1. Introduction
Ever since the discovery of vitamin A by Elmer V. McCollum [
1] in Wisconsin and its synthesis by Paul Karrer in Switzerland in 1937, there has been intense interest in its role in vision. In 1967, the Nobel Prize for Physiology or Medicine was awarded to Ragnar Granit, Haldan Keffer Hartline, and George Wald “for their discoveries concerning the primary physiological and chemical visual processes in the eye”. In his acceptance speech, Wald noted that “events after the activation of rhodopsin
are too slow to explain visual reception” [
2]. Hubel and Weisel later shared another Nobel Prize with David Sperry [
3] in 1981. They showed how specific neurons were responsive to one eye by suturing one eye of a kitten. The current explanation for visual photo-transduction involves the absorption of a photon by 11-
cis retinal, its isomerization to 11-
trans, the breakdown of rhodopsin with the cascade of transducins, G-protein activation, and ion movements. This mechanism and the closing of the cGMP-gated ion channel create a negative potential with hyperpolarization at −65 mV. Its depolarization is considered the signal.
Yet, there are still four unknowns.
2. The Standard Model of Photo-Transduction
2.1. First, Speed of Response
Wald’s question on the speed of transduction can be illustrated by the capture of prey by the barn owl. When diving in darkness to capture a mouse rustling under leaves, the owl must process the sound differential information based on the difference in sound in the two ears, only about 10 cm apart, at a speed of 0.000015 s to locate and stay on track. The coordination of auditory, motor, and visual information demands a rapid processing speed if it is to extend its claws, grab the mouse, and change direction of flight in one sweeping movement. Such speed of processing requires electron function.
2.2. Second, Precision Is Required for the Signal but Is Not Explained by the Standard Model
If two photoreceptors respond differently to photons of the same wavelength, then visual acuity would be lost. Loss of visual acuity is commonly reported in experimental conditions where omega-3 fatty acid deficiency reduces the photoreceptor docosahexaenoic acid (DHA, all-
cis-4,7,10,13,16,19-docosahexaenoic acid) content [
4,
5]. There is no sense of precision in the activation of cGMPs and transducins, nor in the ion movements.
2.3. Third, There Is No Explanation for the Transmission of the Vivid Detail Enabled by the Retina to the Brain for Reconstruction in Our Consciousness
How are images seen by the retina transferred in detail to the receptors in the brain? An attempt to explain the replication of the vivid detail seen by the retina in our consciousness proposed that photons were transferred [
6]. However, Georgiev explained why this could not be [
7]. Neither chemical transmitters nor ion movements explain the transfer. An electrical current per se would not carry specific information, such as wavelength. Yet, there is no explanation for the exquisite transfer of detail observed by the eye to our consciousness.
2.4. Fourth, How Is the Membrane Depolarized?
Membrane depolarization is generally considered to be the visual signal, but its mechanism is largely unknown.
3. Docosahexaenoic Acid (DHA)
The biosynthesis of the omega-3 family of polyunsaturated fatty acids (PUFAs) proceeds via a series of alternating position-specific desaturation and elongation steps from α-linolenic acid (all-
cis-9,12,15-octadecatrienoic acid) to DHA or even longer hexaenoic acids [
8]. However, DHA is preferentially incorporated into the brain during growth by an order of magnitude greater than biosynthesis [
9], and is selectively incorporated into synapses, mitochondria, and microsomes compared to other omega-3 PUFAs [
10].
DHA is densely packed in the membrane surrounding rhodopsin [
11], yet its contribution to the signaling process has seldom been considered, even though it was shown in 1973 to be involved in the electrical function of the photoreceptors [
12]. Moreover, DHA deficiency has been reported to adversely affect visual acuity in infants [
4] and rhesus monkeys [
13]. Klaus Gawrisch and colleagues have detailed how the DHA physical properties in the photoreceptor membrane differ from the omega-6 fatty acid all-
cis-4,7,10,13,16-docosapentaenoic acid (DPAn-6), but not from DHA’s immediate precursor, the omega-3 fatty acid all-
cis-7,10,13,16,19-docosapentaenoic acid (DPAn-3) [
14,
15,
16]. This is a puzzle, as the precursor would originally have been more easily synthesized since the final desaturation is rate-limited and energy-costly. So why is the last double bond so critical and DHA so highly conserved in photoreceptors, neurons, and synapses for over 500 million years of animal evolution?
Bazan and Gordon published radio-autograph evidence of co-migration of rhodopsin and DHA during the reconstruction of the phagocytized material in the pigment epithelium for the rod, where the data indicated non-covalent binding of DHA to rhodopsin [
17]. This evidence of a connection between DHA and rhodopsin is further supported by biophysical analysis from Gawrisch’s group, illustrating phosphatidylethanolamines accumulating preferentially near the protein, and these phosphoglycerides are particularly enriched in DHA. Consequently, there is growing evidence for the role of weakly specific DHA-rhodopsin interactions [
15,
16].
In the following sections, we will present a hypothesis whereby a DHA electron absorbs the surplus energy of the photon-induced isomerization, depolarizes the membrane, and transfers the information of the energizing photon to the brain, thereby resolving some of the unanswered questions and unknowns found with the standard model of photo-transduction.
4. Energetic Considerations
The law of conservation of energy dictates that the surplus energy from the photon energization of the electron and the cis–trans difference in energy must go somewhere and cannot be lost. The surplus energy from the isomerization is the sum of the energy of the photon (its wavelength) plus the difference between cis and trans retinal. The sum of the two functions would be at a higher energy than the exciting photon. A higher energy means a shorter wavelength, which is where DHA absorbs light. However, we calculated that the cis–trans difference is 7.9 kJ/mole. The energy of green light at 550 nm is 2.25 eV. The energy at DHA maximum absorption (240 nm) is about 2.3 times greater at 5.17 eV. The energy from the photon-induced isomerization would therefore be insufficient to shift the wavelength into the UV range. So, we are left with the question: where does the surplus energy go?
