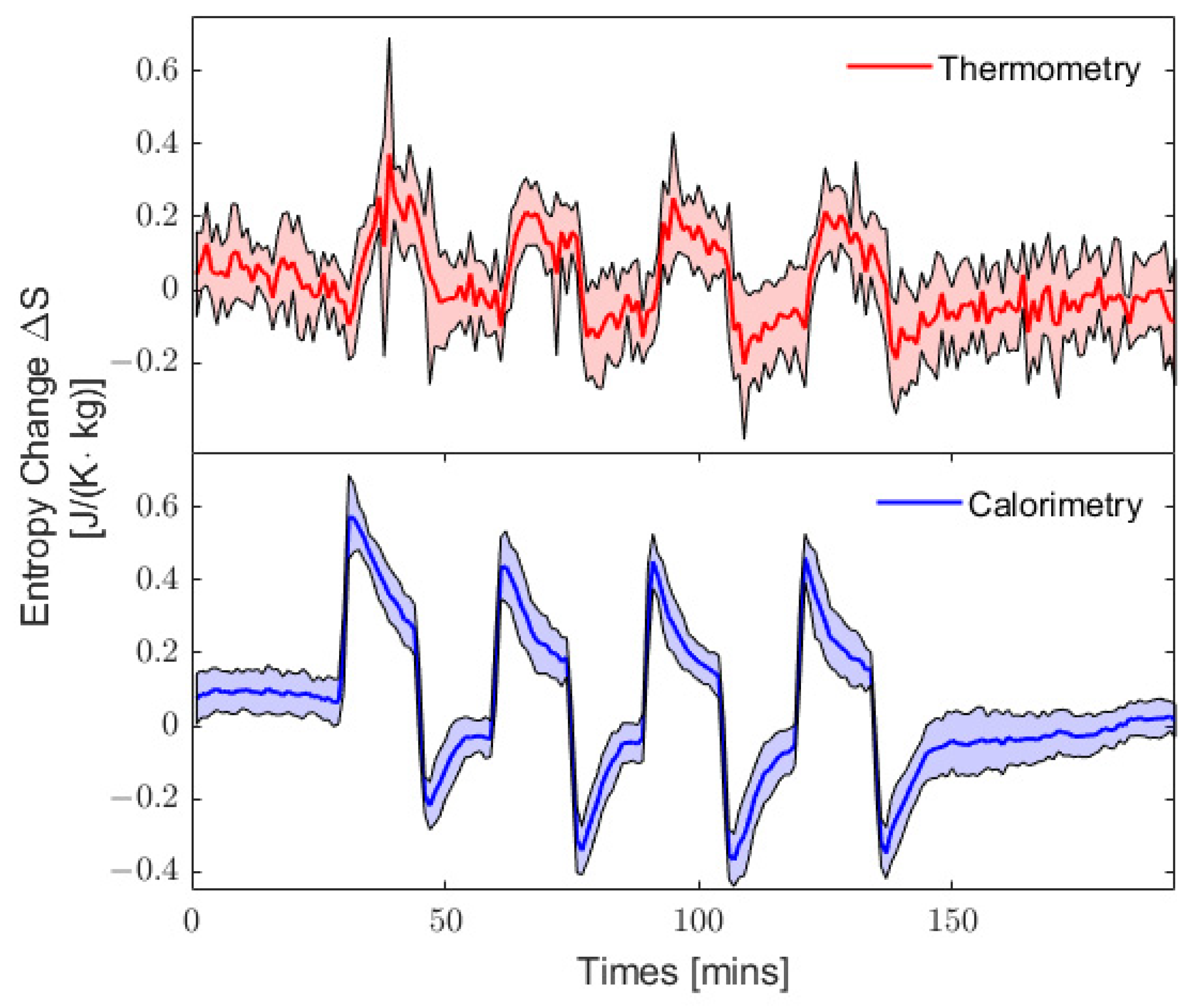
2.5. Temperature Measurements Using Thermometry and Calorimetry
2.6. Two-Compartment Non-Stationary Model of Entropy Production
2.7. Data and Statistical Analysis
3. Results
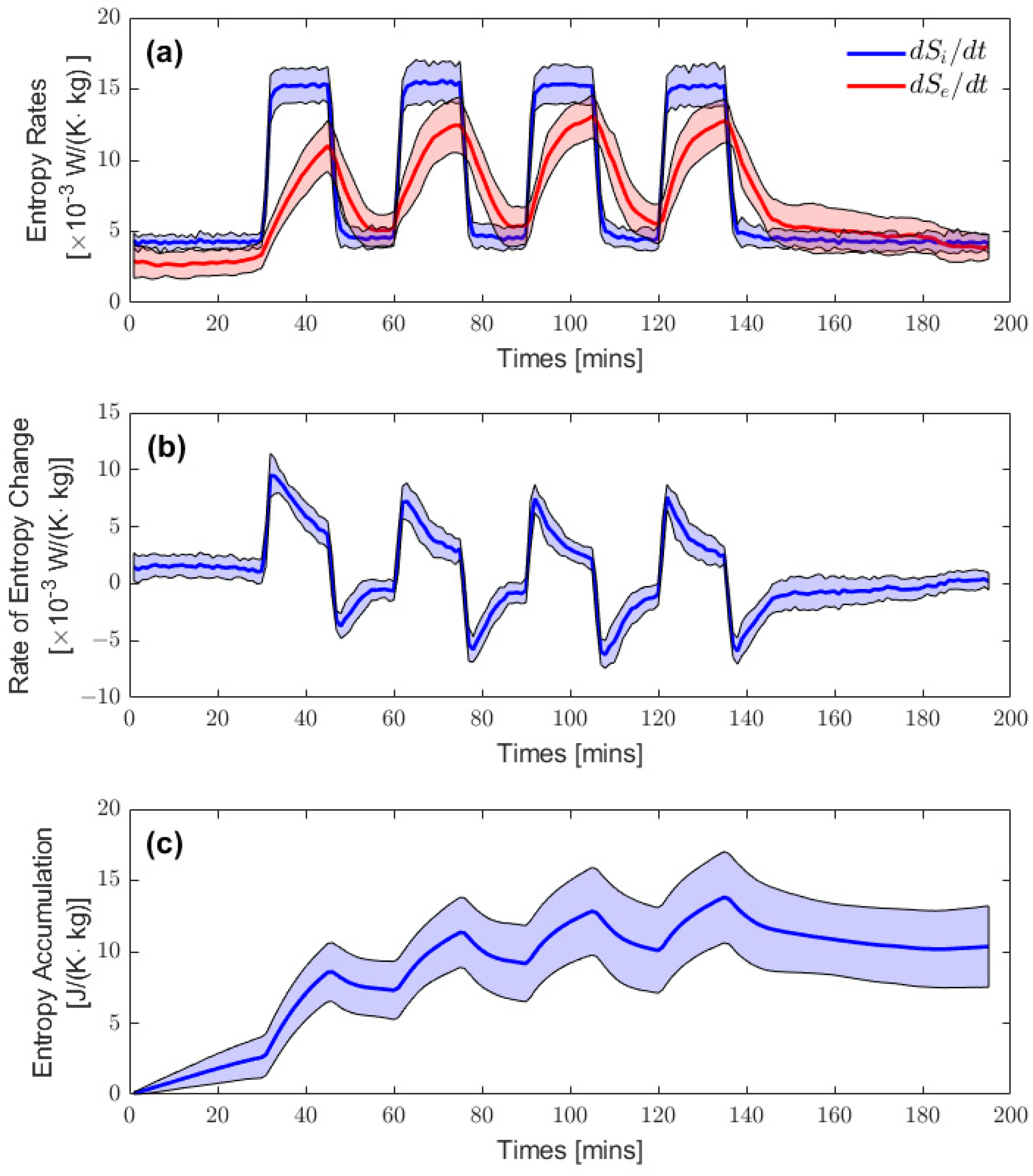
3.1. Resting Entropy Production during Heat Stress
3.2. Entropy Rate Curves
3.3. Quasi-Static Entropy Change Model
4. Discussion
4.1. On the Relevance of the Entropy Analysis
4.2. On the Necessity of Considering Non-Equilibrium States
4.3. Non-Equilibrium Steady State: Temperature Gradient of Subcompartments
4.4. Limitations
4.5. Entropy Production in Living Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prigogine, I. Introduction to Thermodynamics of Irreversible Processes; Wiley: Hoboken, NJ, USA, 1968. [Google Scholar]
- Kleidon, A.; Lorenz, R. Entropy Production by Earth System Processes. In Non-Equilibrium Thermodynamics and the Production of Entropy; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–20. ISBN 3-540-22495-5. [Google Scholar]
- Katchalsky, A.; Curran, P.F. Nonequilibrium Thermodynamics in Biophysics; Harvard University Press: Cambridge, MA, USA, 1975. [Google Scholar]
- Zakharov, M.; Sadovsky, M. Model of Thermal Regulation of Animals Based on Entropy Production Principle. J. Sib. Fed. Univ. Math. Phys. 2013, 6, 381–405. [Google Scholar]
- Aoki, I. Entropy Flow and Entropy Production in the Human Body in Basal Conditions. J. Theor. Biol. 1989, 141, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.D.; Du Bois, E.F.; Soderstrom, G.F. Basal Metabolism, Radiation, Convection and Vaporization at Temperatures of 22 to 35 °C. J. Nutr. 1938, 15, 477–497. [Google Scholar] [CrossRef]
- Silva, C.A.; Annamalai, K. Entropy Generation and Human Aging: Lifespan Entropy and Effect of Diet Composition and Caloric Restriction Diets. J. Thermodyn. 2009, 2009, 186723. [Google Scholar] [CrossRef]
