Practical Considerations for the Application of Nonlinear Indices Characterizing the Atrial Substrate in Atrial Fibrillation
Abstract
:1. Introduction
2. Material and Methods
2.1. Database and Preprocessing
2.2. CFAE Segment Discarding Process
2.3. Sample Entropy
2.4. Recurrence Plots and Recurrence Quantification Analysis Measures
2.5. Atrial Fibrillation Cycle Length
2.6. Dominant Frequency
2.7. Intra-Recording and Intra-Patient Stability Assessment
2.8. Statistical Feature Assessment
2.9. Feature Selection
2.10. Classification of ParAF and PerAF with the Models
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.H.; Franco, O.H.; Hofman, A.; Witteman, J.C.M.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef]
- Blomstrom Lundqvist, C.; Lip, G.Y.H.; Kirchhof, P. What are the costs of atrial fibrillation? EP Eur. 2011, 13, ii9–ii12. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, S.W.; Chouairi, F.; Miller, P.E.; Clark, K.A.A.; Kay, B.; Fuery, M.; Guha, A.; Freeman, J.V.; Ahmad, T.; Desai, N.R.; et al. National Trends in the Burden of Atrial Fibrillation During Hospital Admissions for Heart Failure. J. Am. Heart Assoc. 2021, 10, e019412. [Google Scholar] [CrossRef]
- Lee, E.; Choi, E.K.; Han, K.D.; Lee, H.; Choe, W.S.; Lee, S.R.; Cha, M.J.; Lim, W.H.; Kim, Y.J.; Oh, S. Mortality and causes of death in patients with atrial fibrillation: A nationwide population-based study. PLoS ONE 2018, 13, e0209687. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar]
- Camm, A.J.; Camm, C.F.; Savelieva, I. Medical treatment of atrial fibrillation. J. Cardiovasc. Med. 2012, 13, 97–107. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Hesselson, A.B. Catheter Ablation in the Treatment of Atrial Fibrillation. Int. J. Angiol. 2020, 29, 108–112. [Google Scholar] [CrossRef]
- Prystowsky, E.N.; Padanilam, B.J.; Fogel, R.I. Treatment of Atrial Fibrillation. JAMA 2015, 314, 278–288. [Google Scholar] [CrossRef]
- Shi, R.; Chen, Z.; Pope, M.T.B.; Zaman, J.A.B.; Debney, M.; Marinelli, A.; Boyalla, V.; Sathishkumar, A.; Karim, N.; Cantor, E.; et al. Individualized ablation strategy to treat persistent atrial fibrillation: Core-to-boundary approach guided by charge-density mapping. Heart Rhythm. 2021, 18, 862–870. [Google Scholar] [CrossRef]
