The Impact of Hoffmann Reflex on Standing Postural Control Complexity in the Elderly with Impaired Plantar Sensation
Abstract
1. Introduction
2. Materials and Methods
- Experiment procedure
- Plantar pressure sensitivity test
- Postural control test
- H-reflex test
- Data process
- (1)
- from a vector , two sequences of m consecutive points.
- (2)
- Computed the maximum distance:and then compared with tolerance for repeated sequences counting:where the tolerance equals 0.1−0.2 * SD [29], SD is the standard deviation of .
- (3)
- For the sequence , its count is defined as .is the average amount of for .is the average of .
- (4)
- Statistical analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masud, T.; Morris, R.O. Epidemiology of falls. Age Ageing 2001, 30, 3–7. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.H. Neuropathy: Mobility and quality of life. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. 1), S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.K.; Ashtonmiller, J.A. Peripheral neuropathy: An often-overlooked cause of falls in the elderly. Postgrad. Med. 1996, 99, 161. [Google Scholar] [CrossRef] [PubMed]
- England, J.D.; Asbury, A.K. Peripheral neuropathy. Lancet 2004, 363, 2151–2161. [Google Scholar] [CrossRef]
- Martyn, C.N.; Hughes, R.A. Epidemiology of peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry 1997, 62, 310–318. [Google Scholar] [CrossRef]
- Richardson, J.K. Factors associated with falls in older patients with diffuse polyneuropathy. J. Am. Geriatr. Soc. 2002, 50, 1767–1773. [Google Scholar] [CrossRef]
- Ducic, I.; Short, K.W.; Dellon, A.L. Relationship between loss of pedal sensibility, balance, and falls in patients with peripheral neuropathy. Ann. Plast. Surg. 2004, 52, 535–540. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Dobson, J. The contribution of small and large sensory afferents to postural control in patients with peripheral neuropathy. J. Sport Health Sci. 2019, 8, 218–227. [Google Scholar] [CrossRef]
- Zhang, S.; Manor, B.; Li, L. H-index is important for postural control for people with impaired foot sole sensation. PLoS ONE 2015, 10, e0121847. [Google Scholar] [CrossRef]