- Seely, A.J.E. Optimizing Our Patients’ Entropy Production as Therapy? Hypotheses Originating from the Physics of Physiology. Entropy 2020, 22, 1095. [Google Scholar] [CrossRef]
- Bienertová-Vašků, J.; Zlámal, F.; Nečesánek, I.; Konečný, D.; Vasku, A. Calculating Stress: From Entropy to a Thermodynamic Concept of Health and Disease. PLoS ONE 2016, 11, e0146667. [Google Scholar] [CrossRef]
- Wark, K. Thermodynamics; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Kenny, G.P.; Notley, S.R.; Gagnon, D. Direct Calorimetry: A Brief Historical Review of Its Use in the Study of Human Metabolism and Thermoregulation. Eur. J. Appl. Physiol. 2017, 117, 1765–1785. [Google Scholar] [CrossRef]
- Larose, J.; Wright, H.E.; Stapleton, J.; Sigal, R.J.; Boulay, P.; Hardcastle, S.; Kenny, G.P. Whole Body Heat Loss Is Reduced in Older Males during Short Bouts of Intermittent Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 619–629. [Google Scholar] [CrossRef]
- Reardon, F.D.; Leppik, K.E.; Wegmann, R.; Webb, P.; Ducharme, M.B.; Kenny, G.P. The Snellen Human Calorimeter Revisited, Re-Engineered and Upgraded: Design and Performance Characteristics. Med. Biol. Eng. Comput. 2006, 44, 721–728. [Google Scholar] [CrossRef]
- Kenny, G.P.; Jay, O. Thermometry, Calorimetry, and Mean Body Temperature during Heat Stress. Compr. Physiol. 2013, 3, 1689–1719. [Google Scholar] [CrossRef]
- Mtaweh, H.; Tuira, L.; Floh, A.A.; Parshuram, C.S. Indirect Calorimetry: History, Technology, and Application. Front. Pediatr. 2018, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Matarese, L.E. Indirect Calorimetry: Technical Aspects. J. Am. Diet. Assoc. 1997, 97, S154–S160. [Google Scholar] [CrossRef] [PubMed]