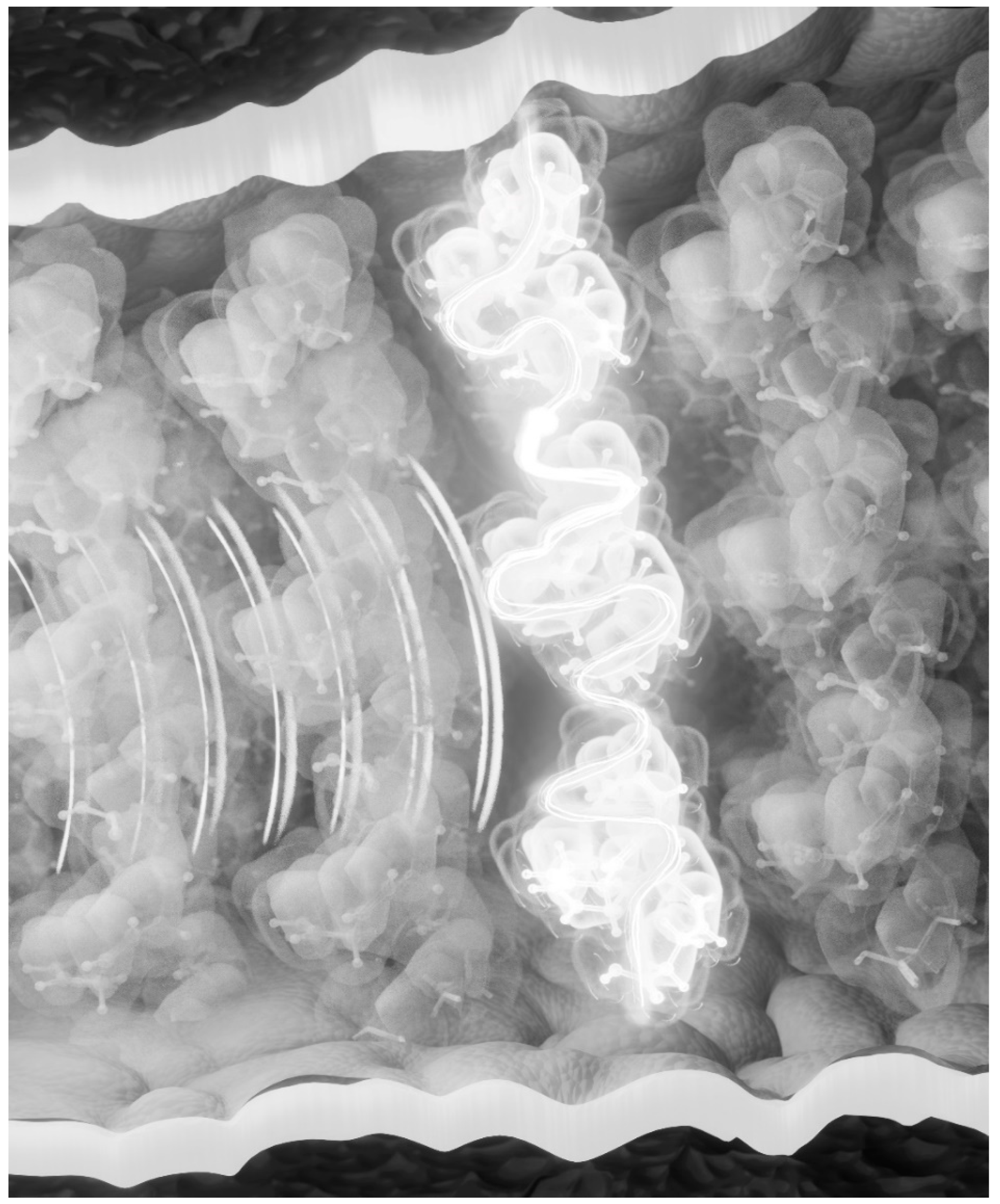
The surplus energy of isomerization must be accounted for by dissipation as heat. If not removed, then would not the constant flood of billions of photons during daylight damage the retina? This surplus energy from a single excitation will be quantized and will include the quantum of the exciting photon. We suggest that the surplus energy is absorbed by a neighboring DHA with the excitation of a π-electron that is then extracted by the hyperpolarization, depolarizes the membrane, and is sent to the brain. An artistic impression of the excitation of DHA in the photoreceptor membrane is shown in
Figure 1.
5. Non-Classicality of Light Harvesting and DHA as an Energy Trap
The methylene group (-CH2-) interruption of the six double bonds found with the DHA molecule is essential to our thesis. It separates the π-electron clouds, creating an energy barrier and confining the electrons to their wells. Hence, DHA is an insulator, preventing the flow of a current across the membrane.
On the other hand, given an electrical potential across the DHA molecule that is sufficient to extract an electron, it will leave a hole that an electron with the same quantum mechanical properties can fill after tunneling through the energy barrier of the methylene groups. In that way, a current can flow. Thus, DHA acts as a semiconductor [
18], with the excitation of a DHA electron lowering the barrier for conduction.