- Kottkamp, H.; Bender, R.; Berg, J. Catheter ablation of atrial fibrillation: How to modify the substrate? J. Am. Coll. Cardiol. 2015, 65, 196–206. [Google Scholar] [CrossRef]
- Quah, J.X.; Dharmaprani, D.; Tiver, K.; Lahiri, A.; Hecker, T.; Perry, R.; Selvanayagam, J.B.; Joseph, M.X.; McGavigan, A.; Ganesan, A. Atrial fibrosis and substrate based characterization in atrial fibrillation: Time to move forwards. J. Cardiovasc. Electrophysiol. 2021, 32, 1147–1160. [Google Scholar] [CrossRef]
- Igarashi, T.; Niwano, S.; Fukaya, H.; Yoshizawa, T.; Nakamura, H.; Fujiishi, T.; Ishizue, N.; Oikawa, J.; Kishihara, J.; Murakami, M.; et al. Discrimination of Paroxysmal and Persistent Atrial Fibrillation in Patients With New-Onset Atrial Fibrillation. Int. Heart J. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Latchamsetty, R.; Morady, F. Catheter Ablation of Atrial Fibrillation. Heart Fail. Clin. 2016, 12, 223–233. [Google Scholar] [CrossRef]
- Ciaccio, E.J.; Biviano, A.B.; Whang, W.; Vest, J.A.; Gambhir, A.; Einstein, A.J.; Garan, H. Differences in repeating patterns of complex fractionated left atrial electrograms in longstanding persistent atrial fibrillation as compared with paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 470–477. [Google Scholar] [CrossRef]
- Nademanee, K.; McKenzie, J.; Kosar, E.; Schwab, M.; Sunsaneewitayakul, B.; Vasavakul, T.; Khunnawat, C.; Ngarmukos, T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004, 43, 2044–2053. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Sanders, P.; Hocini, M.; Hsu, L.F.; Shah, D.C.; Scavée, C.; Takahashi, Y.; Rotter, M.; Pasquié, J.L.; Garrigue, S.; et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation 2004, 109, 3007–3013. [Google Scholar] [CrossRef]
- Cirugeda-Roldán, E.; Novak, D.; Kremen, V.; Cuesta-Frau, D.; Keller, M.; Luik, A.; Srutova, M. Characterization of Complex Fractionated Atrial Electrograms by Sample Entropy: An International Multi-Center Study. Entropy 2015, 17, 7493–7509. [Google Scholar] [CrossRef]