- Jay, O.; Reardon, F.D.; Webb, P.; DuCharme, M.B.; Ramsay, T.; Nettlefold, L.; Kenny, G.P. Estimating Changes in Mean Body Temperature for Humans during Exercise Using Core and Skin Temperatures Is Inaccurate Even with a Correction Factor. J. Appl. Physiol. 2007, 103, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Ohmura, A.; Lorenz, R.D.; Pujol, T. The Second Law of Thermodynamics and the Global Climate System: A Review of the Maximum Entropy Production Principle. Rev. Geophys. 2003, 41, 1018. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. Arch. Intern. Med. 1916, XVII, 863–871. [Google Scholar] [CrossRef]
- Gagnon, D.; Jay, O.; Kenny, G.P. The Evaporative Requirement for Heat Balance Determines Whole-Body Sweat Rate during Exercise under Conditions Permitting Full Evaporation. J. Physiol. 2013, 591, 2925–2935. [Google Scholar] [CrossRef]
- Callen, H.B. Thermodynamics; Wiley: Hoboken, NJ, USA, 1960. [Google Scholar]
- Taylor, N.A.S.; Tipton, M.J.; Kenny, G.P. Considerations for the Measurement of Core, Skin and Mean Body Temperatures. J. Therm. Biol. 2014, 46, 72–101. [Google Scholar] [CrossRef]
- Schrödinger, E. What Is Life? Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Cuddy, J.S.; Hailes, W.S.; Ruby, B.C. A Reduced Core to Skin Temperature Gradient, Not a Critical Core Temperature, Affects Aerobic Capacity in the Heat. J. Therm. Biol. 2014, 43, 7–12. [Google Scholar] [CrossRef]
- Herman, I.P. The Physics of the Human Body; Springer Cham: New York, NY, USA, 2016; ISBN 9783319239309. [Google Scholar]
- Kenny, G.P.; Jay, O.; Journeay, W.S. Disturbance of Thermal Homeostasis Following Dynamic Exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 818–831. [Google Scholar] [CrossRef]
- Saltin, B.; Gagge, A.P.; Bergh, U.; Stolwijk, J.A. Body Temperatures and Sweating during Exhaustive Exercise. J. Appl. Physiol. 1972, 32, 635–643. [Google Scholar] [CrossRef]
- Bełdowski, P.; Gadomski, A. A Quest to Extend Friction Law into Multiscale Soft Matter: Experiment Confronted with Theory—A Review. J. Phys. D Appl. Phys. 2022, 55, 483002. [Google Scholar] [CrossRef]