This idea is based on the extraordinary, high density of DHA in the phosphoglycerides, including DHA uniquely at both the
sn1 and
sn2 positions (di-DHA). Furthermore, in photoreceptor cells, the very long-chain omega-3 PUFAs are in the phosphatidylcholines (PC) of the outer segment membranes, tightly bound to rhodopsin [
19]. Indeed, these retinal very long-chain hexaenoic fatty acids are mainly esterified at the
sn1 position of PC, with DHA in the
sn2 position [
8]. These very long-chain PUFAs are only synthesized in the retina and testes [
20]. It has been shown that loss or reduced levels of these very long-chain omega-3 PUFAs may cause loss of photoreceptors or functional perturbations [
8,
21]. Prominent in this very long-chain omega-3 PUFA family is the C36:6n-3, hexatriacontahexaenoic acid (HTA) [
8,
19,
22], which places its omega-3 hexaenoic sequence between the outer and inner leaflets of the photoreceptor membrane. The location of the hexaenoic motif from HTA creates a stunning image of a high-density electron profile across this signaling membrane. DHA is present in the photoreceptor membrane phosphoglycerides at approximately 50% of the membrane fatty acids. So, virtually every
sn2 position is occupied by a DHA in the outer and inner leaflets. Hence, there will nearly always be 12 π-electrons top and 12 bottom. An arrangement of DHA-HTA-DHA means there are three sets of π-electrons arranged as 12-12-12. If we consider a region of favored di-DHA packing top and bottom with co-existing HTA, the arrangement is 24-12-24 or 24-12-12 or 12-12-24. Even without HTA or other even longer chain PUFAs, there will be 24-24, 24-12, or 12-24.
The outer leaflet, or membrane bilayer, is dominated by PC, and the inner by phosphatidylethanolamine. The phosphates cancel out, and the choline (-C-NH4+), a quaternary amine, is permanently and strongly positive, regardless of pH, with a pKb of 14.1. The ethanolamine of the inner layer is not ionized at physiological pH. Hence, there is always an intrinsic dipole moment across the membrane.
The planar structure of the energy-minimized DHA conformation is a fundamental characteristic of six double bonds separated by methylene groups. The π bonds always have a (+ve) end and a (−ve) end. The shape of their probability orbits will lean towards the (+ve) end of the intrinsic dipole moment. A simple mechanism for absorbing a quantized amount of energy is precisely that amount that will flip the direction of bond polarity to the opposite direction [
23].
This electrical torsion from the inherent membrane dipole creates the image of the mass of DHA π-electrons under tension. Add HTA, placing its double bonds in the space between the bilayers, and there is a remarkable π-electron field density from top to middle and bottom of the membrane under the influence of this electrical torsion: energize an electron, and it will be like a hair trigger. In the presence of hyperpolarization, it would be extracted, and the electron could tunnel from top to bottom, de-polarizing the membrane. Interestingly, depolarization is considered to be the signal, although the mechanism is poorly understood.
The idea that there was a non-classical function at work in light harvesting was put forward by Wilde et al. in 2010 [
24]. Energy transport has been reported to depend on the exciton–phonon interplay of molecular motions [
25], and environmental fluctuations [
26,
27] with the formation of charge density waves at work [
28]. Moreover, vibrations and quantum coherence can enhance energy transfer [
29]. Evidence has been published showing that coupling to quantized vibrations is fundamental for biological function as this generates non-cascaded transport with a rapid and wider spatial distribution of excitation energy [
30].
O’Reilly and Olaya-Castro have made the case for quantized vibrations in coherent light harvesting. They wrote: “we found that the properties of some of the chromophore vibrations that assist energy transfer during photosynthesis can never be described with classical laws, but this non-classical behavior enhances the efficiency of the energy transfer” [
31].
6. Transfer to the Brain
Investigations into photo-transduction have not included any role for the DHA, electron-rich membrane structure surrounding rhodopsin and other opsins. Based on this non-classical behavior described above, it is plausible that a quantum field is responsible for energy transfer from the isomerization to the DHA surrounding rhodopsin. The idea of using quantum field theory in brain function is not new [
32].
On the other hand, the idea is that the isomerization energy surplus is released as heat, which is vibrational energy that is quantized. With electronic/vibronic coherence already demonstrated, we have a novel and plausible mechanism for the quantized transfer of the isomerization energy to DHA.
The energy of the
cis–trans isomerization will be common to all photoreceptions. The variable will be the energy of the photon (
hc/λ). Hence, in each downstream event, we have a discreet, quantized packet of original information, namely the sum of the energy of the exciting photon plus that of the isomerization. This is the first step, as shown by (1).
The energy pulse from a single isomerization will be quantized, and just as the photon ejected from a supernova retains the purity of its wavelength despite starting out several million light years away, this quantized energy cannot be lost. Thus, the electron extracted from DHA carries a quantized function of the original photon, offering a mechanism whereby the wavelength, which means color, is transferred intact to the brain.
Wavelengths are inversely related to energy E.
where h is Plank’s constant, c is the speed of light, f is the frequency, and λ is the wavelength.
Information could be carried by a single electron as a waveform and met by a single electron receptor. There is no explanation for how the color captured by the retina is transferred to our consciousness. The title of the 2007 paper by Rahnama et al. [
33] expressed the question neatly: “How can the visual quantum information be transferred to the brain intact, collapsing there and causing consciousness?”. With the transfer by the actual photons themselves not being viable [
7], the transfer by an electron must be considered.
The DHA electron energized by the isomerization surplus will be at an energy that contains a quantized function of the original photon that initiated the isomerization. The transportation of this single electron as a waveform could be via a tunnelling process or teleportation. It is analogous to a qubit. A flood of electrons will not work, as there is no discrete information. This idea of excess energy disposal is novel, as is the concept of DHA acting as an energy-electron transducer.
Support for this notion comes from direct evidence of quantum transportation [
34], and the teleportation of photons using single photon receptors [
35]. Photons and electrons have much in common. We are suggesting the presence of single electron detectors in the brain. There is, as far as we know, no other explanation for the transfer of imagery from the retina to the brain. The behavior of quantum systems in transferring information has been demonstrated by the Sycamore processor, which was shown to take 200 s to sample one instance of a quantum circuit, which would take a state-of-the-art classical supercomputer approximately 10,000 years to perform an equivalent task [
36].
None of the above challenges the presently accepted model of events following photoreception, the formation of metarhodopsins, or the transducin cascade. It simply adds to the potential involvement of the high density of DHA. The specific, methylene-interrupted sequence of six double bonds in the membrane could act as a photon-energy-electron-transducer, providing a mechanism for quantized electrons to signal to the brain and enabling us to see a reconstruction of the vivid information collected by our retina.