- Ciaccio, E.J.; Biviano, A.B.; Whang, W.; Gambhir, A.; Garan, H. Different characteristics of complex fractionated atrial electrograms in acute paroxysmal versus long-standing persistent atrial fibrillation. Heart Rhythm 2010, 7, 1207–1215. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Ciaccio, E.J.; Koh, J.E.W.; Oh, S.L.; Tan, R.S.; Garan, H. Application of nonlinear methods to discriminate fractionated electrograms in paroxysmal versus persistent atrial fibrillation. Comput. Methods Programs Biomed. 2019, 175, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Karch, M.R.; Schneider, M.A.E.; Weyerbrock, S.; Schreieck, J.; Deisenhofer, I.; Zrenner, B.; Schömig, A.; Schmitt, C. Characterization of paroxysmal and persistent atrial fibrillation in the human left atrium during initiation and sustained episodes. J. Cardiovasc. Electrophysiol. 2002, 13, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.C.; Krummen, D.E.; Tran, A.J.; Bullinga, J.R.; Narayan, S.M. Electrocardiographic measurements of regional atrial fibrillation cycle length. Pacing Clin. Electrophysiol. 2009, 32 (Suppl. S1), S66–S71. [Google Scholar] [CrossRef]
- Sanders, P.; Berenfeld, O.; Hocini, M.; Jaïs, P.; Vaidyanathan, R.; Hsu, L.F.; Garrigue, S.; Takahashi, Y.; Rotter, M.; Sacher, F.; et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005, 112, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Roten, L.; Derval, N.; Pascale, P.; Scherr, D.; Komatsu, Y.; Shah, A.; Ramoul, K.; Denis, A.; Sacher, F.; Hocini, M.; et al. Current hot potatoes in atrial fibrillation ablation. Curr. Cardiol. Rev. 2012, 8, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iniesta, M.; Ródenas, J.; Rieta, J.J.; Alcaraz, R. The stationary wavelet transform as an efficient reductor of powerline interference for atrial bipolar electrograms in cardiac electrophysiology. Physiol. Meas. 2019, 40, 075003. [Google Scholar] [CrossRef] [PubMed]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Alcaraz, R.; Abásolo, D.; Hornero, R.; Rieta, J.J. Optimal parameters study for sample entropy-based atrial fibrillation organization analysis. Comput. Methods Programs Biomed. 2010, 99, 124–132. [Google Scholar] [CrossRef]
- Marwan, N.; Carmen Romano, M.; Thiel, M.; Kurths, J. Recurrence plots for the analysis of complex systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Marwan, N.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Kurths, J. Recurrence-plot-based measures of complexity and their application to heart-rate-variability data. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2002, 66, 026702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noakes, L. The takens embedding theorem. Int. J. Bifurc. Chaos 1991, 1, 867–872. [Google Scholar] [CrossRef]