- Sawka, M.N.; Castellani, J.W. How Hot Is the Human Body? J. Appl. Physiol. 2007, 103, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Montévil, M.; Mossio, M. Biological Organisation as Closure of Constraints. J. Theor. Biol. 2015, 372, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Lotka, A.J. Contribution to the Energetics of Evolution. Proc. Natl. Acad. Sci. USA 1922, 8, 147–151. [Google Scholar] [CrossRef]
- Odum, H.T.; Pinkerton, R.C. Time’s Speed Regulator: The Optimum Efficiency for Maximum Power Output in Physical and Biological Systems. Am. Sci. 1955, 43, 331–343. [Google Scholar]
| Study | Resting Entropy Production per Unit Body Surface Area (W/K/m2) | Resting Entropy Production per Unit Body Mass (×10−3 W/K/kg) |
|---|---|---|
| This study | 0.18 ± 0.01 | 4.3 ± 0.4 |
| Aoki [5] 1 | 0.172 ± 0.003 | 3.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodeur, N.; Notley, S.R.; Kenny, G.P.; Longtin, A.; Seely, A.J.E. Continuous Monitoring of Entropy Production and Entropy Flow in Humans Exercising under Heat Stress. Entropy 2023, 25, 1290. https://doi.org/10.3390/e25091290
Brodeur N, Notley SR, Kenny GP, Longtin A, Seely AJE. Continuous Monitoring of Entropy Production and Entropy Flow in Humans Exercising under Heat Stress. Entropy. 2023; 25(9):1290. https://doi.org/10.3390/e25091290
Chicago/Turabian StyleBrodeur, Nicolas, Sean R. Notley, Glen P. Kenny, André Longtin, and Andrew J. E. Seely. 2023. "Continuous Monitoring of Entropy Production and Entropy Flow in Humans Exercising under Heat Stress" Entropy 25, no. 9: 1290. https://doi.org/10.3390/e25091290
APA StyleBrodeur, N., Notley, S. R., Kenny, G. P., Longtin, A., & Seely, A. J. E. (2023). Continuous Monitoring of Entropy Production and Entropy Flow in Humans Exercising under Heat Stress. Entropy, 25(9), 1290. https://doi.org/10.3390/e25091290