It is plausible that the slower chemical reactions and ion movements on which Wald commented represent the charging of the battery as in membrane hyperpolarization leading to the extraction of an energized electron from DHA, with guidance provided en route to the brain. “Solving the human connectome at the nanoscale level of synaptic wiring will be critical for fully understanding the neural basis of human color vision” [
37]. The novel hypothesis presented here requires further elaboration and testing. We have made a start in the next section with data supporting electron function and the potential of DHA for energy absorption [
37].
7. DHA—Evidence for Electrical Activity
From molecular dynamic calculations, the ground state energy of DHA is 50 kJ/mol, which is in the region of energy reception of the order discussed here. We are proposing the absorption of the energy from the isomerization by DHA, with the hyperpolarization extracting the energized electron and depolarizing the membrane. This requires a condition in which DHA is electrically active. The fact that DHA is electrically active is supported by the first study of DHA deficiency in the photoreceptor performed on rats [
12]. The deficiency did not affect structure but altered the electrical properties. The rod outer segment DHA concentration fell from 45.2% of total fatty acids to 19.0% in the DHA-deficient group. DHA was partially replaced with DPAn-6, which was the only major change identified between the groups. The rhodopsin concentration, the shape of the absorption spectra, and general bleaching characteristics were the same for the DHA-deficient and control groups. The density and packing of rods appeared normal in the DHA-deficient animals, and the ultrastructure of rod outer segments was indistinguishable from control. The electroretinogram (ERG) displayed a reduced A and B wave function. The A-wave of the ERG is a photoreceptor response function, while the B-wave is generated by electrical activity in other neural layers of the retina. Therefore, since these results indicate that specific decreases in membrane DHA lead to abnormal electrical activity, they are consistent with a unique and indispensable role for DHA in the electrical behavior of phototransduction.
Due to its six-methylene interrupted double bonds, DHA has a periodic structure. Any periodic structure on a quantum level can form energy bands and, under the right conditions, conduct electrons. We have previously provided evidence to support the potential of DHA being electrically active by using the calculation of the Kronig–Penney model, which showed that an energy band can exist in DHA [
18]. We also tested the possibility of π-electron behavior using the nuclear Overhauser effect (NOE), which demonstrated polarization buildup at two ethene (CH=CH) sites at 127.6 ppm and at 132.8 ppm, the phosphatidyl, and, surprisingly, the methyl group [