- Rhodes, C.; Morari, M. The false nearest neighbors algorithm: An overview. Comput. Chem. Eng. 1997, 21, S1149–S1154. [Google Scholar] [CrossRef]
- Hoekstra, B.; Diks, C.; Allessie, M.; Degoede, J. Non-linear time series analysis: Methods and applications to atrial fibrillation. Ann. Dell’Ist. Super. Sanit. 2001, 37, 325–333. [Google Scholar]
- Schinkel, S.; Dimigen, O.; Marwan, N. Selection of recurrence threshold for signal detection. Eur. Phys. J. Spec. Top. 2008, 164, 15–53. [Google Scholar] [CrossRef]
- Baumert, M.; Sanders, P.; Ganesan, A. Quantitative-Electrogram-Based Methods for Guiding Catheter Ablation in Atrial Fibrillation. Proc. IEEE 2016, 104, 416–431. [Google Scholar] [CrossRef]
- Botteron, G.W.; Smith, J.M. A Technique for Measurement of the Extent of Spatial Organization of Atrial Activation During Atrial Fibrillation in the Intact Human Heart. IEEE Trans. Biomed. Eng. 1995, 42, 579–586. [Google Scholar] [CrossRef]
- Osorio, D.; Vraka, A.; Quesada, A.; Hornero, F.; Alcaraz, R.; Rieta, J.J. An Efficient Hybrid Methodology for Local Activation Waves Detection under Complex Fractionated Atrial Electrograms of Atrial Fibrillation. Sensors 2022, 22, 5345. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Husser, D.; Mainardi, L.; Lombardi, F.; Langley, P.; Murray, A.; Rieta, J.J.; Millet, J.; Olsson, S.B.; Stridh, M.; et al. Analysis of surface electrocardiograms in atrial fibrillation: Techniques, research, and clinical applications. Europace 2006, 8, 911–926. [Google Scholar] [CrossRef]
- Atienza, F.; Almendral, J.; Jalife, J.; Zlochiver, S.; Ploutz-Snyder, R.; Torrecilla, E.G.; Arenal, A.; Kalifa, J.; Fernández-Avilés, F.; Berenfeld, O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009, 6, 33–40. [Google Scholar] [CrossRef]
- Rieta, J.J.; Castells, F.; Sánchez, C.; Zarzoso, V.; Millet, J. Atrial activity extraction for atrial fibrillation analysis using blind source separation. IEEE Trans. Biomed. Eng. 2004, 51, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargha, A.; Delaney, H.D. The Kruskal-Wallis Test and Stochastic Homogeneity. J. Educ. Behav. Stat. 1998, 23, 170–192. [Google Scholar] [CrossRef]