- Sun, M.; Lewis, K.; Choi, J.; Zhang, F.; Qu, F.; Li, L. The Reduced Adaptability of Hoffmann-reflex Parameters to Postural Change with Deficiency of Foot Plantar Sensitivity. Front. Physiol. 2022, 13, 890414. [Google Scholar] [CrossRef]
- Manor, B.; Lipsitz, L.A.; Wayne, P.M.; Peng, C.K.; Li, L. Complexity-based measures inform tai chi’s impact on standing postural control in older adults with peripheral neuropathy. BMC Complement. Altern. Med. 2013, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.F.; Oddsson, L.I.; De Luca, C.J. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 2004, 156, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.L.; Manor, B.; Li, L. Stance and sensory feedback influence on postural dynamics. Neurosci. Lett. 2007, 423, 104–108. [Google Scholar] [CrossRef]
- Kang, H.G.; Costa, M.D.; Priplata, A.A.; Starobinets, O.V.; Goldberger, A.L.; Peng, C.K.; Kiely, D.K.; Cupples, L.A.; Lipsitz, L.A. Frailty and the degradation of complex balance dynamics during a dual-task protocol. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Mengarelli, A.; Verdini, F.; Cardarelli, S.; Tigrini, A.; Strazza, A.; Di Nardo, F. Complexity Measures of Postural Control in Type-2 Diabetic Subjects. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3527–3530. [Google Scholar]
- Sondergaard, K.H.; Olesen, C.G.; Sondergaard, E.K.; de Zee, M.; Madeleine, P. The variability and complexity of sitting postural control are associated with discomfort. J. Biomech. 2010, 43, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N. Innovative Analyses of Human Movement; Human Kinetics: Champaign, IL, USA, 2003. [Google Scholar]
- Lake, D.E.; Richman, J.S.; Griffin, M.P.; Moorman, J.R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Sepehri, N.; Wu, C.; Szturm, T. Sample entropy of human gait center of pressure displacement: A systematic methodological analysis. Entropy 2018, 20, 579. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Schlosser, F.J.; Sumpio, B.E. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J. Vasc. Surg. 2009, 50, 675–682.e1. [Google Scholar] [CrossRef]
- Mueller, M.J.; Hastings, M.; Commean, P.K.; Smith, K.E.; Pilgram, T.K.; Robertson, D.; Johnson, J. Forefoot structural predictors of plantar pressures during walking in people with diabetes and peripheral neuropathy. J. Biomech. 2003, 36, 1009–1017. [Google Scholar] [CrossRef]