18].
If an energized electron from DHA is followed by a flow of electrons in the membrane depolarization, we are concerned here with this specific electron, which is at a higher energy. According to the law of conservation of energy, the wave function of that electron should carry a quantum of energy belonging to the original photon, thus differentiating it from the pack. It will be traveling at a speed that would certainly be fast enough for transduction, as it could “jump” the synaptic connections in the retina and lateral geniculate nucleus (LGN), rather than having to undergo the slower process of chemical nerve transmission. All that is required is that it be identified by single electron detectors.
For electrons specifically, individual spins in semiconductor quantum dots have been shown to stand out for their long coherence times and potential for scalable fabrication [
35]. Moreover, pulse electron paramagnetic resonance spectroscopy has been used to show that the spin state of an electron can be teleported with high fidelity [
38].
It is known that brain function is dependent on electron movement. If there is none, the brain is dead. In considering the options to explain the purity of the transfer from the retina to the brain, our suggestion here is that the transfer is by an energized electron from DHA as a wave function, which will include the energy quantum of the photon. That is the signal that provides pure information about color from the retina to the brain. Under the condition for DHA to conduct, a flood of or even a burst of electrons would not have the specificity of the signal but could provide a carrier wave to transfer the signal. On the other hand, it is also plausible that the transfer of a single, energized electron to the reception site in the brain could be by teleportation, electron tunnelling, or some other, not yet described, mechanism.
Our understanding of quantum behavior in the brain is in its infancy. The key issue is the question of speed and, secondly, the precision of the wavelength or energy quantum, to enable us to see in color. Both are answered by the transfer of an electron wave function carrying the information of the photon, as described above. Both are absolute requirements for color vision.
8. Raman Spectroscopy and Molecular Dynamics
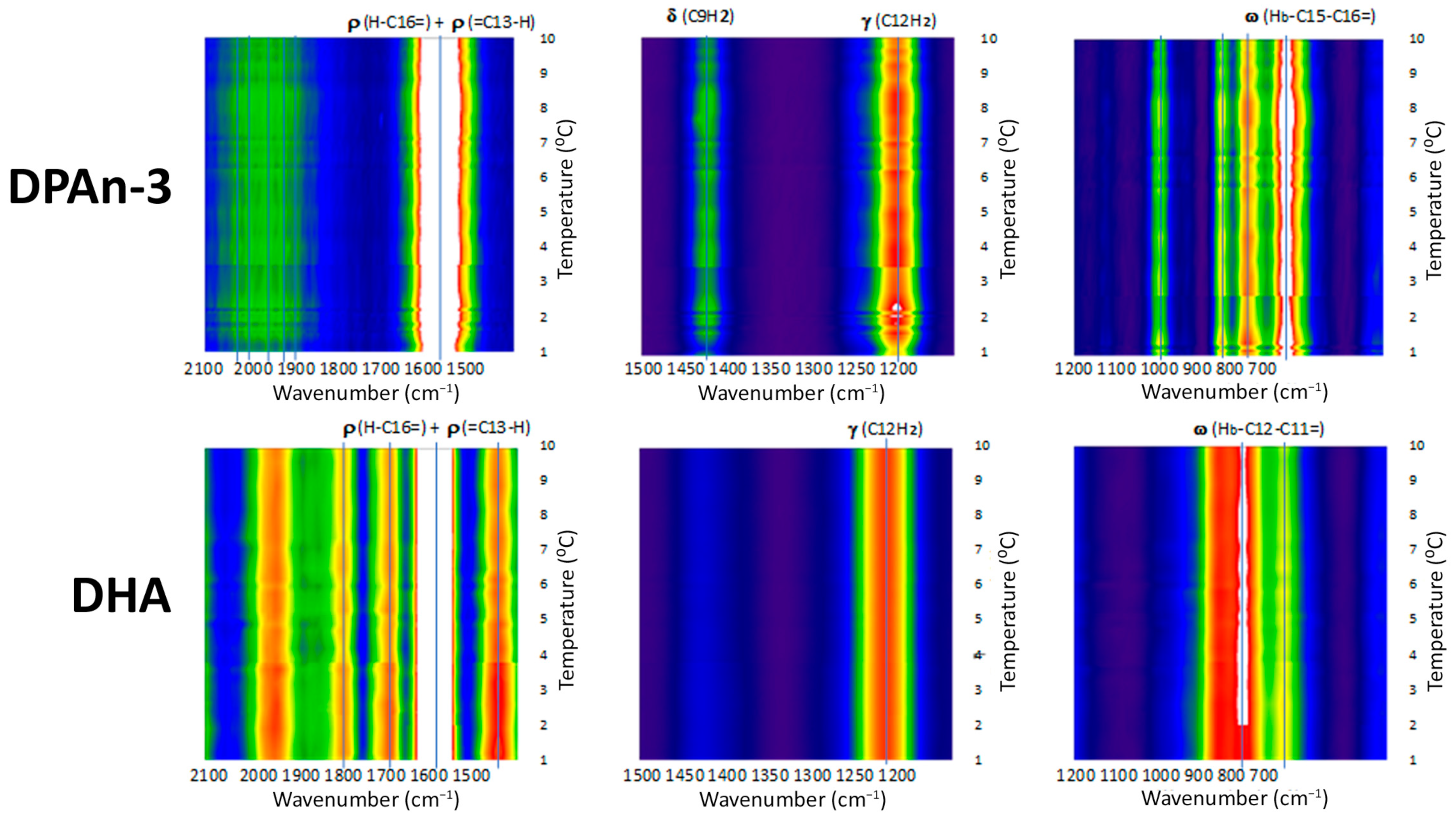
Our data from Raman spectroscopy (see [
39]) supports the idea that DHA could respond to vibrational energy and helps differentiate DHA from DPAn-3, shown in
Figure 2 and
Figure 3. The Raman data is evidence of the vibrational behavior of the hexaenoic acid sequence, which is of a different order than that observed in DPAn-3. In any =C-CH
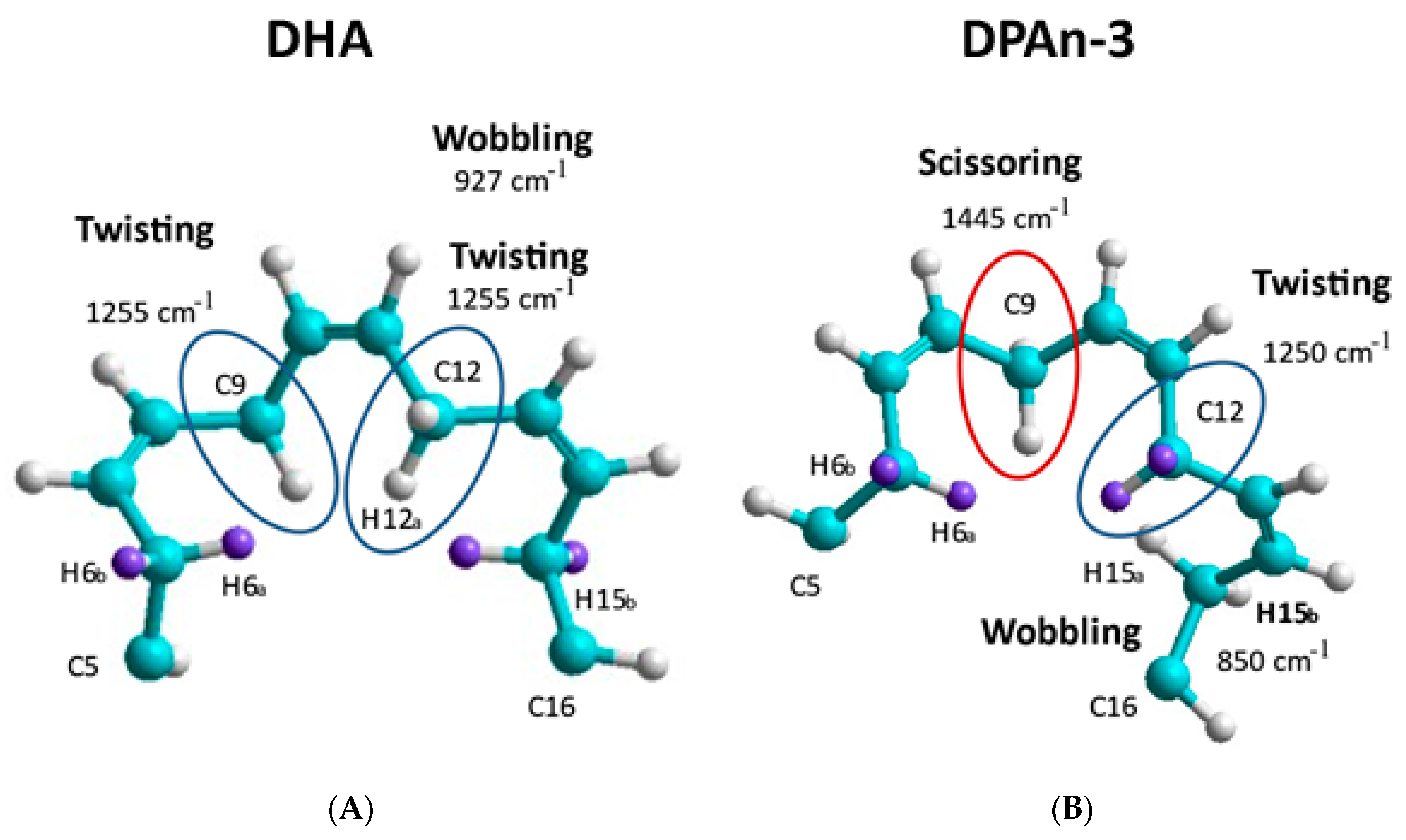
2-C= moiety, unequal =C-C and C-C= lengths result in twisting at the methylene CH
2.