- Honda, Y. Testing the Error Components Model with Non-Normal Disturbances. Rev. Econ. Stud. 1985, 52, 681–690. [Google Scholar] [CrossRef]
- Nachar, N. The Mann-Whitney U: A Test for Assessing Whether Two Independent Samples Come from the Same Distribution. Tutor. Quant. Methods Psychol. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An Introduction of Variable and Feature Selection. J. Mach. Learn. Res. Spec. Issue Var. Feature Sel. 2003, 3, 1157–1182. [Google Scholar]
- Shi, T.; Horvath, S. Unsupervised Learning With Random Forest Predictors. J. Comput. Graph. Stat. 2006, 15, 118–138. [Google Scholar] [CrossRef]
- Gill, J.S. How to perform pulmonary vein isolation. Europace 2004, 6, 83–91. [Google Scholar] [CrossRef]
- Marshall, D.A.; O’Brien, B.J.; Nichol, G. Review of economic evaluations of radiofrequency catheter ablation for cardiac arrhythmias. Can. J. Cardiol. 2003, 19, 1285–1304. [Google Scholar]
- Rodgers, M.; McKenna, C.; Palmer, S.; Chambers, D.; Van Hout, S.; Golder, S.; Pepper, C.; Todd, D.; Woolacott, N. Curative catheter ablation in atrial fibrillation and typical atrial flutter: Systematic review and economic evaluation. Health Technol. Assess. 2008, 12, iii–iv, xi–xiii, 1–198. [Google Scholar] [CrossRef]
- Jarman, J.W.E.; Wong, T.; Kojodjojo, P.; Spohr, H.; Davies, J.E.; Roughton, M.; Francis, D.P.; Kanagaratnam, P.; Markides, V.; Davies, D.W.; et al. Spatiotemporal behavior of high dominant frequency during paroxysmal and persistent atrial fibrillation in the human left atrium. Circ. Arrhythmia Electrophysiol. 2012, 5, 650–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]


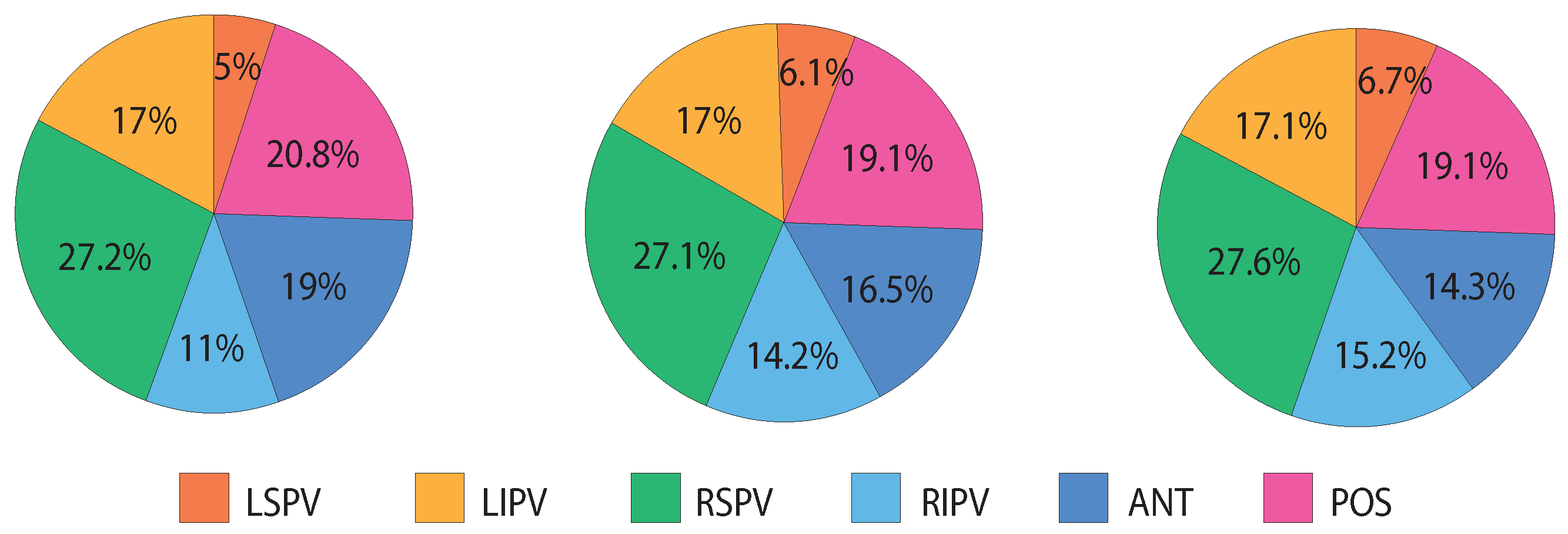
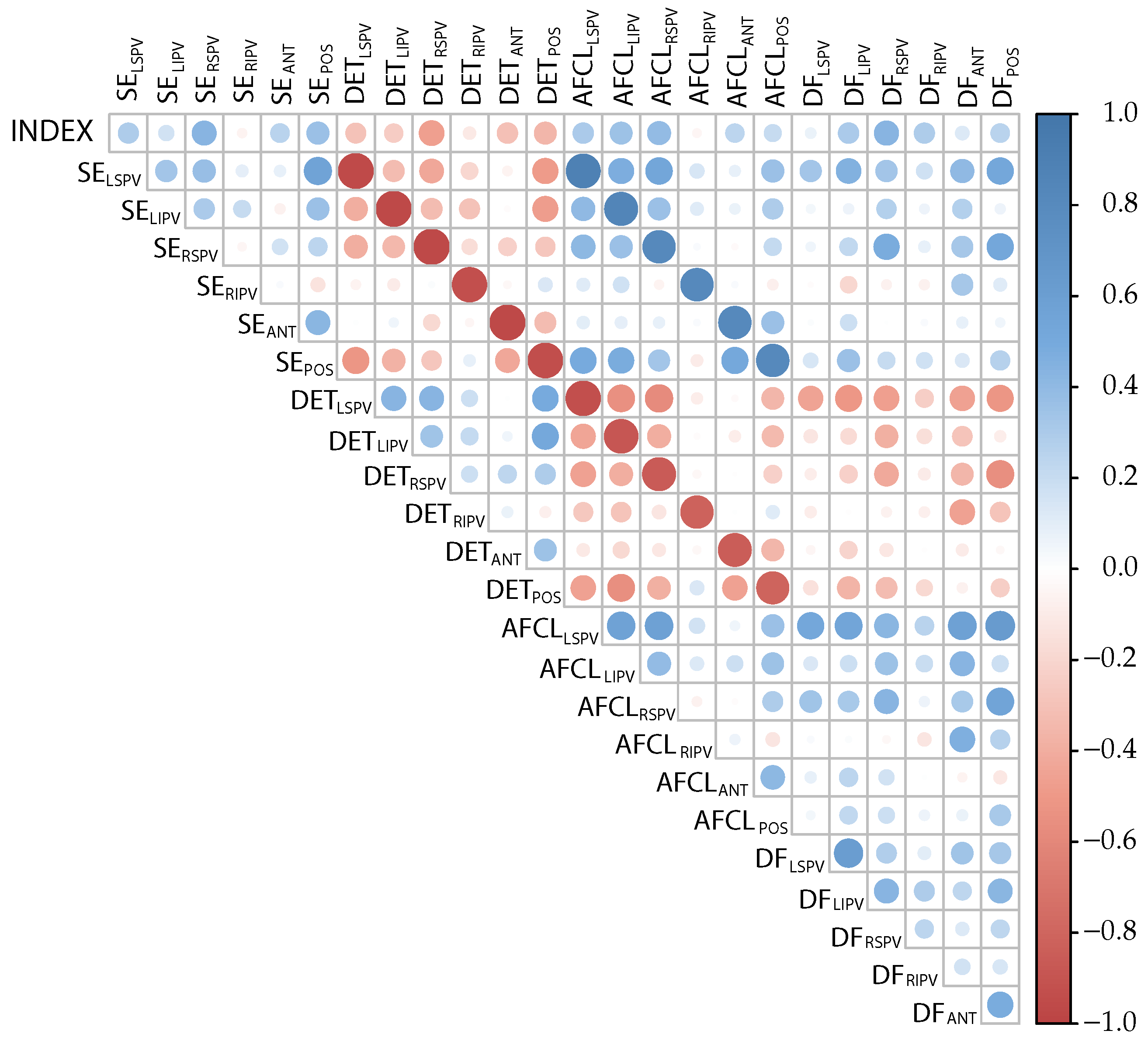

| Length | No Discard | Discard | ||
|---|---|---|---|---|
| Range | Mean ± Std | Range | Mean ± Std | |
| h | [0.004–0.362] | 0.137 ± 0.033 | [0.013–0.362] | 0.138 ± 0.032 |
| 2 s | [0.007–0.312] | 0.134 ± 0.025 | [0.018–0.312] | 0.135 ± 0.023 |
| 4 s | [0.008–0.300] | 0.132 ± 0.017 | [0.020–0.300] | 0.134 ± 0.014 |
| Length | No Discard | Discard | ||
|---|---|---|---|---|