- Nurse, M.A.; Nigg, B.M. The effect of changes in foot sensation on plantar pressure and muscle activity. Clin. Biomech. 2001, 16, 719–727. [Google Scholar] [CrossRef]
- Manor, B.; Doherty, A.; Li, L. The reliability of physical performance measures in peripheral neuropathy. Gait Posture 2008, 28, 343–346. [Google Scholar] [CrossRef]
- Song, Q.; Sun, M.; Lewis, K.; Chioi, J.H.; Manor, B.; Li, L. Hoffmann Reflex Measured from Lateral Gastrocnemius Is More Reliable than from Soleus among Elderly with Peripheral Neuropathy. Front. Aging Neurosci. 2022, 14, 800698. [Google Scholar] [CrossRef]
- Ferris, D.P.; Aagaard, P.; Simonsen, E.B.; Farley, C.T.; Dyhre-Poulsen, P. Soleus H-reflex gain in humans walking and running under simulated reduced gravity. J. Physiol. 2002, 530, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Zhou, S. Soleus H-reflex and its relation to static postural control. Gait Posture 2011, 33, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Laudani, L.; Wood, L.; Casabona, A.; Giuffrida, R.; De Vito, G. Effects of repeated ankle plantar-flexions on H-reflex and body sway during standing. J. Electromyogr. Kinesiol. 2009, 19, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; In, J.; Lee, S. Standard deviation and standard error of the mean. Korean J. Anesthesiol. 2015, 68, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Randall, M.J. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power Analysis for the Behavioral sciences. J. Am. Stat. Assoc. 1989, 14, 499–500. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Evan, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publ. Co.: Monterey, CA, USA, 1996; Volume 91. [Google Scholar]
- Blons, E.; Arsac, L.; Gilfriche, P.; Deschodt-Arsac, V. Multiscale Entropy of Cardiac and Postural Control Reflects a Flexible Adaptation to a Cognitive Task. Entropy 2019, 21, 1024. [Google Scholar] [CrossRef]
- Yamagata, M.; Ikezoe, T.; Kamiya, M.; Masaki, M.; Ichihashi, N. Correlation between movement complexity during static standing and balance function in institutionalized older adults. Clin. Interv. Aging 2017, 12, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, B.; Mansfield, A. Visual feedback of the centre of gravity to optimize standing balance. Gait Posture 2015, 41, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Mercer, V.S.; Stergiou, N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: A methodological report. J. Neuroeng. Rehabil. 