Curvature occurs when the lowest-energy H spin states in the C-H bond are −1/2 and +1/2 (or +1/2 and −1/2), resulting in twisting at the methylene site. Curvature will also result at the methylene site’s carbon atom due to electron spin states. C-C bond lengths will be unequal in C-C= and =C-C when electron spin states in the C-C bond are −1/2 and −1/2 versus +1/2 and +1/2. The spin sets −1/2 and +1/2 (or +1/2 and −1/2) would have an intermediate average bond length.
A change in curvature can be labeled as a change in carbon backbone bending. The accumulation of twisting and bending site-to-site results in the planar C shape of DHA, where three double bonds are planar. This is not possible with DPAn-3 [
18].
Raman spectroscopy provides unequivocal imagery of the utility of DHA’s six-double-bond sequence compared to its five-double-bond precursor, DPAn-3. It also supports the concept of non-classical energy transmission. In addition, the scissoring and twisting on the DPAn-3 molecule with an odd number of double bonds implies it is less of a candidate for wave absorption.
Overall, the data presented in
Figure 2 and
Figure 3 suggest that DHA is a resonant molecule that has all it needs to be positioned at the receiving end of the vibrational energy generated by a photon-induced isomerization event.
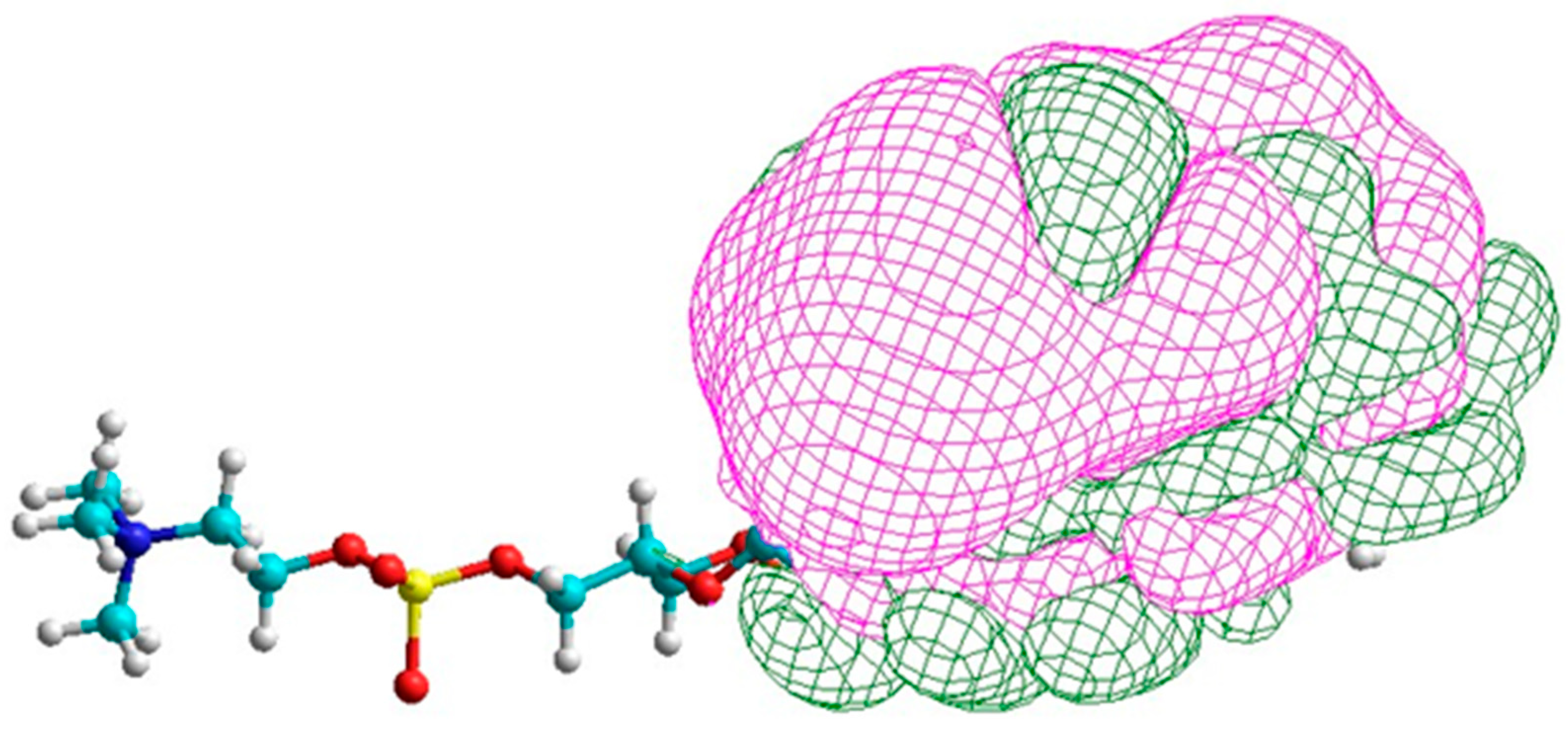
Di-DHA PC
In
Figure 4, the lowest-energy electron spin orbitals in DHA are compared with those of di-DHA PC present in the photoreceptor. The results show that spin orbital energy levels in each of the two DHA moieties in the PC structure remain similar to each other, and both are close to the lowest electron spin orbital in DHA alone. The near absence of electron spin density between 0 eV and −2 eV is common. The energy levels for positive electron spin orbitals from 0 eV to +2 eV (pink) are about 10–20% broader in di-DHA PC than in DHA. Interestingly, DPAn-3 did not exhibit such clear differences, further emphasizing the unique nature of DHA.