| Range | Mean ± Std | Range | Mean ± Std | |
| 1 s | [0.075–0.998] | 0.561 ± 0.120 | [0.075–0.963] | 0.559 ± 0.113 |
| 12 | [0.122–1.000] | 0.600 ± 0.101 | [0.122–0.958] | 0.595 ± 0.091 |
| 4 s | [0.092–1.000] | 0.631 ± 0.072 | [0.124–0.942] | 0.621 ± 0.058 |
| 1 s | CV 1 s | 2 s | CV 2 s | 4 s | CV 4 s | |
|---|---|---|---|---|---|---|
| DET | 23.3% | −15.6% | 19.1% | −22.8% | 13.3% | −47.9% |
| SE | 26.6% | −16.1% | 20.5% | −20.1% | 13.9% | −42.2% |
| 1 s | CV 1 s | 2 s | CV 2 s | 4 s | CV 4 s | |
|---|---|---|---|---|---|---|
| DET | 23.9% | 24.8% | 24.1% | |||
| SE | 34.2% | 34.8% | 35.9% |
| Length | SE | DET | ||
|---|---|---|---|---|
| ParAF | PerAF | ParAF | PerAF | |
| 1 s | 0.123 ± 0.042 | 0.148 ± 0.057 | 0.608 ± 0.135 | 0.524 ± 0.160 |
| 2 s | 0.120 ± 0.043 | 0.145 ± 0.056 | 0.656 ± 0.140 | 0.556 ± 0.177 |
| 4 s | 0.117 ± 0.043 | 0.143 ± 0.057 | 0.688 ± 0.135 | 0.585 ± 0.179 |
| Recording Place | SE | DET | AFCL | DF |
|---|---|---|---|---|
| LSPV | ||||
| LIPV | ||||
| RSPV | ||||
| RIPV | ||||
| ANT | ||||
| POS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finotti, E.; Quesada, A.; Ciaccio, E.J.; Garan, H.; Hornero, F.; Alcaraz, R.; Rieta, J.J. Practical Considerations for the Application of Nonlinear Indices Characterizing the Atrial Substrate in Atrial Fibrillation. Entropy 2022, 24, 1261. https://doi.org/10.3390/e24091261
Finotti E, Quesada A, Ciaccio EJ, Garan H, Hornero F, Alcaraz R, Rieta JJ. Practical Considerations for the Application of Nonlinear Indices Characterizing the Atrial Substrate in Atrial Fibrillation. Entropy. 2022; 24(9):1261. https://doi.org/10.3390/e24091261
Chicago/Turabian StyleFinotti, Emanuela, Aurelio Quesada, Edward J. Ciaccio, Hasan Garan, Fernando Hornero, Raúl Alcaraz, and José J. Rieta. 2022. "Practical Considerations for the Application of Nonlinear Indices Characterizing the Atrial Substrate in Atrial Fibrillation" Entropy 24, no. 9: 1261. https://doi.org/10.3390/e24091261
APA StyleFinotti, E., Quesada, A., Ciaccio, E. J., Garan, H., Hornero, F., Alcaraz, R., & Rieta, J. J. (2022). Practical Considerations for the Application of Nonlinear Indices Characterizing the Atrial Substrate in Atrial Fibrillation. Entropy, 24(9), 1261. https://doi.org/10.3390/e24091261