2007, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-C.; Jiang, B. A Comparison of Postural Stability during Upright Standing between Normal and Flatfooted Individuals, Based on COP-Based Measures. Entropy 2017, 19, 76. [Google Scholar] [CrossRef]
- Ramdani, S.; Seigle, B.; Varoqui, D.; Bouchara, F.; Blain, H.; Bernard, P.L. Characterizing the dynamics of postural sway in humans using smoothness and regularity measures. Ann. Biomed. Eng. 2011, 39, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Sternad, D. Complexity of human postural control in young and older adults during prolonged standing. Exp. Brain Res. 2008, 191, 265–276. [Google Scholar] [CrossRef]
- Montesinos, L.; Castaldo, R.; Pecchia, L. On the use of approximate entropy and sample entropy with centre of pressure time-series. J. Neuroeng. Rehabil. 2018, 15, 116. [Google Scholar] [CrossRef]
- Chen, H.; Nigg, B.; Hulliger, M.; De Koning, J. Influence of sensory input on plantar pressure distribution. Clin. Biomech. 1995, 10, 271–274. [Google Scholar] [CrossRef]
- McKeon, P.O.; Hertel, J. Diminished plantar cutaneous sensation and postural control. Percept. Mot. Ski. 2007, 104, 56–66. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L. The differential effects of foot sole sensory on plantar pressure distribution between balance and gait. Gait Posture 2013, 37, 532–535. [Google Scholar] [CrossRef]
- Meyer, P.F.; Oddsson, L.I.; De Luca, C.J. Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp. Brain Res. 2004, 157, 526–536. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 8) | Less Affected (n = 10) | More Affected (n = 9) | F2,26 | p-Value | |
|---|---|---|---|---|---|
| Body mass (kg) | 68.1 ± 11.7 | 79.9 ± 12.2 | 91.8 ± 21.5 | 4.398 | 0.018 * |
| Height (cm) | 162.5 ± 6.2 | 166.4 ± 7.7 | 173.1 ± 8.7 | 4.356 | 0.028 * |
| BMI (kg/m2) | 25.7 ± 4.2 | 28.8 ± 3.9 | 30.4 ± 5.1 | 2.056 | 0.108 |
| Control | Less Affected | More Affected | ||||
|---|---|---|---|---|---|---|
| W | p-Value | W | p-Value | W | p-Value | |
| SD-AP (mm) | 0.905 | 0.322 | 0.949 | 0.653 | 0.882 | 0.167 |