In
Figure 5, the assignment of positive electron spin orbitals to methine H sites holds for di-DHA PC. The lowest electron spin orbitals include those for carbon atoms as well as H atoms [
40]. The broadening of the negative electron spin orbitals suggests the electron spin states for the carbon atoms in the methine sites again differentiate DHA from di-DHA PC molecular properties. The presence of di-DHA phosphoglycerides in the photo-receptor/rod/cone membranes likely enhances the probability of energy capture.
9. Summary and Conclusions: DHA as a Photon-Energy-Electron Transducer
In this paper, we have set out the limitations of the current model of photo-transduction and provided experimental evidence to support a previously unrecognized key role for DHA in the process. In this revision to the process, the quantum of energy from the photon-induced cis–trans isomerization of retinal is absorbed by a DHA π-electron. Hyperpolarization extracts the energized electron, which then transmits its information to the brain, conserving the fidelity of the original wavelength. This hypothesis provides a clear rationale to support the observations of the extraordinary high density of DHA and the presence of very long-chain omega-3 PUFAs surrounding the opsins, which serve to absorb the energy, and DHA acts as the photon-energy transducer sending visual information to the brain for image reconstruction in our consciousness.
In so doing, this thesis provides an explanation for the previously unresolved issues around the speed of information transfer and the purity of the conservation of a photon’s wavelength. It also explains the extraordinary concentration of the hexaenoic motive in the lipids adjacent to and attached to rhodopsin. This relationship has been conserved throughout the evolution of vertebrate species [
41]. Our proposal that DHA acts as a photon-electron transducer for visual reception places DHA in an unassailable position at the center of visual signaling, and on a par with vitamin A.
Author Contributions
Conceptualization, M.A.C. and J.T.B.; formal analysis, A.J.S.; investigation, W.F.S. and C.L.B.; resources, W.F.S., L.H. and C.L.B.; data curation, Y.W.; writing—original draft preparation, M.A.C. and L.H.; writing—review and editing, S.C.D. and M.R.J.; visualization, S.C.D.; project administration, S.C.D. and M.R.J.; funding acquisition, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Waterloo Foundation, grant number (1155-4723), and Mother and Child Foundation, grant number (FOSS3), including gifts from Pete Linsert, Larry Horn, Rich Radmer, Steve Dubin, John Versteeg and Bud Evans.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The paper is dedicated to Pete Linsert, pioneer in the production of docosahexaenoic acid and arachidonic acid for infant formula, brain and heart health, who passed away before it was completed. We are particularly grateful to the late Letten F. Saugstad, who supported our work in many ways.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McCollum, E.V.; Davis, M. The necessity of certain lipins in the diet during growth. J. Biol. Chem. 1913, 15, 167–175. [Google Scholar] [CrossRef]
- The Nobel Prize in Physiology or Medicine 1967. NobelPrize.org. Nobel Prize Outreach AB 2023. Tue. 17 Oct 2023. Available online: https://www.nobelprize.org/prizes/medicine/1967/summary/ (accessed on 1 August 2023).
- Hubel, D.; Wiesel, T. David Hubel and Torsten Wiesel. Neuron 2012, 75, 182–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birch, E.E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.; Drover, J.R.; Castaneda, Y.S.; Minns, L.; Wheaton, D.K.; Mundy, D.; et al. The DIAMOND (DHA Intake and Measurement of Neural Development) Study: A double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 2010, 91, 848–859. [Google Scholar] [CrossRef]
- Neuringer, M.; Connor, W.E.; Van Petten, C.; Barstad, L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J. Clin. Investig. 1984, 73, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Georgiev, D. Photons do collapse in the retina not in the brain cortex: Evidence from visual illusions. NeuroQuantology 2011, 9, 206–230. [Google Scholar] [CrossRef][Green Version]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J. Incorporation of radioactive polyunsaturated fatty acids into liver and brain of developing rat. Lipids 1975, 10, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Manabe, S.; Wada, O.; Crawford, M.A. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int. J. Vitam. Nutr. Res. 1997, 67, 272–278. [Google Scholar]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef]
- Benolken, R.M.; Anderson, R.E.; Wheeler, T.G. Membrane fatty acids associated with the electrical response in visual excitation. Science 1973, 182, 1253–1254. [Google Scholar] [CrossRef]
- Connor, W.E.; Neuringer, M.; Lin, D.S. Dietary effects on brain fatty acid composition: The reversibilty of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J. Lipid Res. 1990, 31, 237–247. [Google Scholar] [CrossRef]
- Gawrisch, K.; Eldho, N.V.; Holte, L.L. The structure of DHA in phospholipid membranes. Lipids 2003, 38, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Gawrisch, K.; Soubias, O.; Mihailescu, M. Insights from biophysical studies on the role of polyunsaturated fatty acids for function of G-protein coupled membrane receptors. Prostaglandins Leukot Essent Fat. Acids 2008, 79, 131–134. [Google Scholar] [CrossRef]