| SD-ML (mm) | 0.925 | 0.470 | 0.940 | 0.554 | 0.866 | 0.110 |
| SampENAP | 0.933 | 0.545 | 0.904 | 0.239 | 0.914 | 0.343 |
| SampENML | 0.780 | 0.018 * | 0.884 | 0.144 | 0.950 | 0.689 |
| Standing H/M ratio | 0.916 | 0.398 | 0.916 | 0.329 | 0.947 | 0.662 |
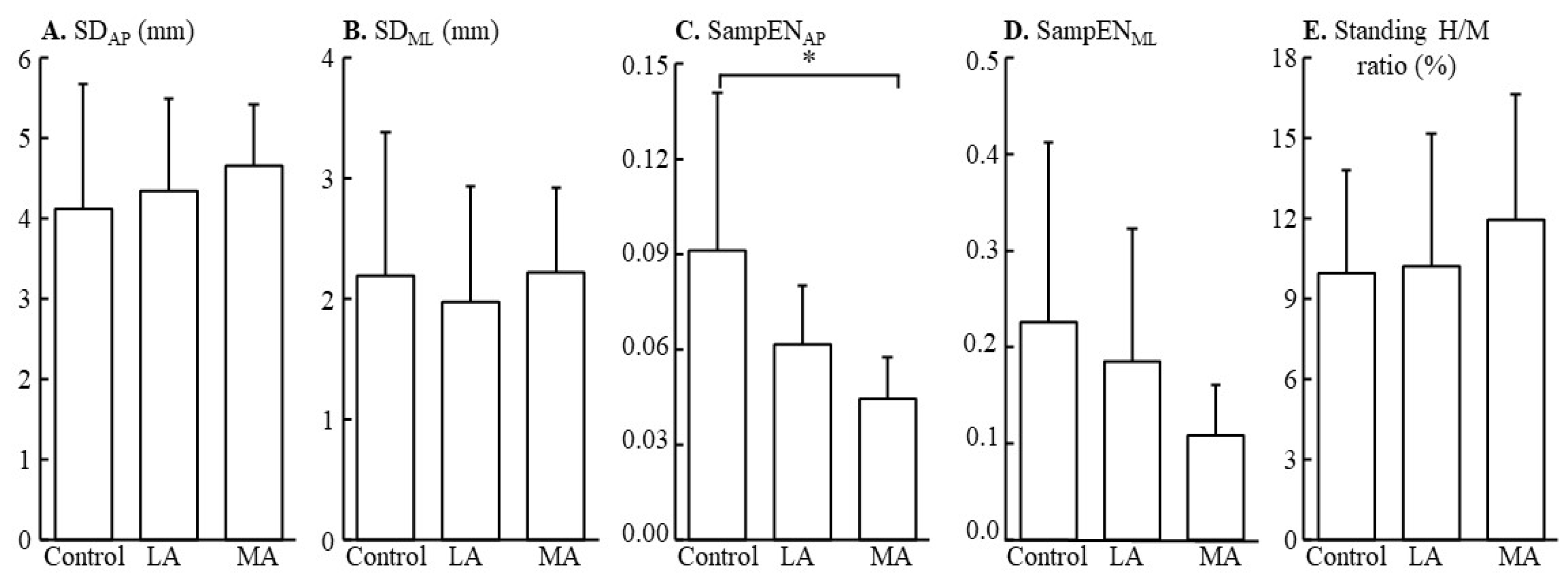
| Control | Less Affected | More Affected | p-Value | |
|---|---|---|---|---|
| SD-AP (mm) | 4 ± 2 | 4 ± 1 | 5 ± 1 | 0.648 |
| SD- ML (mm) | 2 ± 1 | 2 ± 1 | 2 ± 1 | 0.829 |
| SampENAP | 0.09 ± 0.05 | 0.06 ± 0.02 | 0.04 ± 0.01 a | 0.013 * |
| SampENML | 0.23 ± 0.19 | 0.18 ± 0.14 | 0.11 ± 0.05 | 0.358 |
| Standing H/M ratio | 10.0 ± 3.9% | 10.2 ± 5.0% | 11.9 ± 4.7% | 0.619 |
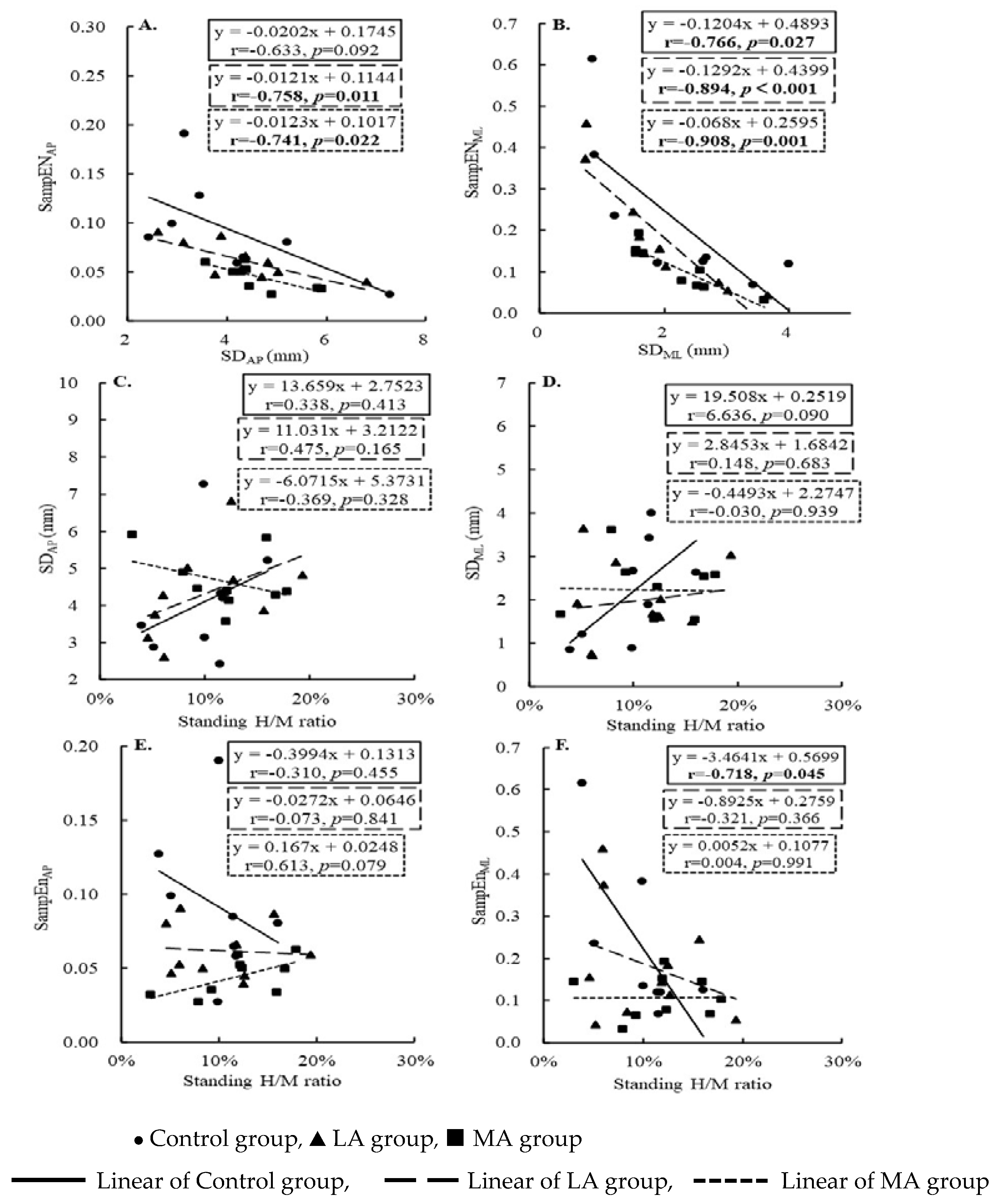
| Control | LA | MA | ||||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| SDAP and SampENAP | −0.633 | 0.092 | −0.758 | 0.011 * | −0.741 | 0.022 * |
| SDML and SampENML | −0.766 | 0.027 * | −0.894 | <0.001 * | −0.908 | 0.001 * |
| SDAP and Standing H/M ratio SDML and Standing H/M ratio SampENAP and Standing H/M ratio SampENML and Standing H/M ratio | 0.338 | 0.413 | 0.475 | 0.165 | −0.369 | 0.328 |
| 0.636 | 0.090 | 0.148 | 0.683 | −0.030 | 0.939 | |
| −0.310 | 0.455 | −0.073 | 0.841 | 0.613 | 0.079 | |
| −0.718 | 0.045 * | −0.321 | 0.366 | 0.004 | 0.991 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Zhang, F.; Lewis, K.; Song, Q.; Li, L. The Impact of Hoffmann Reflex on Standing Postural Control Complexity in the Elderly with Impaired Plantar Sensation. Entropy 2023, 25, 64. https://doi.org/10.3390/e25010064
Sun M, Zhang F, Lewis K, Song Q, Li L. The Impact of Hoffmann Reflex on Standing Postural Control Complexity in the Elderly with Impaired Plantar Sensation. Entropy. 2023; 25(1):64. https://doi.org/10.3390/e25010064
Chicago/Turabian StyleSun, Mengzi, Fangtong Zhang, Kelsey Lewis, Qipeng Song, and Li Li. 2023. "The Impact of Hoffmann Reflex on Standing Postural Control Complexity in the Elderly with Impaired Plantar Sensation" Entropy 25, no. 1: 64. https://doi.org/10.3390/e25010064
APA StyleSun, M., Zhang, F., Lewis, K., Song, Q., & Li, L. (2023). The Impact of Hoffmann Reflex on Standing Postural Control Complexity in the Elderly with Impaired Plantar Sensation. Entropy, 25(1), 64. https://doi.org/10.3390/e25010064