- Eldho, N.V.; Feller, S.E.; Tristram-Nagle, S.; Polozov, I.; Gawrisch, K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. J. Am. Chem. Soc. 2003, 125, 6409–6421. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.C.; Bazan, N.G. Docosahexaenoic acid utilization during rod photoreceptor cell renewal. J. Neurosci. 1990, 10, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Broadhurst, C.L.; Guest, M.; Nagar, A.; Wang, Y.; Ghebremeskel, K.; Schmidt, W.F. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot Essent Fat. Acids 2013, 88, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Agbaga, M.P.; Mandal, M.N.; Anderson, R.E. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J. Lipid Res. 2010, 51, 1624–1642. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.B.; Brush, R.S.; Sullivan, M.T.; Zavy, M.T.; Agbaga, M.P.; Anderson, R.E. Decreased very long chain polyunsaturated fatty acids in sperm correlates with sperm quantity and quality. J. Assist. Reprod. Genet. 2019, 36, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.D.; Brush, R.S.; Chan, M.; Lydic, T.A.; Reese, K.; Reid, G.E.; Busik, J.V.; Elliott, M.H.; Anderson, R.E. Effect of reduced retinal VLC-PUFA on rod and cone photoreceptors. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3150–3157. [Google Scholar] [CrossRef][Green Version]
- Bazan, N.G. Overview of how N32 and N34 elovanoids sustain sight by protecting retinal pigment epithelial cells and photoreceptors. J. Lipid Res. 2021, 62, 100058. [Google Scholar] [CrossRef]
- Crawford, M.A.; Bloom, M.; Broadhurst, C.L.; Schmidt, W.F.; Cunnane, S.C.; Galli, C.; Gehbremeskel, K.; Linseisen, F.; Lloyd-Smith, J.; Parkington, J.E. Evidence for the unique function of docosahexaenoic acid during the evolution of modern hominid brain. Lipids 1999, 34, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.M.; McCracken, J.; Mizel, A. Could light harvesting complexes exhibit non-classical effects at room temperature? Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 1347–1363. [Google Scholar] [CrossRef]
- Renger, T.; May, V.; Kuhn, O. Ultrafast excitation energy transfer dynamics in photosynthetic pigment–protein complexes. Phys. Rep. 2001, 343, 137–254. [Google Scholar] [CrossRef]
- Plenio, M.B.; Huelga, S.F. Dephasing-assisted transport: Quantum networks and biomolecules. New J. Phys. 2008, 10, 113019. [Google Scholar] [CrossRef]
- Rebentrost, P.; Mohseni, M.; Kassal, I.; Lloyd, S.; Aspuru-Guzik, A. Environment-assisted quantum transport. New J. Phys. 2009, 11, 033003. [Google Scholar] [CrossRef]
- Lee, M.H.; Troisi, A. Vibronic enhancement of excitation energy transport: Interplay between local and non-local exciton-phonon interactions. J. Chem. Phys. 2017, 146, 075101. [Google Scholar] [CrossRef]
- Duan, H.G.; Nalbach, P.; Miller, R.J.D.; Thorwart, M. Intramolecular vibrations enhance the quantum efficiency of excitonic energy transfer. Photosynth. Res. 2020, 144, 137–145. [Google Scholar] [CrossRef]
- Kolli, A.; O’Reilly, E.J.; Scholes, G.D.; Olaya-Castro, A. The fundamental role of quantized vibrations in coherent light harvesting by cryptophyte algae. J. Chem. Phys. 2012, 137, 174109. [Google Scholar] [CrossRef]
- O’Reilly, E.J.; Olaya-Castro, A. Non-classicality of the molecular vibrations assisting exciton energy transfer at room temperature. Nat. Commun. 2014, 5, 3012. [Google Scholar] [CrossRef]
- Nishiyama, A.; Tanaka, S.; Tuszynski, J.A. Non-Equilibrium Quantum Electrodynamics in Open Systems as a Realizable Representation of Quantum Field Theory of the Brain. Entropy 2019, 22, 43. [Google Scholar] [CrossRef]
- Rahnama, M.; Salari, V.; Tuszynski, J.A. How Can the Visual Quantum Information be Transferred to The Brain Intact, Collapsing There and Causing Consciousness? Neuroquantology 2009, 7, 491–499. [Google Scholar] [CrossRef]
- Perdomo, A.; Vogt, L.; Najmaie, A.; Aspuru-Guzik, A. Engineering directed excitonic energy transfer. Appl. Phys. Lett. 2010, 96, 093114. [Google Scholar] [CrossRef]
- Valivarthi, R.; Davis, S.I.; Peña, C.; Xie, S.; Lauk, N.; Narváez, L.; Allmaras, J.P.; Beyer, A.D.; Gim, Y.; Hussein, M.; et al. Teleportation Systems Toward a Quantum Internet. PRX Quantum 2020, 1, 020317. [Google Scholar] [CrossRef]
- Arute, F.; Arya, K.; Babbush, R.; Bacon, D.; Bardin, J.C.; Barends, R.; Biswas, R.; Boixo, S.; Brandao, F.; Buell, D.A.; et al. Quantum supremacy using a programmable superconducting processor. Nature 2019, 574, 505–510. [Google Scholar] [CrossRef]
- Kim, Y.J.; Packer, O.; Pollreisz, A.; Martin, P.R.; Grunert, U.; Dacey, D.M. Comparative connectomics reveals noncanonical wiring for color vision in human foveal retina. Proc. Natl. Acad. Sci. USA 2023, 120, e2300545120. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Kandel, Y.P.; Manikandan, S.K.; Jordan, A.N.; Fallahi, S.; Gardner, G.C.; Manfra, M.J.; Nichol, J.M. Conditional teleportation of quantum-dot spin states. Nat. Commun. 2020, 11, 3022. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Schmidt, W.F.; Nguyen, J.K.; Qin, J.; Chao, K.; Aubuchon, S.R.; Kim, M.S. Continuous gradient temperature Raman spectroscopy and differential scanning calorimetry of N-3DPA and DHA from −100 to 10 degrees C. Chem. Phys. Lipids 2017, 204, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Head-Gordon, M. Restricted active space spin-flip configuration interaction approach: Theory, implementation and examples. Phys. Chem. Chem. Phys. 2009, 11, 9779–9790. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Schmidt, W.F.; Broadhurst, C.L.; Wang, Y. Lipids in the origin of intracellular detail and speciation in the Cambrian epoch and the significance of the last double bond of docosahexaenoic acid in cell signaling. Prostaglandins Leukot Essent Fat. Acids 2021, 166, 102230. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).